- 1Department of Cardiovascular Diseases, Guang anmen Hospital Affiliated to China Academy of Chinese Medical Sciences, Beijing, China
- 2College of Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
- 3Department of Cardiovascular Diseases, Guangwai Hospital, Beijing, China
- 4Shanghai Qianhe Technology Co., Ltd., Shanghai, China
Background: The relationship between sleep characteristics and cardiovascular disease (CVD) risk has yet to reach a consistent conclusion, and more research needs to be carried out. This study aimed to explore the relationship between snoring, daytime sleepiness, bedtime, sleep duration, and high-risk sleep patterns with CVD risk.
Methods: Data from the National Health and Nutrition Examination Survey (NHANES) 2015–2018 were collected and analyzed. Multivariable logistic regression was used to evaluate the relationship between snoring, daytime sleepiness, bedtime, sleep duration, high-risk sleep patterns, and CVD risk. Stratified analysis and interaction tests were carried out according to hypertension, diabetes and age.
Results: The final analysis contained 6,830 participants, including 1,001 with CVD. Multivariable logistic regression suggested that the relationship between snoring [OR = 7.37,95%CI = (6.06,8.96)], daytime sleepiness [OR = 11.21,95%CI = (9.60,13.08)], sleep duration shorter than 7 h [OR = 9.50,95%CI = (7.65,11.79)] or longer than 8 h [OR = 6.61,95%CI = (5.33,8.19)], bedtime after 0:00 [OR = 13.20,95%CI = (9.78,17.80)] compared to 22:00–22:59, high-risk sleep patterns [OR = 47.73,95%CI = (36.73,62.04)] and CVD risk were statistically significant. Hypertension and diabetes interacted with high-risk sleep patterns, but age did not.
Conclusions: Snoring, daytime sleepiness, excessive or short sleep duration, inappropriate bedtime, and high-risk sleep patterns composed of these factors are associated with the CVD risk. High-risk sleep patterns have a more significant impact on patients with hypertension and diabetes.
1 Introduction
Cardiovascular disease (CVD) is the leading cause of morbidity, disability, and death worldwide, including in low-income countries, and has become a significant public health problem (1). In 2023, the American Heart Association reported that the number of CVD patients, including coronary heart disease (CHD), heart failure, hypertension, and stroke, in the U.S. was 127.9 million (2). Early intervention in CVD risk factors can prevent or reduce CVD incidence, which could benefits population health and relieve economic burden (3, 4). Therefore, identifying risk factors for CVD as much as possible is crucial.
About one-third of an individual's life is dedicated to sleep, emphasizing the criticality of quality sleep for overall health and well-being. Short-term sleep deprivation, long-term sleep restriction, circadian rhythm disturbances and untreated sleep disorders have been associated with far-reaching harmful effects on physical health, mental health, and public safety (5). Long-term lack of sleep will increase personal risks and social burdens related to several medical epidemics, including CVD, diabetes, obesity, and cancer (6). Previous studies have also discussed the relationship between sleep characteristics and CVD risk. A large cohort study found that insomnia was an independent risk factor for CVD, particularly in adults without hypertension (7). Another cohort study established a significant correlation between chronic snoring and an increased risk of ischemic heart disease and ischemic stroke, particularly among individuals under 50 years old (8). Other studies have also found that sleep time and daytime sleepiness were related to the increased risk of CVD (9–11).
However, previous studies assessing the relationship between sleep characteristics and CVD risk have yielded inconsistent results. A Dutch cohort study found that shorter sleep duration increased the risk of CVD compared with 7 h of sleep, while longer sleep duration had no such effect (12). Another cohort study involving multi-ethnic people found that both shorter and longer sleep durations were associated with an increased risk of CVD compared with 7 h of sleep duration, and this relationship was not affected by gender (13). In addition, most previous studies only focused on one sleep characteristic, despite various sleep factors being interactive rather than independent. Therefore, evaluating the correlation between multiple sleep characteristics and CVD risk may be more practical and feasible. For example, a study scored five sleep characteristics, such as insomnia and snoring, and found that healthy sleep patterns were related to reduced risk of CVD (14). Therefore, further study on the relationship between sleep and CVD is of great necessity, especially the comprehensive study of multiple sleep characteristics.
Therefore, we used data from the National Health and Nutrition Examination Survey (NHANES) 2015–2018, to assess the correlation between snoring, daytime sleepiness, bedtime, sleep duration, and CVD. This study also evaluated the association between high-risk sleep patterns and CVD risk by combining the above factors.
2 Methods
2.1 Study design
The data for this study were collected from the NHANES, a cross-sectional survey designed to assess the health and nutritional status of adults and children in the U.S. NHANES collects data every two years using multistage stratified sampling. The survey is unique in that it combines the home interview and the mobile examination. The NHANES interview includes demographic, socioeconomic, dietary, and health-related questionnaires. The examination includes medical, dental, and physiologic measurements and laboratory tests performed by trained medical personnel (15). The National Center for Health Statistics Ethical Review Board approved the study. Informed consent was obtained from all study participants (16). Therefore, no additional informed consent and ethical review were required for our research.
2.2 Study population
In this study, information was collected from participants in the 2017–2018 and 2015–2016 survey cycles, and participants were screened according to the following criteria: 1. participants without sleep information were excluded; 2. Participants without cardiovascular disease information were excluded; 3. Participants under the age of 40 were excluded (17). All remaining participants were included in the study.
2.3 Independent variable
Participants' sleep characteristics were obtained through questionnaires from trained interviewers at home using a computer-assisted personal interviewing (CAPI) system. The CAPI system was programmed with built-in consistency checks to minimize data entry errors. This study collected information on snoring, daytime sleepiness, bedtime, and sleep duration. Snoring was determined by asking participants, “ How often did you snore while you were sleeping?” and participants answered “ Never” or “Rarely, 1–2 nights a week” were defined as “no”, while responses of “Occasionally, 3–4 nights a week” or “Frequently, 5 nights a week and more” were defined as “yes”. Daytime sleepiness was determined by asking participants, “ How often did you feel excessively or overly sleepy during the day?” and participants answered “ Never” or “Rarely, 1–2 nights a week” were defined as “no”, while responses of “Occasionally, 3–4 nights a week” or “Frequently, 5 nights a week and more” were defined as “yes”. Bedtime was obtained by asking participants, “ What time do you usually sleep on weekdays or workdays?”. We categorized bedtimes into five categories using 21:00, 22:00, 23:00, and 00:00 as cutoff points. After asking participants about their weekday awakening time, the interval between awakening time and bedtime was sleep duration, which we categorized into three classifications using 7 and 8 h as cutoff points. The division of rest and sleep duration was determined by previous studies (18, 19). In addition, we defined sleep patterns by combining the four sleep characteristics mentioned above and classified sleep patterns as medium-low-risk and high-risk. The simultaneous occurrence of snoring, daytime drowsiness, sleeping for less than 7 or more than 8 h, and not going to bed between 22:00 and 23:00 was considered high-risk sleep patterns; otherwise, it was regarded as the medium-low-risk sleep pattern.
2.4 Dependent variable
CVD information was obtained through interviews between interviewers and participants. CVD in this study refers to CHD and stroke. CHD information was obtained by asking participants: “Has a doctor or other health professional ever told you that you had coronary heart disease?” “ Has a doctor or other health professional ever told you that you had angina, also called angina pectoris?” or “Has a doctor or other health professional ever told you that you had a heart attack (also called myocardial infarction)?” If they answered “Yes” to any of the above questions, they were considered CHD. Stroke was defined by asking the participant, “ Has a doctor or other health professional ever told you that you had a stroke?” if the participant answered “yes”, they were considered to have a stroke. Participants with CHD or stroke were defined to have CVD.
2.5 Covariates
Covariates included three components: demographics, history, and laboratory tests. Demographic information included gender, age, race, marriage, education, and family poverty income ratio (PIR). Medical history included hypertension, diabetes, cancer, smoking and alcohol use, and body mass index (BMI). Laboratory tests collected data on triglyceride (TG) and low density lipoprotein cholesterol (LDL-C). The trained interviewers used the CAPI system to conduct demographic and history questionnaires at the respondents' homes to obtain corresponding information. This study reclassified some of the original data. Marital status was divided into two categories according to whether the participant had a sexual partner. Participants who were married and cohabiting were defined as “yes”, and participants who were widowed, divorced, separated, and unmarried was described as “no”. Education level was divided into two categories, with high school as the boundary. PIR is divided into three equal parts. History of hypertension, diabetes, and cancer was obtained by asking participants the following questions: “Have you ever been told by a doctor or other health professional that you had hypertension, also called high blood pressure?” “Have you ever been told by a doctor or health professional that you had diabetes or sugar diabetes?” and “Have you ever been told by a doctor or other health professional that you had cancer or a malignancy of any kind?”. Smoking and drinking history were determined by asking participants, “Have you smoked at least 100 cigarettes in your entire life?” or “Was there ever a time or times in your life when you drank 4(female)/5(male) or more drinks of any kind of alcoholic beverage almost every day?”. Participants in the above questions answered “yes” or “no”, and refusing to answer was regarded as missing relevant data. After measuring the participants’ standing height and weight, BMI was calculated using the formula weight (Kg)/height (m) *2. Serum samples for laboratory examination were processed, stored, and shipped to the University of Minnesota for analysis. LDL-C and TG were obtained by standard biochemical profiling using a Beckman Synchron LX20.
2.6 Statistic analysis
Participants were divided into two groups according to whether they had CVD or not to describe the characteristics of the study population. Continuous variables were expressed as mean and standard deviation or median and quartile, and the t-test or Kruskal–Wallis rank sum test was selected for hypothesis testing according to applicable conditions. Classified variables were expressed as absolute numbers and percentages, and the chi-square test was used for hypothesis testing.
Multivariable logistics regression evaluated the relationship between snoring, daytime sleepiness, bedtime, sleep duration and CVD risk respectively. Bedtime and sleep duration were set as dummy variables when included in the model as multi-classification variables. Sleep patterns based on the four sleep characteristics above were also evaluated with the risk of CVD. In addition, CHD and stroke were separately analyzed as dependent variables.
To ensure the robustness of the results, we constructed several regression models. In Model 1, we only adjusted for demographic variables, including age, gender, race, marriage, education level, and PIR. Age was converted into a binary variable and included in the model. In Model 2, we further adjusted for smoking, drinking, and BMI in addition to variables in Model 1. BMI was converted into categorical variables using 18.5 and 25 as cut-off points and then included in the model. We additionally included the history of hypertension, diabetes, and cancer in Model 3 in addition to variables adjusted in Model 2. TG and LDL-C were converted into three-category variables and included in Model 3.
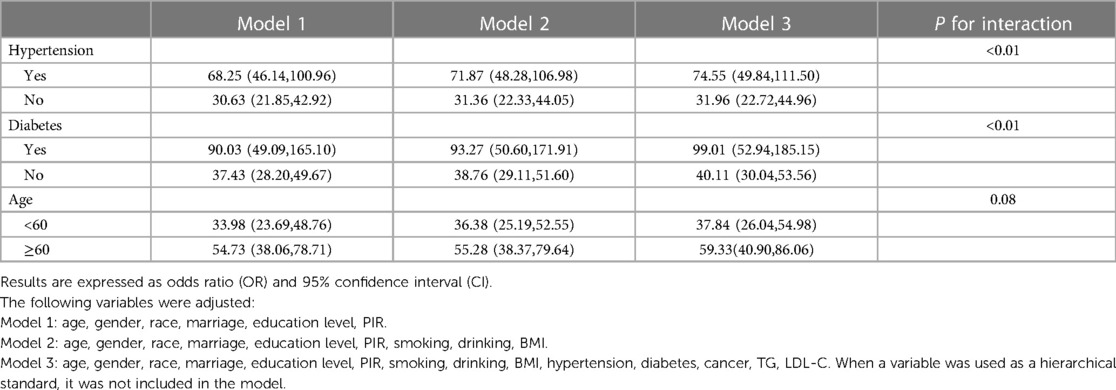
To further explore whether the influence of high-risk sleep patterns on CVD risk was consistent among different populations, we also conducted stratified analysis according to hypertension, diabetes, and age. To verify the interaction, we added the multiplication term of sleep patterns and the above factors to model 3.
Statistics and theory drove the screening of covariates While we incorporated variables with statistical significance in univariate analysis into the regression model, we also included some covariates that may theoretically affect the outcome variables, to maximize the control of confounding bias. The sample size should meet the principle of 10 events per variable (EPV), which means that the group with less sample size of dependent variables was at least ten times the number of variables in the model.
To avoid the selection bias that the lack of covariate data may cause, we treated the missing variables as follows: for classified variables, the missing values were recorded as the “missing” group and included in the analysis; for continuous variables, after being converted into classified variables, the missing values were recorded as the “missing” group and then included in the study.
Data analysis was completed using the IBM SPSS Statistics software version 26.0 (IBM Corp., Armonk, N.Y., USA). P < 0.05 on both sides was considered statistically significant. Depending on the rules for selecting weight values offered on the NHANES website, “Full Sample Two-Year Mobile Examination Center Exam Weight” was Selected as representative weighting value (20).
3 Results
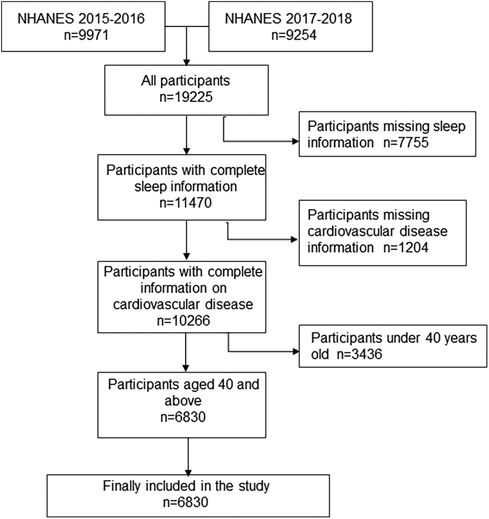
The study population screening process is shown in Figure 1. A total of 19,225 participants took part in the NHANES 2015–2018. After initial screening according to inclusion and exclusion criteria, 6,830 participants, with complete information on independent and dependent variables, were included in the study.
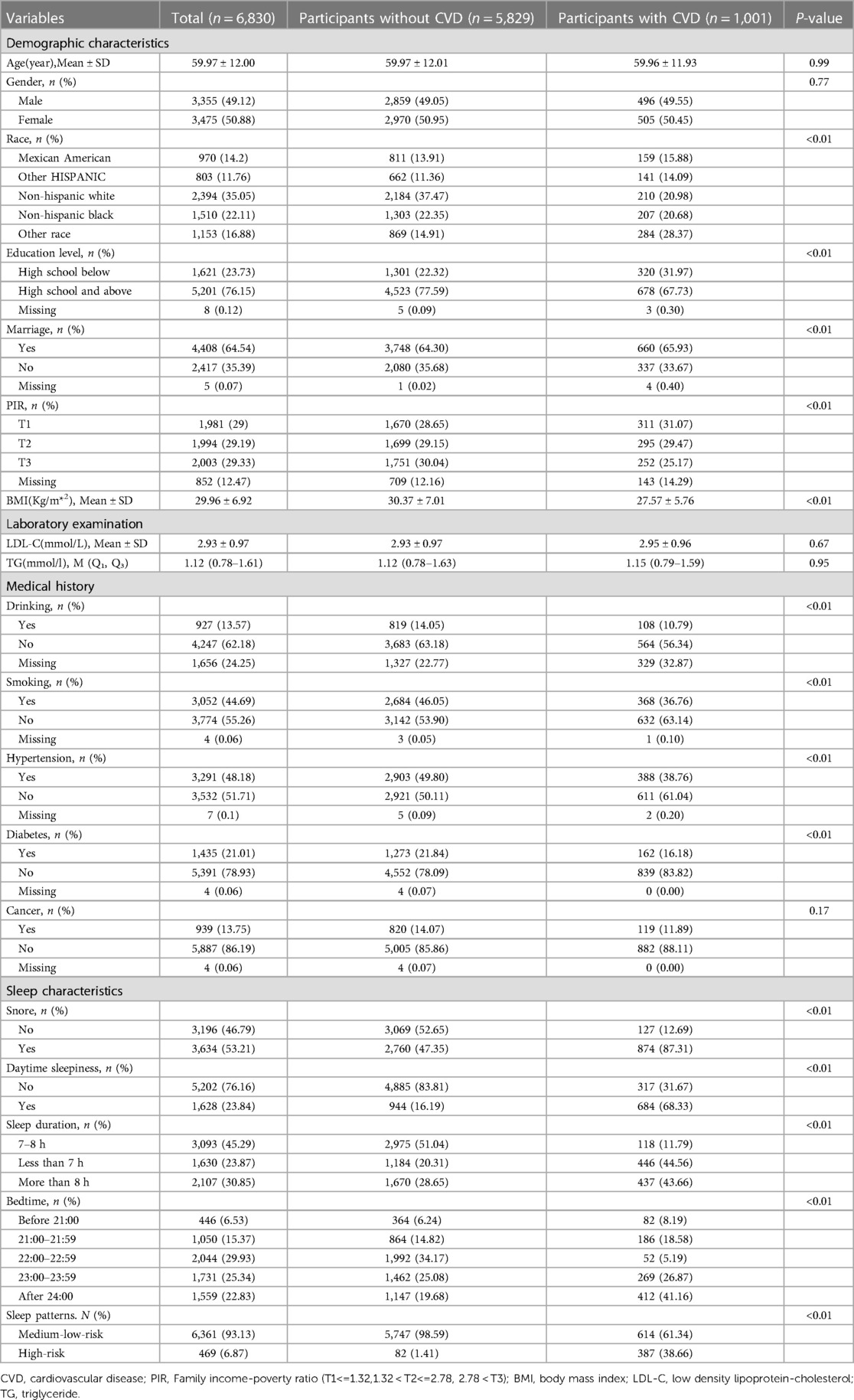
The participants were divided into two groups based on whether they had cardiovascular disease. One thousand and one participants had cardiovascular disease. They had higher likelihoods of snoring, daytime sleepiness, short or long sleep durations, and going to bed after midnight, constituting a high proportion of their high-risk sleep patterns. Univariate analysis showed that race, marital status, education level, PIR, smoking, alcohol use, BMI, hypertension, and diabetes might affect cardiovascular disease, so they were included in the multiple regression model. Additionally, age, sex, LDL-C, TG, and cancer were also included in the multiple regression model because, according to theoretical knowledge they might also be associated with cardiovascular disease. The detailed characteristics of the study population are shown in Table 1.
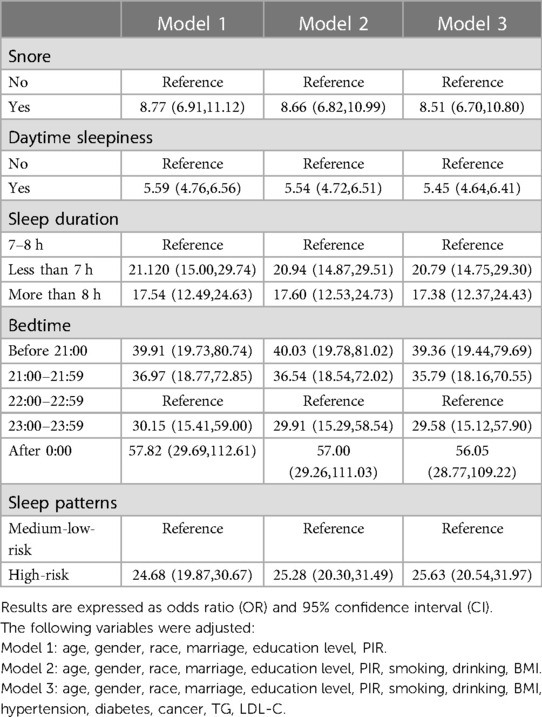
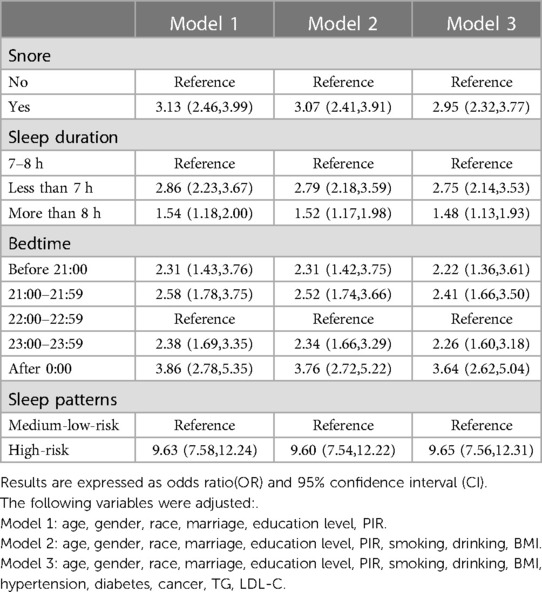
The relationship between different sleep characteristics and CVD risk is shown in Table 2. Although we have constructed several regression models to examine the robustness of the results, their results were similar. After fully adjusting various potential confounding factors, snoring [OR = 7.37,95%CI = (6.06,8.96)] and daytime sleepiness [OR = 11.21,95%CI = (9.60,13.08)] were positively correlated with CVD. Compared with the participants who went to bed from 22: 00 to 22: 59, those who went to bed at other periods also had a higher CVD risk. The effect values were before 21:00 [OR = 8.29,95%CI = (5.73,11.99)], 21:00–21:59 [OR = 7.95,95%CI = (5.77,10.95)], 23:00–23:59 [OR = 6.78,95%CI = (4.99,9.20)], after 0:00 [OR = 13.20,95%CI = (9.78,17.80)] respectively. Sleep duration shorter [OR = 9.50,95%CI = (7.65,11.79)], or longer [OR = 6.61,95%CI = (5.33,8.19)], than 7–8 h was positively associated with CVD risk. The high-risk sleep patterns [OR = 47.73,95%CI = (36.73,62.04)] formed by the superposition of the above dangerous sleep characteristics greatly increased the CVD risk. CHD and stroke also showed similar results when analyzed separately as dependent variables. Negative sleep characteristics have a greater influence on CHD than stroke, as shown in Tables 3, 4.
The results of stratified analysis showed that high-risk sleep patterns and increased risk of CVD were population-specific. The CVD risk of participants with hypertension [OR = 74.55,95%CI = (49.84,111.50)] was more affected by high-risk sleep patterns than those without hypertension [OR = 31.96,95%CI = (22.72,44.96)]. Similarly, the CVD risk of diabetic participants was more easily affected by high-risk sleep patterns [OR = 99.01,95%CI = (52.94,185.15) vs. OR = 40.11,95%CI = (30.04,53.56)]. Participants over 60 years old were also more susceptible to the impact of high-risk sleep patterns on CVD risk [OR = 59.33,95%CI = (40.90,86.06) vs. OR = 37.84,95%CI = (26.04,54.98)]. Still, interaction analysis between age and sleep patterns was not statistically significant (P = 0.08), which was worth further exploration. The above results are shown in Table 5.
4 Discussion
This study found that snoring, daytime sleepiness, a sleep duration of less than 7 h or more than 8 h, as well as going to bed outside the 22:00–23:00 time frame, were associated with a higher risk of CVD. The high-risk sleep pattern, characterized by the above sleep factors, significantly increased the risk of CVD. Stratified analysis showed that high-risk sleep patterns significantly impacted CVD risk among people with hypertension and diabetes. No interaction between high-risk sleep patterns and age was found.
Univariate analysis found that participants with CVD had lower educational levels and higher poverty rates. However, unlike previous findings, this study's results showed that CVD participants had lower BMI, smoking and drinking proportion, which may be because participants with CVD have received good health education and given up these unhealthy living habits. Still, the cross-sectional survey research design cannot demonstrate this causal relationship.
The mechanisms of the association between sleep and CVD remain unknown. Sleep is an essential part of biological rhythm. Healthy sleep requires proper bedtime, enough sleep time and high-quality sleep without abnormal behaviors such as snoring and nightmares, which is essential for cognitive function, mental health, physical health, and mood (21). Some unhealthy sleep characteristics can harm health in multiple ways. When habitual snoring occurs during sleep, the upper airway is narrowed or blocked, resulting in hypoxia. Chronic hypoxia can lead to inflammatory reactions, oxidative stress and chronic sympathetic nerve activation, which can induce or aggravate atherosclerosis. Moreover, snoring-related energy may cause atherosclerotic plaque rupture (22–25). Shortening sleep duration is related to endocrine disorders and circadian rhythm disorders, which may increase the CVD risk (26). For example, shortening sleep time will decrease testosterone, melatonin and leptin secretion and increase auxin levels, which are risk factors for CVD (27–31). The above mechanisms may synergistically increase CVD risk (14).
Our findings were consistent with findings from previous studies. Two meta-analyses found that longer sleep time was related to an increased risk of CHD and stroke (32, 33). A large cohort study found that insomnia can increase the incidence of acute myocardial infarction (34). Insomnia and stroke also showed a similar relationship (35). Daytime sleepiness was associated with new strokes and cardiovascular events, and this relationship was more evident in older people (36). Habitual snoring and obstructive sleep apnea were risk factors for stroke and CHD (37). The above research only focuses on one sleep characteristic, but various sleep risk factors are not independent. For example, people who habitually snore are more likely to be sleepy during the day and have longer sleep durations (38, 39). Therefore, studying the superposition of various sleep characteristics is more practical. A cohort study in China found that there was a dose-response relationship between sleep health score and CVD risk after adding snoring, daytime sleepiness, insomnia, and sleep duration. This was consistent with our research conclusion, that was, the superposition of various dangerous sleep factors would increase the CVD risk (40). Our study further carried out hierarchical analysis and found that this relationship was more significant among participants with hypertension and diabetes. A survey among people with diabetes also confirmed our conclusion (19).
Although sleep health is getting more and more attention, more attention should be paid to clinical and public health efforts. Many resources are invested in promoting healthy nutrition, regular exercise, and reducing risky behaviors like smoking. In contrast, projects to promote healthy sleep still need to be developed (5). Sleep is a multidisciplinary field, and cognitive behavioral intervention is the first choice to treat sleep problems. However, only 6% of clinical psychology programs offer formal teaching courses in sleep medicine (41). Another study found that 95% of the respondents said they had yet to receive clinical sleep training during their postgraduate years and internship years (42).
Our study found that adverse sleep characteristics, such as snoring, daytime sleepiness, excessive or insufficient sleep duration, and inappropriate bedtime, were CVD risks. Furthermore, the accumulation of these adverse sleep characteristics can significantly increase CVD risk. Our research results indicate that in addition to traditional CVD risk factors, above sleep characteristics may also contribute to the development of CVD. Therefore, it is important to consider incorporating sleep monitoring and sleep therapy into CVD prevention and treatment strategies. Previous small-scale clinical studies have found that patients with obstructive sleep apnea syndrome (OSAS) who receive continuous positive airway pressure (CPAP) treatment can improve insulin resistance, oxidative stress, and inflammatory responses, thereby reducing CVD risk (43). However, other studies have also found that treating OSAS does not improve endothelial function (43). Whether improving sleep can reduce CVD risk remains controversial, which points to the need for further research in this area.
The strength of this study is to construct multiple regression models with different independent and dependent variables and fully adjust the potential confounding factors to describe the relationship between other sleep characteristics and CVD risk to the maximum extent. Secondly, we evaluated the relationship between individual sleep characteristics, high-risk sleep patterns formed by the superposition of various dangerous sleep characteristics and CVD risk, and carried out a hierarchical analysis to identify population specificity, which made the research more practical. In addition, the data of this study comes from NHANES, of which the research design is rigorous, and the data is reliable. As for limitations, this study, as a cross-sectional survey, cannot make causal inferences, therefore the results and conclusions of this study cannot clearly explain the causal relationship between various sleep characteristics and CVD risk. In addition, due to the limitation of sample size, sleep patterns are only divided into high-risk and medium-low-risk groups. High-risk sleep patterns contain various dangerous characteristics, which may lead to a relatively large effect value. Subsequent research can further refine sleep patterns by expanding the sample size. Finally, the data on sleep from NHANES comes from a questionnaire survey. During the survey, CAPI system was programmed with limited built-in consistency checks to reduce data entry errors. Other measures are taken to ensure the integrity, consistency, and analytical usefulness of the data. These measures can control system errors (44). Numerous studies have been published based on the sleep survey. However, there may still be information bias compared to objective data obtained through sleep breathing detection.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
X-ZH: Data curation, Formal Analysis, Software, Writing – original draft, Writing – review & editing, Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization. Y-SL: Data curation, Formal Analysis, Software, Writing – review & editing. QW: Writing – original draft, Writing – review & editing. Q-YL: Supervision, Writing – review & editing. Y-TY: Supervision, Writing – review & editing. L-LL: Supervision, Writing – review & editing. X-JY: Supervision, Writing – review & editing. C-YY: Supervision, Writing – review & editing. M-SW: Supervision, Writing – review & editing. Y-FL: Data curation, Formal Analysis, Software, Visualization, Writing – review & editing. L-LC: Funding acquisition, Supervision, Writing – review & editing. S-HW: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by grants from the General Project of National Natural Science Foundation of China (No.81473465) and Science and technology innovation project of China Academy of Chinese Medical Sciences (No.C12021A00921)
Acknowledgments
We would like to acknowledge the following financial support: the General Project of National Natural Science Foundation of China (No.81473465) and Science and technology innovation project of China Academy of Chinese Medical Sciences (No.C12021A00921). We thank the NHANES database for sharing the data.
Conflict of interest
Y-FL was employed by Shanghai Qianhe Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.’
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Raleigh V, Colombo F. Cardiovascular disease should be a priority for health systems globally. Br Med J. (2023) 382:e076576. doi: 10.1136/bmj-2023-076576
2. Tsao CW, Aday AW, Almarzooq ZI, Anderson C, Arora P, Avery CL, et al. Heart disease and stroke statistics-2023 update: a report from the American heart association. Circulation. (2023) 147:e93–621. doi: 10.1161/CIR.0000000000001123
3. Zhou D, Xi B, Zhao M, Wang L, Veeranki SP. Uncontrolled hypertension increases risk of all-cause and cardiovascular disease mortality in US adults: the nhanes iii linked mortality study. Sci Rep. (2018) 8:9418. doi: 10.1038/s41598-018-27377-2
4. Smith L, Atherly A, Campbell J, Flattery N, Coronel S, Krantz M. Cost-effectiveness of a statewide public health intervention to reduce cardiovascular disease risk. BMC Public Health. (2019) 19:1234. doi: 10.1186/s12889-019-7573-8
5. Ramar K, Malhotra RK, Carden KA, Martin JL, Abbasi-Feinberg F, Aurora RN, et al. Sleep is essential to health: an American academy of sleep medicine position statement. J Clin Sleep Med. (2021) 17:2115–9. doi: 10.5664/jcsm.9476
6. Luyster FS, Strollo PJ, Zee PC, Walsh JK. Sleep: a health imperative. Sleep. (2012) 35:727–34. doi: 10.5665/sleep.1846
7. Zheng B, Yu C, Lv J, Guo Y, Bian Z, Zhou M, et al. Insomnia symptoms and risk of cardiovascular diseases among 0.5 million adults: a 10-year cohort. Neurology. (2019) 93:e2110–20. doi: 10.1212/WNL.0000000000008581
8. Wei Y, Lv J, Guo Y, Bian Z, Fan J, Du H, et al. Age-specific associations between habitual snoring and cardiovascular diseases in China: a 10-year cohort study. Chest. (2021) 160:1053–63. doi: 10.1016/j.chest.2021.04.070
9. Fernandez-Mendoza J, He F, Vgontzas AN, Liao D, Bixler EO. Interplay of objective sleep duration and cardiovascular and cerebrovascular diseases on cause-specific mortality. J Am Heart Assoc. (2019) 8:e013043. doi: 10.1161/JAHA.119.013043
10. Kuehn BM. Sleep duration linked to cardiovascular disease. Circulation. (2019) 139:2483–4. doi: 10.1161/CIRCULATIONAHA.119.041278
11. Wang L, Liu Q, Heizhati M, Yao X, Luo Q, Li N. Association between excessive daytime sleepiness and risk of cardiovascular disease and all-cause mortality: a systematic review and meta-analysis of longitudinal cohort studies. J Am Med Dir Assoc. (2020) 21:1979–85. doi: 10.1016/j.jamda.2020.05.023
12. Hoevenaar-Blom MP, Spijkerman AM, Kromhout D, van den Berg JF, Verschuren WM. Sleep duration and sleep quality in relation to 12-year cardiovascular disease incidence: the morgen study. Sleep. (2011) 34:1487–92. doi: 10.5665/sleep.1382
13. Kim Y, Wilkens LR, Schembre SM, Henderson BE, Kolonel LN, Goodman MT. Insufficient and excessive amounts of sleep increase the risk of premature death from cardiovascular and other diseases: the multiethnic cohort study. Prev Med. (2013) 57:377–85. doi: 10.1016/j.ypmed.2013.06.017
14. Fan M, Sun D, Zhou T, Heianza Y, Lv J, Li L, et al. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: a prospective study of 385 292 UK biobank participants. Eur Heart J. (2020) 41:1182–9. doi: 10.1093/eurheartj/ehz849
15. Deng X, Tan Y. A national cross-sectional analysis of selenium intake and risk of osteoarthritis: nhanes 2003–2016. Front Public Health. (2022) 10:1047605. doi: 10.3389/fpubh.2022.1047605
16. Shi JW, Wu JN, Zhu XY, Zhou WH, Yang JY, Li MQ. Association of serum 25-hydroxyvitamin d levels with all-cause and cause-specific mortality among postmenopausal females: results from nhanes. J Transl Med. (2023) 21:629. doi: 10.1186/s12967-023-04413-y
17. Odland ML, Gassama K, Bockarie T, Wurie H, Ansumana R, Witham MD, et al. Cardiovascular disease risk profile and management among people 40 years of age and above in bo, Sierra Leone: a cross-sectional study. PloS One. (2022) 17:e0274242. doi: 10.1371/journal.pone.0274242
18. Diao T, Liu K, Wang Q, Lyu J, Zhou L, Yuan Y, et al. Bedtime, sleep pattern, and incident cardiovascular disease in middle-aged and older Chinese adults: the dongfeng-tongji cohort study. Sleep Med. (2023) 110:82–8. doi: 10.1016/j.sleep.2023.08.002
19. Li J, Yin J, Luo Y, Ma T, He L, Xie H, et al. Association of healthy sleep pattern with the risk of cardiovascular disease and all-cause mortality among people with diabetes: a prospective cohort study. Diabetes Res Clin Pract. (2022) 186:109822. doi: 10.1016/j.diabres.2022.109822
20. Liu E, Hou X, Liu S, Han J, Lv H. Association of hemoglobin levels with bone mineral density for adults over 18 years of age: a cross-sectional study. Sci Rep. (2022) 12:9975. doi: 10.1038/s41598-022-13973-w
21. Foster RG. Sleep, circadian rhythms and health. Interface Focus. (2020) 10:20190098. doi: 10.1098/rsfs.2019.0098
22. Xie A, Skatrud JB, Puleo DS, Morgan BJ. Exposure to hypoxia produces long-lasting sympathetic activation in humans. J Appl Physiol (1985). (2001) 91:1555–62. doi: 10.1152/jappl.2001.91.4.1555
23. Tauman R, Ivanenko A, O'Brien LM, Gozal D. Plasma c-reactive protein levels among children with sleep-disordered breathing. Pediatrics. (2004) 113:e564–9. doi: 10.1542/peds.113.6.e564
24. Fernandez AR, Rubinos CG, Alonso AR, Cascon HJ, Palomo AB, Iscar UM, et al. Snoring as a determinant factor of oxidative stress in the airway of patients with obstructive sleep apnea. Lung. (2016) 194:469–73. doi: 10.1007/s00408-016-9869-0
25. Cho JG, Witting PK, Verma M, Wu BJ, Shanu A, Kairaitis K, et al. Tissue vibration induces carotid artery endothelial dysfunction: a mechanism linking snoring and carotid atherosclerosis? Sleep. (2011) 34:751–7. doi: 10.5665/SLEEP.1042
26. Yin J, Jin X, Shan Z, Li S, Huang H, Li P, et al. Relationship of sleep duration with all-cause mortality and cardiovascular events: a systematic review and dose-response meta-analysis of prospective cohort studies. J Am Heart Assoc. (2017) 6:e005947. doi: 10.1161/JAHA.117.005947
27. Leproult R, Van Cauter E. Effect of 1 week of sleep restriction on testosterone levels in young healthy men. JAMA. (2011) 305:2173–4. doi: 10.1001/jama.2011.710
28. Brugger P, Marktl W, Herold M. Impaired nocturnal secretion of melatonin in coronary heart disease. Lancet. (1995) 345:1408. doi: 10.1016/s0140-6736(95)92600-3
29. Kloner RA, Carson CR, Dobs A, Kopecky S, Mohler ER. Testosterone and cardiovascular disease. J Am Coll Cardiol. (2016) 67:545–57. doi: 10.1016/j.jacc.2015.12.005
30. Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. Plos Med. (2004) 1:e62. doi: 10.1371/journal.pmed.0010062
31. Rodríguez A. Novel molecular aspects of ghrelin and leptin in the control of adipobiology and the cardiovascular system. Obes Facts. (2014) 7:82–95. doi: 10.1159/000360837
32. He Q, Sun H, Wu X, Zhang P, Dai H, Ai C, et al. Sleep duration and risk of stroke: a dose-response meta-analysis of prospective cohort studies. Sleep Med. (2017) 32:66–74. doi: 10.1016/j.sleep.2016.12.012
33. Wang D, Li W, Cui X, Meng Y, Zhou M, Xiao L, et al. Sleep duration and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Int J Cardiol. (2016) 219:231–9. doi: 10.1016/j.ijcard.2016.06.027
34. Laugsand LE, Vatten LJ, Platou C, Janszky I. Insomnia and the risk of acute myocardial infarction: a population study. Circulation. (2011) 124:2073–81. doi: 10.1161/CIRCULATIONAHA.111.025858
35. Wu MP, Lin HJ, Weng SF, Ho CH, Wang JJ, Hsu YW. Insomnia subtypes and the subsequent risks of stroke: report from a nationally representative cohort. Stroke. (2014) 45:1349–54. doi: 10.1161/STROKEAHA.113.003675
36. Blachier M, Dauvilliers Y, Jaussent I, Helmer C, Ritchie K, Jouven X, et al. Excessive daytime sleepiness and vascular events: the three city study. Ann Neurol. (2012) 71:661–7. doi: 10.1002/ana.22656
37. Li D, Liu D, Wang X, He D. Self-reported habitual snoring and risk of cardiovascular disease and all-cause mortality. Atherosclerosis. (2014) 235:189–95. doi: 10.1016/j.atherosclerosis.2014.04.031
38. Chen Y, Kartsonaki C, Clarke R, Guo Y, Yu C, Bian Z, et al. Characteristics and correlates of sleep duration, daytime napping, snoring and insomnia symptoms among 0.5 million Chinese men and women. Sleep Med. (2018) 44:67–75. doi: 10.1016/j.sleep.2017.11.1131
39. Hägg SA, Ilieva E, Ljunggren M, Franklin KA, Middelveld R, Lundbäck B, et al. The negative health effects of having a combination of snoring and insomnia. J Clin Sleep Med. (2022) 18:973–81. doi: 10.5664/jcsm.9764
40. Zhong Q, Qin Z, Wang X, Lan J, Zhu T, Xiao X, et al. Healthy sleep pattern reduce the risk of cardiovascular disease: a 10-year prospective cohort study. Sleep Med. (2023) 105:53–60. doi: 10.1016/j.sleep.2023.03.003
41. Meltzer LJ, Phillips C, Mindell JA. Clinical psychology training in sleep and sleep disorders. J Clin Psychol. (2009) 65:305–18. doi: 10.1002/jclp.20545
42. Zhou ES, Mazzenga M, Gordillo ML, Meltzer LJ, Long KA. Sleep education and training among practicing clinical psychologists in the United States and Canada. Behav Sleep Med. (2021) 19:744–53. doi: 10.1080/15402002.2020.1860990
43. Chen J, Wu K, Lin Y, Huang M, Xie S. Association of triglyceride glucose index with all-cause and cardiovascular mortality in the general population. Cardiovasc Diabetol. (2023) 22:320. doi: 10.1186/s12933-023-02054-5
Keywords: snoring, daytime sleepiness, bedtime, sleep duration, sleep patterns, cardiovascular disease
Citation: Hou X-Z, Li Y-S, Wu Q, Lv Q-Y, Yang Y-T, Li L-L, Ye X-J, Yang C-Y, Wang M-S, Lv Y-F, Cao L-L and Wang S-H (2024) Association of sleep characteristics with cardiovascular disease risk in adults over 40 years of age: a cross-sectional survey. Front. Cardiovasc. Med. 11:1308592. doi: 10.3389/fcvm.2024.1308592
Received: 6 October 2023; Accepted: 15 January 2024;
Published: 24 January 2024.
Edited by:
Stefano Palermi, University of Naples Federico II, ItalyReviewed by:
Steve Noumegni, University of Florida, United StatesIzolde Bouloukaki, University of Crete, Greece
© 2024 Hou, Li, Wu, Lv, Yang, Li, Ye, Yang, Wang, Lv, Cao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shi-Han Wang d2FuZ3NoaWhhbjkxQDEyNi5jb20=
 Xin-Zheng Hou
Xin-Zheng Hou Yu-Shan Li2
Yu-Shan Li2 Qian Wu
Qian Wu Qian-Yu Lv
Qian-Yu Lv Ying-Tian Yang
Ying-Tian Yang Lan-Lan Li
Lan-Lan Li Xue-Jiao Ye
Xue-Jiao Ye Chen-Yan Yang
Chen-Yan Yang Man-Shi Wang
Man-Shi Wang Lin-Lin Cao
Lin-Lin Cao Shi-Han Wang
Shi-Han Wang