
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 23 February 2024
Sec. Heart Valve Disease
Volume 11 - 2024 | https://doi.org/10.3389/fcvm.2024.1297304
 Prajwal Reddy1
Prajwal Reddy1 Vidhu Anand1
Vidhu Anand1 Prabhakar Rajiah2
Prabhakar Rajiah2 Nicholas B. Larson3
Nicholas B. Larson3 Jared Bird1
Jared Bird1 James M. Williams2
James M. Williams2 Eric E. Williamson2
Eric E. Williamson2 Rick A. Nishimura1
Rick A. Nishimura1 Juan A. Crestanello4
Juan A. Crestanello4 Arman Arghami4
Arman Arghami4 Jeremy D. Collins2
Jeremy D. Collins2 Alex Bratt2*
Alex Bratt2*
Introduction: Volume overload from mitral regurgitation can result in left ventricular systolic dysfunction. To prevent this, it is essential to operate before irreversible dysfunction occurs, but the optimal timing of intervention remains unclear. Current echocardiographic guidelines are based on 2D linear measurement thresholds only. We compared volumetric CT-based and 2D echocardiographic indices of LV size and function as predictors of post-operative systolic dysfunction following mitral repair.
Methods: We retrospectively identified patients with primary mitral valve regurgitation who underwent repair between 2005 and 2021. Several indices of LV size and function measured on preoperative cardiac CT were compared with 2D echocardiography in predicting post-operative LV systolic dysfunction (LVEFecho <50%). Area under the curve (AUC) was the primary metric of predictive performance.
Results: A total of 243 patients were included (mean age 57 ± 12 years; 65 females). The most effective CT-based predictors of post-operative LV systolic dysfunction were ejection fraction [LVEFCT; AUC 0.84 (95% CI: 0.77–0.92)] and LV end systolic volume indexed to body surface area [LVESViCT; AUC 0.88 (0.82–0.95)]. The best echocardiographic predictors were LVEFecho [AUC 0.70 (0.58–0.82)] and LVESDecho [AUC 0.79 (0.70–0.89)]. LVEFCT was a significantly better predictor of post-operative LV systolic dysfunction than LVEFecho (p = 0.02) and LVESViCT was a significantly better predictor than LVESDecho (p = 0.03). Ejection fraction measured by CT demonstrated significantly greater reproducibility than echocardiography.
Discussion: CT-based volumetric measurements may be superior to established 2D echocardiographic parameters for predicting LV systolic dysfunction following mitral valve repair. Validation with prospective study is warranted.
Mitral regurgitation (MR) is the most frequently recognized valvular heart disease in the United States (1). Left untreated, chronic left ventricular (LV) volume overload from MR eventually leads to irreversible myocardial remodeling and contractile dysfunction (2). Early surgical repair has been shown to improve survival (3) though the optimal timing of operation is not well understood. Current guidelines from the American College of Cardiology and American Heart Association recommend mitral valve repair based on 2D echocardiographic thresholds of left ventricular ejection fraction (LVEFecho) and end systolic diameter (LVESDecho) as these have been shown to predict the occurrence of post-operative LV dysfunction (4, 5). Current thresholds are 60% for LVEFecho and 40 mm for LVESDecho. By nature, 2D measurements contain only a small fraction of the information necessary to fully describe ventricular size and function and therefore likely fail to capture all the adverse myocardial remodeling that occurs in MR. Thus, we hypothesize that volumetric parameters are better predictors of outcome and could improve risk stratification in patients with MR.
Unlike echocardiography, Cardiac CT is inherently volumetric and insensitive to operator skill. Cardiac CT is already used to preoperatively evaluate coronary artery disease in appropriately selected patients undergoing mitral repair since it reduces the need for invasive coronary angiography (6). At our institution, pre-procedural cardiac CT is routinely performed with time-resolved retrospective ECG gating to allow for characterization of mitral leaflet structure and function and also to compensate for the high prevalence of arrhythmia in these patients (7–9). Time-resolved imaging also enables volumetric assessment of ventricular chamber size and function. This gives a more complete picture of LV status than 2D echocardiographic measurements and could clarify the optimal timing of intervention by improving prediction of irreversible LV remodeling. Motivated by this possibility, we sought to compare CT- and echocardiography-based measures of left ventricular size and function as predictors of LV systolic dysfunction following repair of primary MR.
This research protocol was carried out under the supervision of our Institutional Review Board (IRB), which approved retrospective analysis of pre-existing datasets and waived the requirement for informed consent (IRB# 21-001262, deemed exempt 02/25/21). Patients who refused research participation were excluded from analysis according to state law.
Patient selection is summarized in Figure 1. Briefly, a medical record query was performed to identify patients for analysis. A patient was tentatively deemed eligible if her medical record contained a cardiac CT report issued between 2005 and 2022 that contained the words “mitral” and “prolapse”, along with a pre-operative diagnosis note that mentioned “mitral” (n = 806). Patients were excluded whose medical records did not contain at least one echocardiogram (transthoracic or transesophageal) performed between 6 and 36 months following the operation (n = 490). The charts of the remaining 316 patients were manually analyzed for additional exclusion criteria, including: insufficient CT image quality (n = 33; usually poor gating related to arrhythmia), incomplete pre-operative CT or echocardiographic data (n = 11), non-annuloplasty mitral operation (n = 7), ischemic etiology of MR (n = 1), acute illness at echo follow-up (n = 1), and previous mitral repair (n = 1). Additionally, 19 patients were excluded for recurrent post-operative MR of greater than mild severity since this could mask underlying systolic dysfunction. All cases of recurrent MR were independently reviewed by a level three trained echocardiographer blinded to outcome. The relatively high observed prevalence of recurrent MR (∼6%) was likely related to the criterion of a 6–36-month post-operative echocardiogram, which biases toward high-risk patients (e.g., those with symptoms, murmur, and residual regurgitation at hospital discharge). The total number of eligible patients after exclusion was 243.
Cardiac CT scans were performed according to an established protocol described elsewhere (6). Briefly, all examinations were performed using a dual-source, 64- or 96-detector row CT scanner (Siemens SOMATOM Force, Definition Flash, Definition; Forchheim, Germany). Patients were positioned supine with electrocardiogram (ECG) leads placed on the anterior chest wall. Scan parameters included variable kV (80–120) and mAs/rotation (150–400) as determined by patient size (using standard technique charts) to maintain a consistent quality reference mass. Pitch was also variable and was auto selected based on the patient's heart rate. All studies utilized intravenous iodinated contrast administration and were acquired in arterial phase. All studies employed retrospective ECG gating with tube current pulsing. An analysis of radiation dose for these exams has been previously described (6).
Manual CT analysis was carried out by two raters blinded to patient outcomes, one of which a board-certified cardiothoracic radiologist with 2 years of post-fellowship experience (AB) and the other a fourth-year cardiology fellow undergoing subspecialty training in multimodality cardiovascular imaging (PRe). End-systolic volume (LVESVCT) and end-diastolic volume (LVEDVCT) were derived from CT scans using commercial semi-automated 3-D segmentation software (Visage, Richmond, Australia; Figure 2). LV short axis area was manually segmented at a few locations along the ventricle and the software interpolated between the manually segmented slices. After applying slight manual corrections to the interpolated segmentation maps, LV chamber volume was calculated by taking the integral of short axis area over the length of the ventricle. Chamber segmentation maps included trabeculation and papillary muscle as part of the chamber. LV ejection fraction (LVEFCT) was calculated in the standard fashion. The maximum LV short axis diameter was recorded in end-systole and end-diastole (LVEDDCT, LVESDCT, Figure 2).
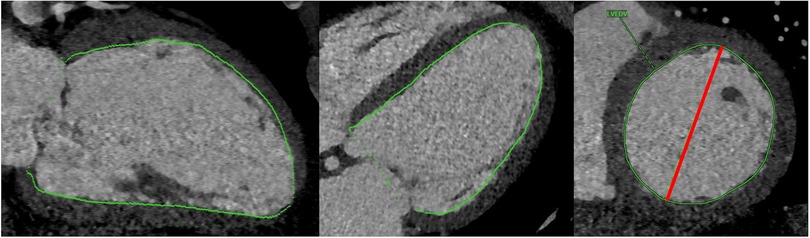
Figure 2. Depiction of chamber volume segmentation (green) and short axis diameter measurement (red).
Pre- and post-operative echocardiographic parameters were obtained from existing interpretive reports in the electronic medical record, including LVEFecho (2D linear or biplane), 2D linear LV end diastolic diameter (LVEDDecho), and 2D linear LV end systolic diameter (LVESDecho). Echocardiographic volumes (LVEDV and LVESV) were not recorded because they were only available for a small minority of cases, even upon re-review of the original images. The endpoint of the study was post-operative left ventricular dysfunction. This was defined as a post-operative LVEFecho of less than 50% measured on echocardiography performed between 6 and 36 months after the operation. If multiple post-operative echocardiograms were available during this time interval, we used LVEFecho from the echocardiogram performed nearest to one year after the operation. All echocardiographic measurements were performed according to current American Society of Echcardiography guidelines (10).
All patients underwent surgical repair of the mitral valve using a combination of leaflet resection, artificial chordae placement and flexible posterior annuloplasty band placement. Most operations were performed robotically (n = 188, 78%) (11), while 34 (14%) were performed via sternotomy and the remainder via port access. The robotic platform was introduced at our institution in 2008.
A random 50-patient cohort was identified for inter-rater reproducibility analysis. A single cardiothoracic radiologist with 13 years of post-fellowship experience (PRa) remeasured LVESVCT, LVEDVCT, and LVEFCT for all patients in the reproducibility cohort. Another rater (PRe) remeasured preoperative LVEDDecho, LVESDecho, and LVEFecho for all patients in the reproducibility cohort.
Diagnostic performance was assessed by means of receiver operating characteristic (ROC) curve analysis, with area under the curve (AUC) as the primary metric. Comparison of paired AUC estimates was carried out using the method of DeLong (12). Comparisons between groups were made using the Wilcoxon signed-rank test for paired data. Probability of post-operative systolic dysfunction over ranges of individual LV parameters was estimated univariably with logistic regression under a generalized additive model using flexible thin-plate splines. Lin's concordance correlation coefficient (CCC) was used for assessment of inter-rater reproducibility. To compare inter-rater reproducibility across modalities, we applied a bootstrapping approach to characterize the precision about the difference in CCC estimates between CT and echocardiography while taking into consideration the paired nature of the data by using patient as the resampling unit. The 95% confidence interval (CI) was calculated using the adjusted bootstrap percentile method based on B = 5,000 bootstrap samples. Inter-modality (i.e., CT vs. echocardiography) reproducibility was assessed using the method of Bland and Altman (13), which yielded a mean difference and limits of agreement (LoA; mean ± 1.96 standard deviation). Baseline variables were normalized to BSA (14) where appropriate. For all comparisons a two-sided p-value threshold of 0.05 was used to indicate statistical significance. Statistical analyses were performed with R v4.0.3 (15) as well as the Python packages NumPy (16), SciPy (17), and Statsmodels (18).
Baseline characteristics are shown in Table 1. Mean age was 57 ± 12 years and 65 (27%) patients were female. Atrial Fibrillation was present in 15 (6%) patients, diabetes in 3 (1%) patients, and only 4 (2%) patients had a diagnosis of congestive heart failure. Pulmonary hypertension, defined as right ventricular systolic pressure (RVSP) greater than 50 mmHg, was present in 17/215 (8%) of patients in whom RVSP was available on preoperative echocardiography. Mitral prolapse was present in all cases. In most patients, MR was severe or greater by preoperative echocardiography (n = 240, 99%) with the remainder of cases moderate or moderate-severe. Among patients with preoperative NYHA functional classification documented in the medical record (n = 160/243), the mean NYHA class was 2.0 with 142/160 patients class II or greater (i.e., symptomatic). The mean Society of Thoracic Surgeons score in the cohort was 0.6%.
There was no mortality in 30 days, nor did any patient require dialysis post-operatively. Median cardiopulmonary bypass time was 72 (IQR 59–87) minutes and cross clamp time was 50 (IQR 41–61) minutes. The most common concomitant procedure was closure of a patent foramen ovale or atrial septal defect (n = 35, 14%). Ten patients (4%) had concomitant coronary artery bypass, 15 (6%) had left atrial appendage ligation, 8 (3%) had the Cox maze procedure, and 6 (2%) had tricuspid annuloplasty. Postoperative atrial fibrillation occurred in 51 (21%) patients while only 15 (6%) had a history of atrial fibrillation preoperatively. On pre-discharge echocardiography, 228 patients (94%) had less than moderate residual MR and no patient had severe regurgitation. Median length of hospital stay was 3 (IQR 3–4) days.
Twenty-five (10%) patients developed post-operative LV systolic dysfunction, defined as LVEFecho less than 50%. Results from ROC analysis are shown in Table 2. BSA-indexed LVESVCT (LVESViCT) was a significantly better predictor of post-operative LV systolic dysfunction than LVESDecho (AUC 0.88 vs. 0.79, p = 0.03; Figure 3). Non-indexed LVESVCT was not a significantly better predictor than LVESDecho (0.86 vs. 0.79, p = 0.08). Note that LVESDecho was used for both comparisons since it was superior to indexed LVESDecho in terms of AUC (0.79 vs. 0.68, p = 0.03). LVEFCT was a significantly better predictor of post-operative LV systolic dysfunction than LVEFecho (0.84 vs. 0.70, p = 0.02; Figure 3). Figure 4 shows the log-odds of post-operative systolic dysfunction over ranges of LVESViCT and LVEFCT, respectively. The log-odds of systolic dysfunction changes linearly with both parameters over both ranges of values, indicating that there was no threshold above or below which the risk of systolic dysfunction ceased to change as a function of these measurements.
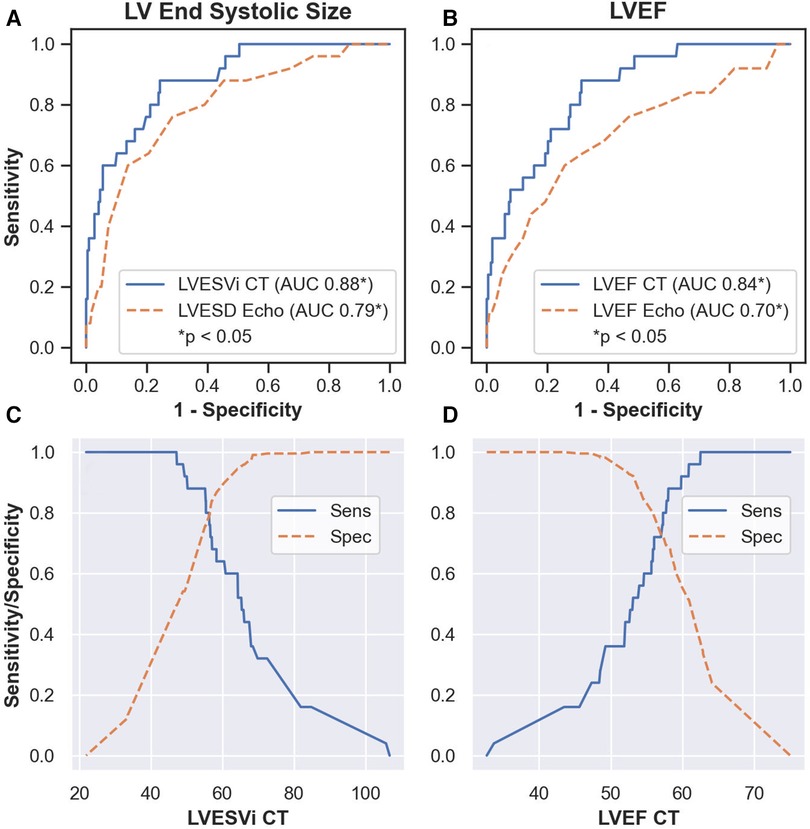
Figure 3. Results of receiver operating characteristic analysis. LVESViCT was a significantly better predictor of post-operative left ventricular dysfunction than LVESDecho (p = 0.03, A). LVEFCT was a significantly better predictor than LVEFecho (p = 0.02, B). Sensitivity and specificity values over ranges of LVESViCT (C) and LVEFCT (D) AUC, area under the receiver operating characteristic curve. LVEFecho, left ventricular ejection fraction on echocardiography. LVESDecho, left ventricular end systolic diameter on echocardiography. LVEFCT, left ventricular ejection fraction on CT. LVESViCT, indexed left ventricular end systolic volume on CT.
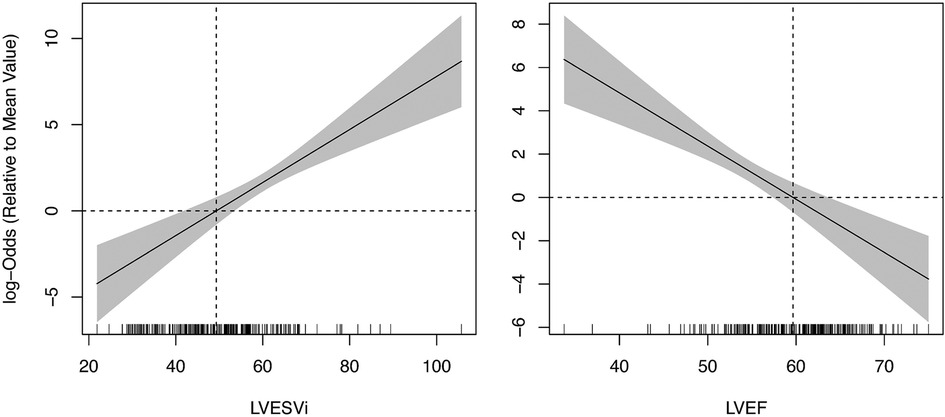
Figure 4. Logistic regression of post-operative systolic dysfunction vs. pre-operative CT-based LV measurements. The log-odds ratio of dysfunction (LVEF < 50%) increases linearly with LVESVi (left pane) and decreases with LVEF (right pane) across the entire range of observed values. Vertical dashed lines represent mean values of the study population. Horizontal dashed lines represent zero log odds relative to the sample mean. Hash marks along the x-axis represent individual data points. LV, left ventricle; LVEF, left ventricular ejection fraction; LVESVi, indexed left ventricular end systolic volume.
The LVESDecho threshold value of 40 mm suggested by current guidelines (4, 5) yielded 60% sensitivity and 86% specificity in our cohort. At the same specificity level, LVESViCT gave a sensitivity of 68% (threshold value 58 ml/m2), identifying two additional patients with underlying systolic dysfunction (17 patients vs. 15). We propose 47 ml/m2 as a tentative LVESViCT threshold in our cohort as it provided 100% sensitivity with 50% specificity (Figure 3), while being over three standard deviations greater than the mean for healthy women and two standard deviations above the mean for healthy men (19). In our sample, this operative threshold would theoretically ensure that no patients develop post-operative LV dysfunction while exposing only 50% of patients to an earlier-than-ideal operation. Compare this to LVESDecho, which was only 13% specific at 100% sensitivity (cutoff 31 mm). This cutoff value is consistent with studies in patients with aortic regurgitation showing elevated risk at similar levels of indexed left ventricular end systolic volume (20–22).
The LVEFecho threshold value of 60% suggested by current guidelines (4, 5) yielded 44% sensitivity and 85% specificity in our cohort. At the same specificity level, LVEFCT gave a sensitivity of 56% (threshold LVEFCT = 54%), identifying three additional patients with underlying systolic dysfunction (14 patients vs. 11). We propose 61% as a tentative LVEFCT threshold in our cohort as this provides comparable specificity to the LVESViCT threshold described above (51%) while maintaining excellent sensitivity (96%, Figure 3). This cutoff value is consistent with findings in patients with aortic regurgitation showing elevated risk at a similar ejection fraction threshold (22).
Post-operative LV systolic dysfunction was associated with all baseline variables on univariate regression [p < 0.05; age, gender, LVEFCT, LVEDVCT, LVEDVCT indexed to BSA (LVEDViCT), LVESVCT, LVESViCT, LVEDDCT, LVESDCT, BSA, LVEFecho, LVEDDecho, LVEDSecho]. However, the event rate was too limited to investigate multivariable models.
Correspondence between echocardiographic and CT measures of LV ejection fraction is shown in Supplementary Figure S2. The two modalities were significantly correlated (Pearson r = 0.4, p < 1e–11) with an estimated mean difference of −5% (CT minus echocardiography, p < 1e–26) and LoA ±11%.
Inter-rater reproducibility is summarized in Table 3. The 95% CI for the difference in CCC between EF measured by CT and echocardiography did not overlap zero (0.011, 0.522), indicating significantly greater reproducibility for LVEFCT (0.76) than LVEFecho (0.52).
This is the first study to compare preoperative CT to echocardiography in predicting the outcome of surgical repair in patients with primary MR. The goal of surgical intervention in primary MR is to correct it before the onset of irreversible LV dysfunction (5). Early identification of patients at risk can be challenging because the chronic volume overload that accompanies MR artificially inflates LVEF, concealing underlying contractile dysfunction (23–26).
Current guidelines rely on preoperative 2D echocardiographic predictors of post-operative LV dysfunction (4, 23, 24, 27). However, technological advancements have enabled reproducible volumetric left ventricular measurements especially in patients who have an indication for multimodality imaging prior to surgical intervention. Preoperative cardiac CT not only allows for accurate assessment of coronary arteries, but retrospectively gated exams also allow for time-resolved volumetric assessment of the left ventricle. Our study suggests that CT-derived volumetric measurements better predict post-operative LV systolic dysfunction than established 2D echocardiographic parameters, which may clarify the optimal timing of repair. Importantly, most patients in our cohort began with normal or supranormal LVEF (Supplementary Figure S2), making this the exact group most likely to benefit from improved risk stratification. We also found that ejection fraction measured by CT demonstrated significantly greater reproducibility than echocardiography, highlighting another potential advantage of CT.
Use of CT for preoperative risk stratification may enable earlier intervention and thereby reduce the incidence of postoperative LV dysfunction. For example, since the LVEFecho threshold of 60% suggested by current guidelines was only 44% sensitive in our cohort, 56% of patients who developed systolic dysfunction may have been incorrectly classified (14/25). With CT, we could have theoretically identified and intervened upon three additional patients at risk with similar specificity (only 11/25 misclassified). We note that these thresholds could be adjusted to reduce the number of misclassified patients further, at the cost of specificity.
We identify volumetric LV measurement thresholds (LVESViCT > 47 ml/m2, LVEFCT < 61%) that may provide a more favorable balance of sensitivity and specificity in our cohort. These align with prior studies, which identified similar risk thresholds in patients with aortic regurgitation (20–22). Our results show that cardiac CT is a promising avenue toward improving outcomes for patients with MR, though further validation is necessary in prospective randomized cohorts.
This study has several limitations. First, 2D transthoracic echocardiography was used to measure post-operative LV ejection fraction, which may be less accurate than CT, MRI, or 3D echocardiography (28). Future studies will assess the latter modalities as post-operative endpoints. Second, we did not evaluate preoperative 3D echocardiographic LV chamber measurements. This is because our goal was to compare with established guidelines (5), which are based on 2D measurements. Third, 11% of patients were excluded due to poor CT image quality, which could limit the benefit of CT. Given that CT exams in our study were not explicitly optimized for chamber volume measurement at the time of image acquisition, we feel that this could be mitigated with appropriate planning. Fourth, this study has all the inherent limitations associated with retrospective analysis of a relatively small sample size, including bias related to selection criteria. We plan to address this in future prospective studies.
Finally, CT exposes patients to ionizing radiation while echocardiography does not (29). The slight incremental risk incurred by radiation exposure is offset by the fact that preoperative cardiac CT reduces the need for invasive coronary angiography in patients undergoing mitral repair (6). One could argue that prospective gating would be a better choice for coronary evaluation since it delivers less radiation and maintains similar diagnostic accuracy (29). However, the additional radiation exposure of retrospective gating as compared with prospective gating is offset by several factors. First, retrospectively gated cardiac CT provides time-resolved information about mitral valve anatomy and function while prospectively gated CT does not. Second, retrospective gating is often already necessary for coronary evaluation in this patient population because of the relatively high prevalence of arrhythmia (7–9). Third, use of prospective gating does not allow dynamic assessment of LV volume and function, which improves prediction of post-operative LV dysfunction, as we show. If radiation remains a concern in certain settings, 3D echocardiography or cardiac MRI could be considered, especially given the value of the latter in assessing the severity of MR (30). This could be a subject for further study.
We provide evidence that CT-derived parameters may be more effective than standard of care echocardiographic evaluation of LV size and function in predicting post-operative left ventricular systolic dysfunction following mitral repair. With further validation, CT could inform clinical decision making by increasing diagnostic confidence and thereby enabling earlier intervention for patients at risk. After validation in larger prospective studies, incorporating volumetric CT-based measures of cardiac chamber size and function into routine preoperative evaluation may improve outcomes by reducing the incidence of post-operative LV systolic dysfunction.
The data analyzed in this study is subject to the following licenses/restrictions: Mayo Clinic patient data. Requests to access these datasets should be directed toYnJhdHQuYWxleGFuZGVyQG1heW8uZWR1.
The studies involving humans were approved by Mayo Clinic Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Retrospective analysis of existing medical data with minimal/no risk.
PR: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. VA: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing. PR: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Writing – review & editing. NL: Conceptualization, Data curation, Formal Analysis, Investigation, Software, Validation, Visualization, Writing – review & editing. JB: Conceptualization, Formal Analysis, Investigation, Methodology, Writing – review & editing. JW: Conceptualization, Data curation, Formal Analysis, Investigation, Validation, Writing – review & editing. EW: Conceptualization, Data curation, Investigation, Methodology, Resources, Supervision, Validation, Writing – review & editing. RN: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – review & editing. JC: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – review & editing. AA: Conceptualization, Investigation, Methodology, Project administration, Supervision, Validation, Writing – review & editing. JC: Conceptualization, Formal Analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. AB: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
PR: royalties from Elsevier for book and journal editing (not related to present manuscript). JC: co-investigator for an institutional grant from Siemens Healthineers; stock owner in Ceta, a non-publicly traded healthcare I company; travel costs from Siemens Healthineers to attend a CT research summit in Germany in October 2019 (none related to present manuscript).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2024.1297304/full#supplementary-material
Supplemental Figure S1
Non-log odds ratio of post-operative systolic dysfunction versus CT-based LV measurements. The reference value for odds of post-operative systolic dysfunction (i.e., odds ratio = 1, horizontal dashed lines) was arbitrarily assigned to the sample mean for each measurement parameter (vertical dashed lines). Hash marks along the x-axis represent individual data points. The fact that these curves appear to have relatively flat segments as well as relatively vertical segments is an artifact of truncating the x-axes. As shown in Figure 4, consistent impacts on risk were observed across the spectrum of observed values. LV, left ventricle; LVEF, left ventricular ejection fraction; LVESVi, indexed left ventricular end systolic volume.
Supplemental Figure S2
Correspondence between echocardiographic and CT measures of left ventricular ejection fraction. The two modalities were significantly correlated (left plot) with mean difference −5% (CT minus echo) and limits of agreement ±11% (right plot). LVEF, left ventricular ejection fraction.
1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. (2006) 368:1005–11. doi: 10.1016/S0140-6736(06)69208-8
2. Spinale FG, Ishihra K, Zile M, DeFryte G, Crawford FA, Carabello BA. Structural basis for changes in left ventricular function and geometry because of chronic mitral regurgitation and after correction of volume overload. J Thorac Cardiovasc Surg. (1993) 106:1147–57. doi: 10.1016/S0022-5223(19)33992-3
3. Mohty D, Enriquez-Sarano M. The long-term outcome of mitral valve repair for mitral valve prolapse. Curr Cardiol Rep. (2002) 4:104–10. doi: 10.1007/s11886-002-0021-9
4. Tribouilloy C, Rusinaru D, Szymanski C, Mezghani S, Fournier A, Lévy F, et al. Predicting left ventricular dysfunction after valve repair for mitral regurgitation due to leaflet prolapse: additive value of left ventricular end-systolic dimension to ejection fraction. Eur J Echocardiogr. (2011) 12:702–10. doi: 10.1093/ejechocard/jer128
5. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2021) 143:e72–227. doi: 10.1161/CIR.0000000000000923
6. Morris MF, Suri RM, Akhtar NJ, Young PM, Gruden JF, Burkhart HM, et al. Computed tomography as an alternative to catheter angiography prior to robotic mitral valve repair. Ann Thorac Surg. (2013) 95:1354–9. doi: 10.1016/j.athoracsur.2012.12.010
7. Kligfield P, Hochreiter C, Kramer H, Devereux RB, Niles N, Kramer-Fox R, et al. Complex arrhythmias in mitral regurgitation with and without mitral valve prolapse: contrast to arrhythmias in mitral valve prolapse without mitral regurgitation. Am J Cardiol. (1985) 55:1545–9. doi: 10.1016/0002-9149(85)90970-1
8. Turker Y, Ozaydin M, Acar G, Ozgul M, Hoscan Y, Varol E, et al. Predictors of ventricular arrhythmias in patients with mitral valve prolapse. Int J Cardiovasc Imaging. (2010) 26:139–45. doi: 10.1007/s10554-009-9514-6
9. Turker Y, Ozaydin M, Acar G, Ozgul M, Hoscan Y, Varol E, et al. Predictors of atrial arrhythmias in patients with mitral valve prolapse. Acta Cardiol. (2009) 64:755–60. doi: 10.2143/AC.64.6.2044739
10. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2015) 28:1–39.e14. doi: 10.1016/j.echo.2014.10.003
11. Arghami A, Jahanian S, Daly RC, Hemmati P, Lahr BD, Rowse PG, et al. Robotic mitral valve repair: a decade of experience with echocardiographic follow-up. Ann Thorac Surg. (2022) 114:1587–95. doi: 10.1016/j.athoracsur.2021.08.083
12. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. (1988) 44:837–45. doi: 10.2307/2531595
13. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. (1986) 1:307–10. doi: 10.1016/S0140-6736(86)90837-8
14. Du Bois D, Du Bois EF. Clinical calorimetry: tenth paper a formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. (1916) XVII(6_2):863–71. doi: 10.1001/archinte.1916.00080130010002
15. R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2020). Available online at: https://www.R-project.org/
16. Harris CR, Millman KJ, van der Walt SJ, Gommers R, Virtanen P, Cournapeau D, et al. Array programming with NumPy. Nature. (2020) 585:357–62. doi: 10.1038/s41586-020-2649-2
17. Virtanen P, Gommers R, Oliphant TE, Haberland M, Reddy T, Cournapeau D, et al., SciPy 1.0 Contributors. Scipy 1.0: fundamental algorithms for scientific computing in python. Nat Methods. (2020) 17:261–72. doi: 10.1038/s41592-019-0686-2
18. Seabold S, Perktold J. Statsmodels: econometric and statistical modeling with python. 9th Python in Science Conference (2010).
19. Kawel-Boehm N, Hetzel SJ, Ambale-Venkatesh B, Captur G, Francois CJ, Jerosch-Herold M, et al. Reference ranges (“normal values”) for cardiovascular magnetic resonance (CMR) in adults and children: 2020 update. J Cardiovasc Magn Reson. (2020) 22:87. doi: 10.1186/s12968-020-00683-3
20. Anand V, Yang L, Luis SA, Padang R, Michelena HI, Tsay JL, et al. Association of left ventricular volume in predicting clinical outcomes in patients with aortic regurgitation. J Am Soc Echocardiogr. (2021) 34:352–9. doi: 10.1016/j.echo.2020.11.014
21. Hashimoto G, Enriquez-Sarano M, Stanberry LI, Oh F, Wang M, Acosta K, et al. Association of left ventricular remodeling assessment by cardiac magnetic resonance with outcomes in patients with chronic aortic regurgitation. JAMA Cardiol. (2022) 7:924–33. doi: 10.1001/jamacardio.2022.2108
22. Yang L-T, Anand V, Zambito EI, Pellikka PA, Scott CG, Thapa P, et al. Association of echocardiographic left ventricular end-systolic volume and volume-derived ejection fraction with outcome in asymptomatic chronic aortic regurgitation. JAMA Cardiol. (2021) 6:189–98. doi: 10.1001/jamacardio.2020.5268
23. Enriquez-Sarano M, Tajik AJ, Schaff HV, Orszulak TA, Bailey KR, Frye RL. Echocardiographic prediction of survival after surgical correction of organic mitral regurgitation. Circulation. (1994) 90:830–7. doi: 10.1161/01.CIR.90.2.830
24. Flemming MA, Oral H, Rothman ED, Briesmiester K, Petrusha JA, Starling MR. Echocardiographic markers for mitral valve surgery to preserve left ventricular performance in mitral regurgitation. Am Heart J. (2000) 140:476–82. doi: 10.1067/mhj.2000.108242
25. Carabello BA, Nolan SP, McGuire LB. Assessment of preoperative left ventricular function in patients with mitral regurgitation: value of the end-systolic wall stress-end-systolic volume ratio. Circulation. (1981) 64:1212–7. doi: 10.1161/01.CIR.64.6.1212
26. Gaasch WH, Meyer TE. Left ventricular response to mitral regurgitation. Circulation. (2008) 118:2298–303. doi: 10.1161/CIRCULATIONAHA.107.755942
27. Suri RM, Schaff HV, Dearani JA, Sundt TM, Daly RC, Mullany CJ, et al. Recovery of left ventricular function after surgical correction of mitral regurgitation caused by leaflet prolapse. J Thorac Cardiovasc Surg. (2009) 137:1071–6. doi: 10.1016/j.jtcvs.2008.10.026
28. Pickett CA, Cheezum MK, Kassop D, Villines TC, Hulten EA. Accuracy of cardiac CT, radionucleotide and invasive ventriculography, two-and three-dimensional echocardiography, and SPECT for left and right ventricular ejection fraction compared with cardiac MRI: a meta-analysis. Eur Heart J Cardiovasc Imaging. (2015) 16:848–52. doi: 10.1093/ehjci/jeu313
29. Menke J, Unterberg-Buchwald C, Staab W, Sohns JM, Seif Amir Hosseini A, Schwarz A. Head-to-head comparison of prospectively triggered vs. retrospectively gated coronary computed tomography angiography: meta-analysis of diagnostic accuracy, image quality, and radiation dose. Am Heart J. (2013) 165:154–163.e3. doi: 10.1016/j.ahj.2012.10.026
Keywords: computed tomography, mitral annuloplasty, echocardiography, ejection fraction, systolic dysfunction
Citation: Reddy P, Anand V, Rajiah P, Larson NB, Bird J, Williams JM, Williamson EE, Nishimura RA, Crestanello JA, Arghami A, Collins JD and Bratt A (2024) Predicting postoperative systolic dysfunction in mitral regurgitation: CT vs. echocardiography. Front. Cardiovasc. Med. 11:1297304. doi: 10.3389/fcvm.2024.1297304
Received: 19 September 2023; Accepted: 7 February 2024;
Published: 23 February 2024.
Edited by:
Roney Orismar Sampaio, University of São Paulo, BrazilReviewed by:
Yukiharu Sugimura, University Hospital RWTH Aachen, Germany© 2024 Reddy, Anand, Rajiah, Larson, Bird, Williams, Williamson, Nishimura, Crestanello, Arghami, Collins and Bratt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alex Bratt YnJhdHQuYWxleGFuZGVyQG1heW8uZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.