- Department of Vascular Surgery, West China Hospital, Sichuan University, Chengdu, China
Non-traumatic lower limb ischemic diseases are extremely rare among young people. Clinically, they are mainly seen in the form of popliteal artery entrapment syndrome (PAES). In addition, with the prevalence of COVID-19 infection, more and more studies report that COVID-19 infection may lead to arteriovenous thrombosis, which could cause lower limb ischemia. This case reported that a 31-year-old male amateur football player who developed intermittent claudication after recovering from COVID-19. After 2 months of consultation, he was ultimately diagnosed with PAES. As is well known, PAES is mostly caused by long-term compression of the popliteal artery by abnormal anatomical structures, resulting in thickening of the vascular outer membrane and progression of the disease until intimal damage and thrombosis, leading to lower limb ischemia. During the progression of the disease, there may be multiple factors that accelerate its progression. Therefore, combined with the patient's clinical history and related studies on confirmed thrombosis caused by COVID-19, we can infer that COVID-19 could accelerate the occurrence of PAES.
Introduction
Popliteal artery entrapment syndrome (PAES) is a rare clinical ischemic disease of the lower limbs, mainly caused by various reasons such as popliteal artery compression and stenosis and secondary thrombosis caused by an injury of the vascular endothelium due to exercise and other factors (1–3). It is more common among young people, especially sports professionals and enthusiasts (3). The main manifestation is progressive motor pain in the lower limbs, and the combination of high-risk factors such as infection and smoking for thrombosis will exacerbate lower limb ischemia. If not diagnosed and treated appropriately, it may lead to disability and even serious consequences, such as amputation (4). This disease is easily confused with lower extremity arterial ischemic diseases such as lower extremity arterial thromboangiitis obliterans, lower extremity arterial embolism, and lower extremity atherosclerosis obliterans (5, 6). Therefore, clinical diagnosis is difficult and prone to misdiagnosis, which can affect treatment decisions and clinical prognosis. At present, there are many reports on cases of PAES, but there is no report on cases of acute exacerbation of lower limb ischemia induced by COVID-19.
Case description
A 31-year-old male amateur football player who took part in football 1 week after recovering from COVID-19 developed intermittent claudication, accompanied by pain and discomfort of the left lower limb. The symptoms of claudication did not subside for 2 months, and the pulsation of the left dorsal foot artery was decreased. During this period, he had been traveling to many hospitals without a clear diagnosis. After admission to our hospital, lower limb computed tomography angiography (CTA) indicated thrombosis in the P2 segment of the left popliteal artery, with complete occlusion of the lumen (Figure 1A). Magnetic resonance imaging (MRI) of the left knee joint indicated the filling defect in the P2 segment of the left popliteal artery was considered to be possibly due to thrombosis, and the superficial surface of the P2 segment of the popliteal artery was suspected to be crossed by an abnormal ribbon of fibers (Figure 1B). Furthermore, functional color doppler ultrasound revealed that the bilateral popliteal arteries showed compression and stenosis of the lumen in the extended position. We performed left popliteal artery lesion resection and autologous saphenous vein bypass surgery for the patient. Exploring the popliteal fossa confirmed that the strip-like ligament passed through the surface of the popliteal artery, and the affected popliteal artery showed a strip-like change without pulsation (Figure 1C). The fibrous strips on the surface of the popliteal artery were severed and loosened, and an organic thrombus was found within the popliteal artery (Figure 1D). The bypass popliteal artery was well filled and pulsated after surgery (Figure 1E). After 3 months of anticoagulation therapy with 20 mg rivasaban tablets once a day, the patient started playing football without symptoms in the lower limbs. Moreover, the lower limb CTA showed that a patent great saphenous vein bypassed the left popliteal artery (Figure 1F). Figure 2 shows the timeline diagram of the diagnosis, treatment, and follow-up of the patient.
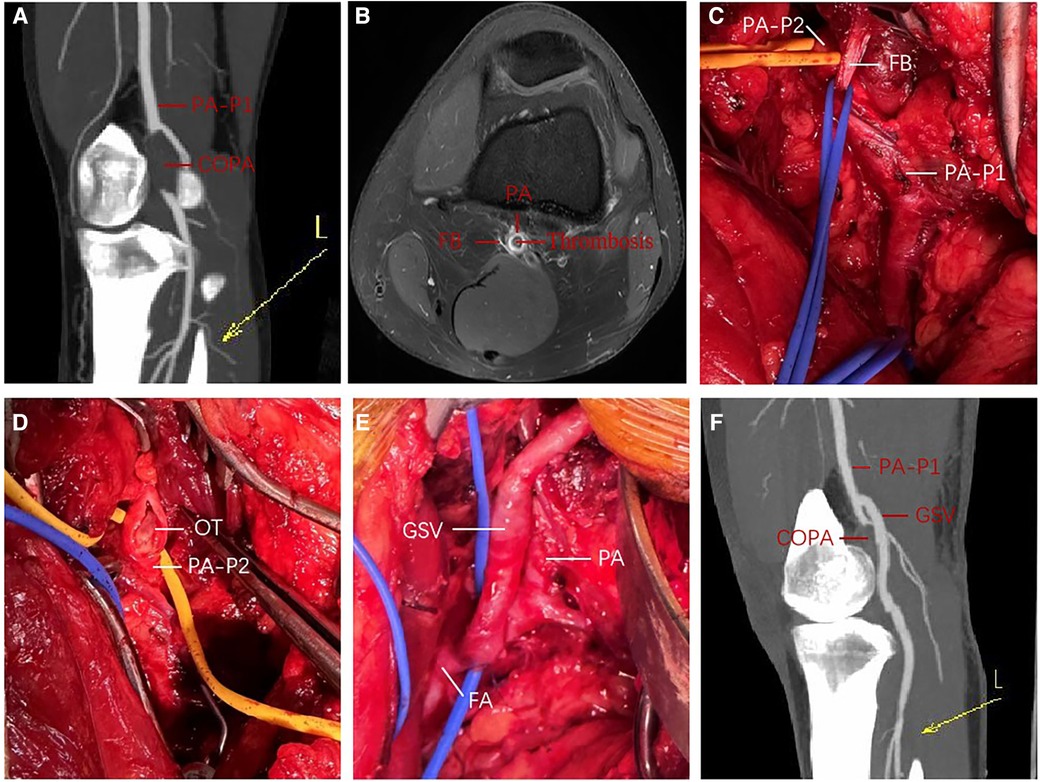
Figure 1. Perioperative findings in a young man with PAES; (A) preoperative 3D contrast-enhanced CTA of left lower extremity; (B) preoperative enhanced MRI T1W imaging of left knee joint; (C,E) intraoperative exploration and left popliteal artery autologous saphenous vein bypass; (F) 3D contrast-enhanced CTA of left lower extremity at 3 months after operation. COPA, completely occluded popliteal artery; CTA, computed tomography angiography; FA, femoral artery; FB, fibrous band; GSV, great saphenous vein; L, left lower extremity; MRI, magnetic resonance imaging; OT, organic thrombus; PA, popliteal artery; PA-P1, the P1 segment of the popliteal artery; PA-P2, the P2 segment of the popliteal artery; PAES, popliteal artery entrapment syndrome; 3D, three-dimensional.
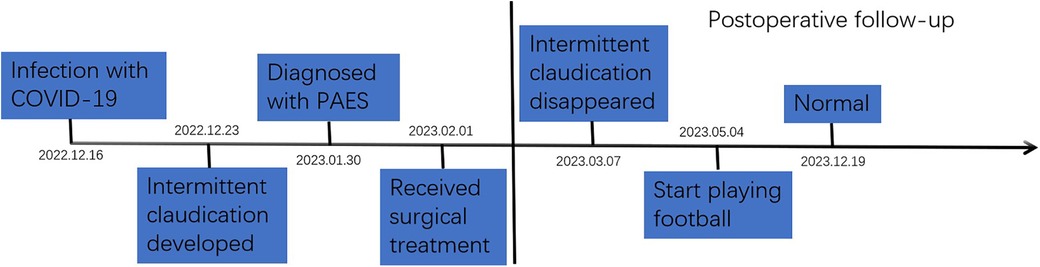
Figure 2. The timeline diagram of the diagnosis, treatment, and follow-up of the patient with PAES. PAES, popliteal artery entrapment syndrome.
Discussion
PAES is a rare disease that often causes intermittent claudication of the lower limbs and is common among athletes. Its incidence rate is 0.6%–3.5% (3, 4). PAES is based on congenital anatomical abnormalities, such as abnormal development of the popliteal fossa structure that compresses the popliteal artery. These cases are classified according to the Love and Whelan classification modified by Rich (7). As it was found that the fibrous cord had collapsed through the popliteal artery, this case was type IV. The preoperative diagnosis of PAES mainly relies on imaging examinations such as lower limb ultrasound, CTA, and, especially, MRI, due to its advantages in soft tissue imaging (8, 9). Even so, the imaging manifestations of the soft tissue around the compressed popliteal artery are still difficult to detect (8). Therefore, it is easily misdiagnosed as lower limb arterial thromboangiitis obliterans, lower limb arterial embolism, and other ischemic diseases (5, 6). Furthermore, certain factors may lead to the worsening of PAES ischemia. These include blood hypercoagulability and vascular endothelial damage (10). Interestingly, this case reported that the patient developed intermittent claudication 1 week after COVID-19 infection and had no symptoms before that. At present, some studies have reported that patients with COVID-19 have arteriovenous thrombosis (11–14). COVID-19 causes endothelial cell damage, exposes collagen, causes coagulation activation, and then induces thrombosis (11–14). Generally speaking, the abnormal fibrous band crossing the superficial surface of the popliteal artery in patients is innate. In addition, it is well known that PAES is often caused by long-term compression of the popliteal artery by abnormal anatomical structures, resulting in thickening of the outer membrane of the blood vessels and progression of the disease until intimal damage and thrombosis, leading to lower limb ischemia. During the progression of the disease, there may be multiple factors that accelerate the progression of the disease. Therefore, combined with the patient's clinical history and related studies on confirmed thrombosis caused by COVID-19, we can infer that COVID-19 could accelerate the occurrence of PAES. Of course, these are just speculations, and there is currently no relevant laboratory test to support them. Ultimately, large-scale clinical studies are needed for validation.
When patients have early symptoms of lower limb pain, it is necessary to actively perform relevant examinations and perform a clear diagnosis to avoid delaying treatment. Anticoagulants and vasodilators are the conventional therapy. Furthermore, open surgery and endovascular intervention are the fundamental therapies to cure patients. The key to treating PAES lies in relieving popliteal artery compression and restoring the blood supply to the affected limb. Clinically, for patients with acute ischemia whose disease duration is less than 2 weeks, femoral artery incision thrombectomy or catheterization thrombolysis of the affected limb can be selected to restore the blood supply of the lower limb (15, 16). Furthermore, for patients with a disease duration of more than 2 weeks, thrombectomy, venous patch arterioplasty, and autologous/artificial vessel bypass surgery can be adopted to restore the blood supply of the affected lower limb (17, 18). Moreover, based on the current literature results, vascular bypass surgery is recommended as a priority for blood flow reconstruction (18). Thus, popliteal artery bypass surgery may be the best treatment for PAES (19, 20).
Conclusion
PAES is the most common non-traumatic lower limb ischemic disease in young people, and its diagnosis relies on CTA/MRI and clinical history during exercise. All patients with clear PAES should undergo surgical treatment. COVID-19 may increase the risk of arteriovenous thrombosis, which may further aggravate the acute ischemia of lower limbs in people with PAES. Thus, these people tend to be given preventive anticoagulant treatment to avoid serious complications such as amputation. However, due to the limitations of case reports, this still needs further research with large samples for further confirmation.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
The studies involving humans were approved by West China Hospital of Sichuan University Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LB: Writing – original draft. DX: Conceptualization, Funding acquisition, Investigation, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bouhoutsos J, Daskalakis E. Muscular abnormalities affecting the popliteal vessels. Br J Surg. (1981) 68(7):501–6. doi: 10.1002/bjs.1800680720
2. Gibson MH, Mills JG, Johnson GE, Downs AR. Popliteal entrapment syndrome. Ann Surg. (1977) 185(3):341–8. doi: 10.1097/00000658-197703000-00016
3. Sinha S, Houghton J, Holt PJ, Thompson MM, Loftus IM, Hinchliffe RJ. Popliteal entrapment syndrome. J Vasc Surg. (2012) 55(1):252–62.e30. doi: 10.1016/j.jvs.2011.08.050
4. Collins PS, Mcdonald PT, Lim RC. Popliteal artery entrapment: an evolving syndrome. J Vasc Surg. (1989) 10(5):484–9; discussion 489–90. doi: 10.1016/0741-5214(89)90129-8
5. Igari K, Sugano N, Kudo T, Toyofuku T, Jibiki M, Inoue Y, et al. Surgical treatment for popliteal artery entrapment syndrome. Ann Vasc Dis. (2014) 7(1):28–33. doi: 10.3400/avd.oa.13-00081
6. Song X, Zhou M, Tang L, Liu Z, Zheng Y, Chen Y. Popliteal artery entrapment syndrome as a cause of failed treatment of a false popliteal aneurysm. J Int Med Res. (2020) 48(2):300060519868628. doi: 10.1177/0300060519868628
7. Rich NM, Hughes CW. Popliteal artery and vein entrapment. Am J Surg. (1967) 113(5):696–8. doi: 10.1016/0002-9610(67)90323-6
8. Chernoff DM, Walker AT, Khorasani R, Polak JF, Jolesz FA. Asymptomatic functional popliteal artery entrapment: demonstration at MR imaging. Radiology. (1995) 195(1):176–80. doi: 10.1148/radiology.195.1.7892463
9. Longchamp A, Longchamp J, Manzocchi BS, Danzer D. Trapped by the entrapment. EJVES Vasc Forum. (2020) 49:1–3. doi: 10.1016/j.ejvsvf.2020.07.031
10. Kayssi A, Shaikh F, Roche-Nagle G, Brandao LR, Williams SA, Rubin BB. Management of acute limb ischemia in the pediatric population. J Vasc Surg. (2014) 60(1):106–10. doi: 10.1016/j.jvs.2014.01.051
11. Berchiolli R, Torri L, Adami D, Bertagna G, Canovaro F, Troisi N. Inflammatory popliteal aneurysm associated with SARS-CoV-2 infection. Eur Rev Med Pharmacol Sci. (2023) 27(4):1708–12. doi: 10.26355/eurrev_202302_31414
12. Capoccia L, Mansour W, Di Marzo L, Grimaldi S, Di Girolamo A. Symptomatic popliteal artery aneurysms in recently SARS-CoV-2-infected patients: the microangiopathic thrombosis that undermines treatment. Diagnostics (Basel). (2023) 13(4):647–61. doi: 10.3390/diagnostics13040647
13. Hanff TC, Mohareb AM, Giri J, Cohen JB, Chirinos JA. Thrombosis in COVID-19. Am J Hematol. (2020) 95(12):1578–89. doi: 10.1002/ajh.25982
14. Sastry S, Cuomo F, Muthusamy J. COVID-19 and thrombosis: the role of hemodynamics. Thromb Res. (2022) 212:51–7. doi: 10.1016/j.thromres.2022.02.016
15. Settembre N, Bouziane Z, Bartoli MA, Nabokov V, Venermo M, Feugier P, et al. Popliteal artery entrapment syndrome in children: experience with four cases of acute ischaemia and review of the literature. Eur J Vasc Endovasc Surg. (2017) 53(4):576–82. doi: 10.1016/j.ejvs.2016.12.032
16. Skeik N, Thomas TM, Engstrom BI, Alexander JQ. Case report and literature review of popliteal artery entrapment syndrome. Int J Gen Med. (2015) 8:221–5. doi: 10.2147/IJGM.S82067
17. di Marzo L, Cavallaro A, O’donnell SD, Shigematsu H, Levien LJ, Rich NM. Endovascular stenting for popliteal vascular entrapment is not recommended. Ann Vasc Surg. (2010) 24(8):1135.e1–3. doi: 10.1016/j.avsg.2010.03.010
18. Lejay A, Delay C, Georg Y, Gaertner S, Ohana M, Thaveau F, et al. Five year outcomes of surgical treatment for popliteal artery entrapment syndrome. Eur J Vasc Endovasc Surg. (2016) 51(4):557–64. doi: 10.1016/j.ejvs.2015.12.015
19. Fujimura N, Obara H, Takahashi A, Miyata H, Hosaka A, ObitsU Y, et al. Surgical treatment for popliteal artery entrapment syndrome in Japan: a retrospective, multicentre study using a national clinical registry. Eur J Vasc Endovasc Surg. (2023) 66(3):381–8. doi: 10.1016/j.ejvs.2023.05.031
Keywords: case report, non-traumatic lower limb ischemic diseases, young adults, popliteal artery entrapment syndrome (PAES), COVID-19
Citation: Bo L and Xiaojiong D (2024) Case Report: COVID-19 exacerbates acute lower limb ischemia in patients with popliteal artery entrapment syndrome. Front. Cardiovasc. Med. 11:1329863. doi: 10.3389/fcvm.2024.1329863
Received: 31 October 2023; Accepted: 17 January 2024;
Published: 2 February 2024.
Edited by:
Pasqualino Sirignano, Sapienza University of Rome, ItalyReviewed by:
Alberto Settembrini, IRCCS Ca ‘Granda Foundation Maggiore Policlinico Hospital, ItalyAlessia Di Girolamo, Sapienza University of Rome, Italy
© 2024 Bo and Xiaojiong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Du Xiaojiong ZHV4aWFvamlvbmdAMTYzLmNvbQ==
 Li Bo
Li Bo Du Xiaojiong*
Du Xiaojiong*