- 1Department of Cardiovascular Surgery, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou, China
- 2Department of Thoracic and Cardiac Surgery, The Eighth Affiliated Hospital, Sun Yat-Sen University, Shenzhen, China
Aims: Perioperative stroke remains a devastating complication after transcatheter aortic valve implantation (TAVI), and using a cerebral embolic protection device (CEPD) during TAVI may reduce the occurrence of stroke according to some studies. Therefore, we conducted this meta-analysis to determine whether CEPD should be routinely used during TAVI.
Methods and results: The inclusion criteria for this study were randomized controlled trials (RCTs) that examined the outcome of stroke with or without CEPD during TAVI, with a minimum follow-up period of 30 days. The primary endpoint was the occurrence of stroke (including both cerebrovascular accidents and death due to cerebrovascular accidents). The risk of stroke was lower in the CEPD group: RR 0.68, 95% CI 0.49–0.96, p = 0.03, I2 = 0%. A subgroup analysis was conducted according to the type of CEPD. The risk of stroke was lower in the I&LCCA (filter cover the innominate and the left common carotid arteries) type CEPD group: RR 0.66, 95% CI 0.49–0.96, p = 0.03, I2 = 36%. However, there was no statistically significant difference in the risk of stroke in the TMCA [filter cover the three major cerebral arteries (innominate, left common carotid, and subclavian arteries)] type CEPD group: RR 0.81, 95% CI 0.36–1.80, p = 0.60, I2 = 0%.
Conclusions: In this meta-analysis, the I&LCCA-type CEPD can reduce the risk of stroke within 30 days following TAVI, but the TMCA type cannot.
1 Introduction
Transcatheter aortic valve implantation (TAVI) was initially introduced in 2002 for the treatment of severe aortic stenosis (1). Over time, its utilization has extended beyond high-risk patients and may now also be used for low-risk patients.
Although the technology of TAVI equipment, the experience of operators, and the use of antithrombotic drugs had all been greatly improved, the incidence of stroke after TAVI was not significantly reduced (2, 3), and studies have shown that it was as high as 2%–5% (4, 5). Attention was drawn to the need to reduce the risk of stroke during TAVI, leading to the development of the cerebral embolic protection device (CEPD).
According to the operating principle of CEPD, using CEPD during TAVI can prevent the entry of various emboli (such as thrombus, vascular fragments, heart tissue) into the cerebral arteries, thereby reducing the incidence of stroke (6–8).
Published randomized controlled trials (RCTs) and retrospective cohort studies were insufficient to demonstrate that CEPD avoids or lowers the incidence of stroke, death, and other complications in patients after TAVI. In addition, the published meta-analysis may not draw firm conclusions due to the insufficient sample size and short follow-up time (4, 5, 9, 10).
A recent RCT enrolled 3,000 patients, which has the potential to provide valuable insights into the true efficacy of CEPD (11). Therefore, we conducted this updated meta-analysis to examine the efficacy of CEPD during TAVI. In addition, we also evaluated different types of CEPD.
2 Methods
This meta-analysis adhered to the guidelines established by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (12) for the development of programs, data analysis, and reporting.
2.1 Search strategy
A systematic search of relevant publications was conducted in the following databases: Embase, PubMed, Cochrane Library, Wanfang database, and China National Knowledge Infrastructure (CNKI) from April 2013 to April 2023. We used a combination of keywords and Medical Subject Heading (MeSH) terms to represent the following concepts: [(cerebral embolic protection device) OR (cerebral embolic protection system)] AND [(transcatheter aortic valve implantation) OR (transcatheter aortic valve replacement)].
2.2 Study selection
2.2.1 Inclusion criteria
This analysis exclusively comprised RCTs, which were required to meet two specific criteria: (1) comparative studies investigating stroke outcomes with or without CEPD during TAVI and (2) a minimum of 30 days of follow-up. The primary endpoint was stroke (not only cerebrovascular accidents but also death due to cerebrovascular accidents). Including stroke-related deaths in the analysis was important as they serve as an indication that the patient suffered from a stroke. Additional clinical outcomes included major bleeding, acute kidney injury, and major vascular complications.
2.2.2 Exclusion criteria
The following are the exclusion criteria for the study: (1) studies that are not written in English or Chinese, (2) studies where the primary endpoint was not stroke, (3) studies with overlapping articles, replicated data, or replicated studies, and (4) studies where the valve model or CEPD was uncertain.
2.3 Data extraction
Two authors (CW and JH) extracted the data from the included studies. The extracted data included the study design, year of publication, number of patients, patient demographic characteristics, valve type, CEPD type, TAVI route, outcome definitions, and clinical outcomes (stroke). Any disputes could be resolved by a third author (LL) after discussions.
2.4 Quality assessment
The Cochrane collaboration's tool was used to assess the risk of bias in RCTs (13). Funnel plots were used to demonstrate the publication bias of the included RCTs (13).
2.5 Statistical analysis
The RevMan 5.4 software was used for this meta-analysis. The effect size was determined using 95% confidence intervals (95% CI) and risk ratios (RR). We used the I-squared (I2) statistic to evaluate the statistical heterogeneity. When I2 < 50%, the fixed-effects model will be used; otherwise, the random-effects model will be selected. Sensitivity analyses were performed by removing one study at a time. A significance level of P < 0.05 was used to determine statistical significance.
Mantel–Haenszel methods were used for this meta-analysis. These methods employed fixed-effect meta-analysis methods with different weighting schemes depending on which effect measure (e.g., risk ratio, odds ratio, risk difference) was used. They have been shown to have better statistical properties when there are few events (10).
3 Results
3.1 Search results and inclusion studies
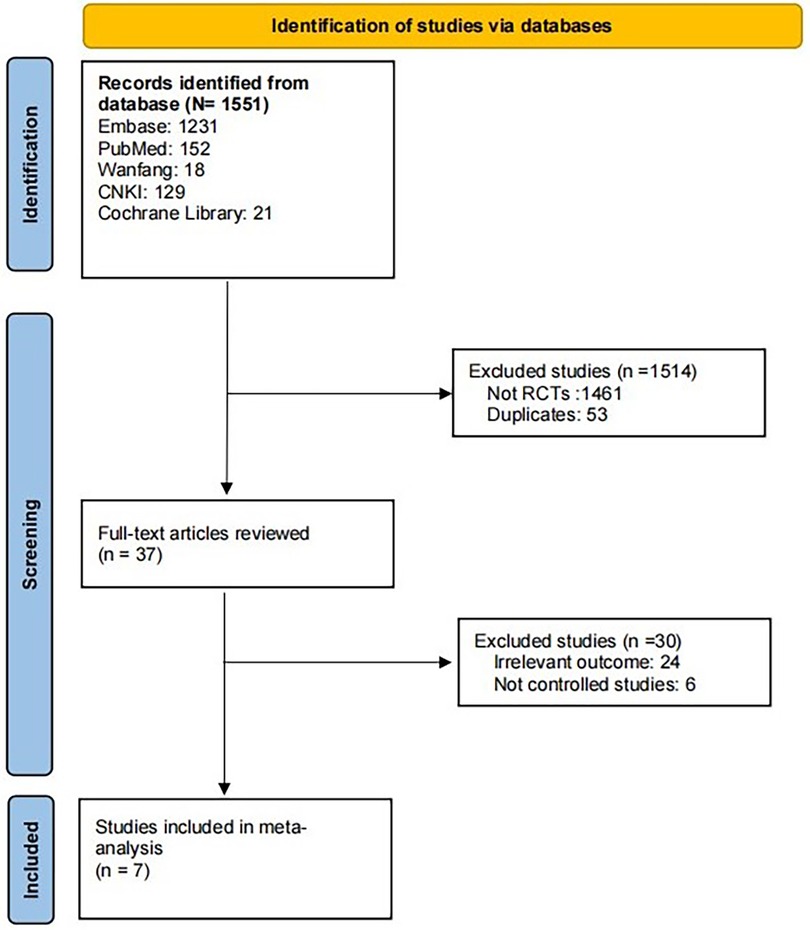
Through the above search method, a total of 1,551 publications were obtained. Upon thorough examination of the abstract and full text, seven articles (11, 14–19) with a total of 4,048 patients were included. The article screening process is shown in Figure 1.
3.2 Study characteristics and quality assessment
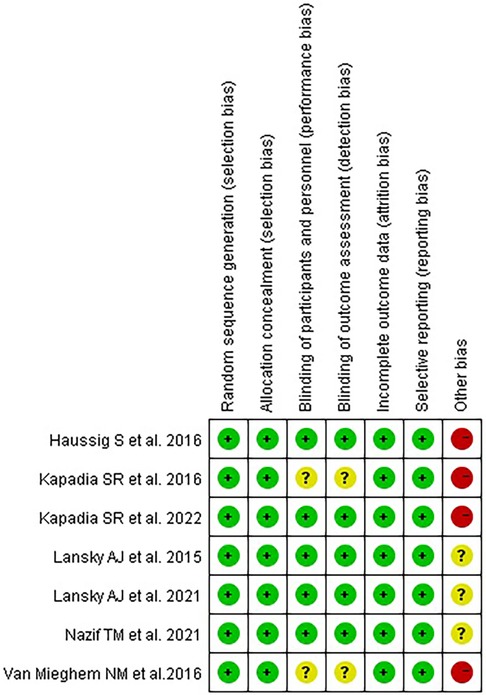
The characteristics of the included studies are shown in Table 1, and the quality assessment is shown in Figure 2.
3.3 Outcomes
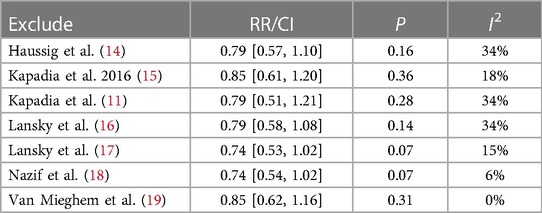
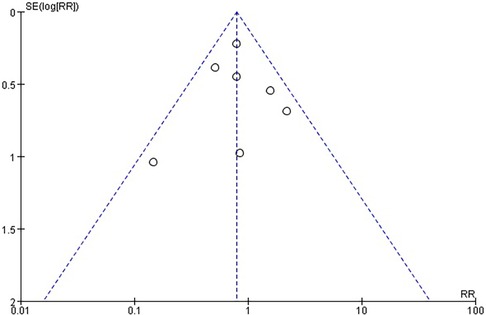
There was no statistically significant difference in the risk of stroke within 30 days between the use of CEPD during TAVI and the control group: RR 0.79, 95% CI 0.58–1.08, p = 0.14, I2 = 21% (Figure 3). A sensitivity analysis was conducted by systematically excluding one study at a time, and the results were not significantly changed (Table 2). An inspection of the funnel plot (Figure 4) showed no apparent asymmetry, indicating a possible absence of publication bias.
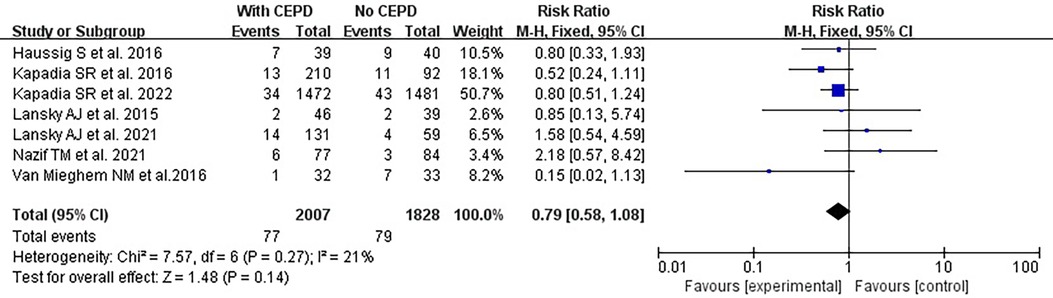
Figure 3. Effects of a 30-day stroke occurrence when comparing the use of CEPD and NOT during TAVI in RCTs. CEPD, cerebral embolic protection devices; RR, risk ratio; M–H, Mantel–Haenszel; TAVI, transcatheter aortic valve implantation.
However, due to the misplacement of certain CEPDs or vascular damage observed in the studies conducted by Lansky et al. (17) and Nazif et al. (18), the efficacy of CEPD was decreased and the risk of stroke was increased. Consequently, a high-quality meta-analysis was performed after excluding the two RCTs mentioned above. The risk of stroke was lower in the CEPD group: RR 0.68, 95% CI 0.49–0.96, p = 0.03, I2 = 0% (Figure 5).
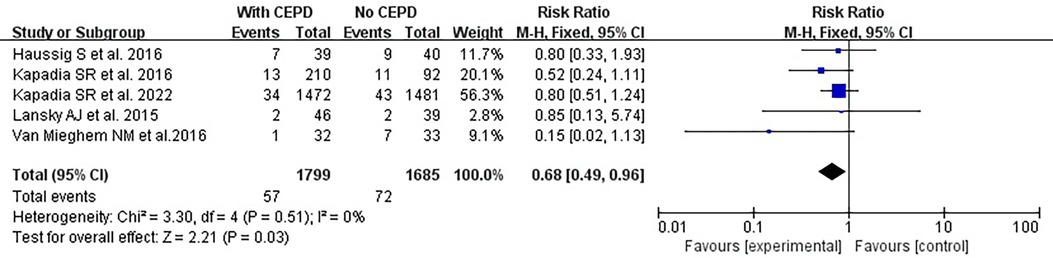
Figure 5. High-quality meta-analysis: effects of a 30-day stroke occurrence between using CEPD and NOT during TAVI in RCTs. CEPD, cerebral embolic protection devices; RR, risk ratio; M–H, Mantel–Haenszel; TAVI, transcatheter aortic valve implantation.
Based on the CEPD design types of the enrolled studies, there were two categories: (1) The I&LCCA group mostly utilized the SentinelTM CEPD, which covered the innominate and the left common carotid artery and (2) the TMCA group mainly used the TriGuardTM CEPD, which covered the three major cerebral arteries (innominate, left common carotid, and subclavian arteries) (Figure 6). A subgroup analysis was conducted according to the type of CEPD. The risk of stroke was lower in the I&LCCA-type CEPD group: RR 0.66, 95% CI 0.49–0.96, p = 0.03, I2 = 36%. However, there was no significant difference in the risk of stroke when using the TMCA-type CEPD group: RR 0.81, 95% CI 0.36–1.80, p = 0.60, I2 = 0% (Figure 7).
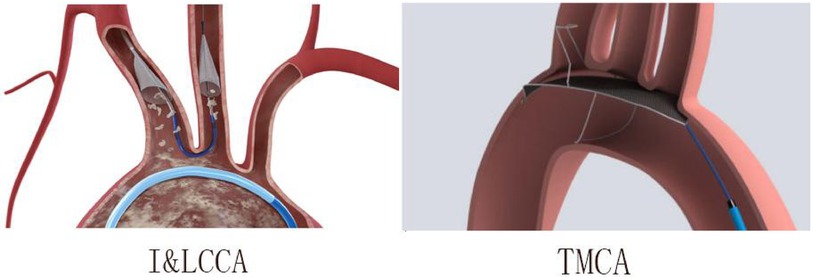
Figure 6. The innominate–left common carotid artery type (I&LCCA) and the three major cerebral arteries type (TMCA) in this meta-analysis.
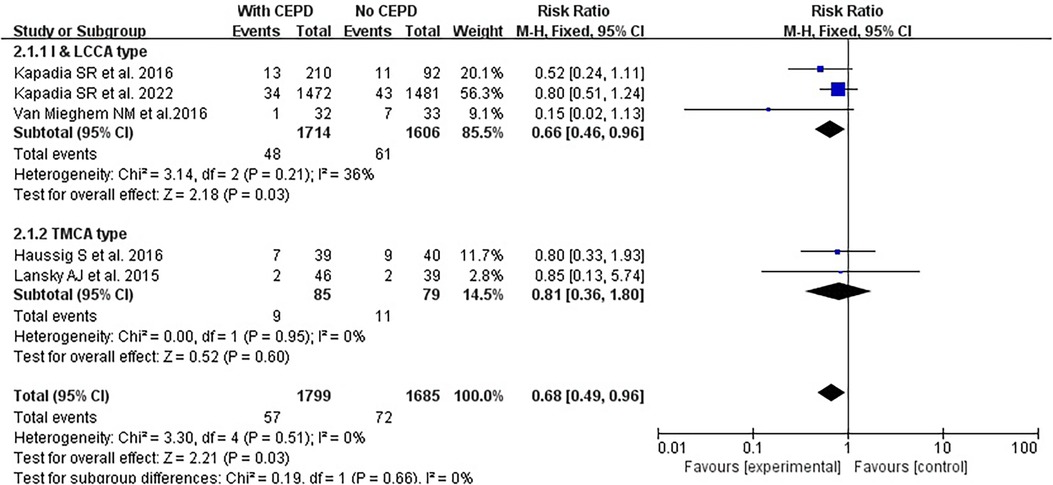
Figure 7. Subgroup analyses of the I&LCCA type and the TMCA type. I&LCCA: A type of CEPD that filter cover the innominate and the left common carotid artery; TMCA: A type of CEPD that filter cover the three major cerebral arteries (innominate, left common carotid, and subclavian arteries). CEPD, cerebral embolic protection devices; RR, risk ratio; M–H, Mantel–Haenszel; TAVI, transcatheter aortic valve implantation.
4 Discussion
In this meta-analysis, we analyzed the incidence of stroke within 30 days after TAVI, and the risk of stroke was found to be lower in the CEPD group during TAVI. Furthermore, the subgroup analysis indicated that the I&LCCA-type CEPD could reduce the risk of stroke during TAVI, but the TMCA type could not.
After our discussion, the reasons were as follows:
1. The products design of the I&LCCA type consists of two independent funnel-shaped strainers that are designed to better align with vessels and be positioned more accurately. Moreover, the two funnels were fixed to the target vessel through blood flow pressure, resulting in less pressure and minimal damage to the vascular endothelium. However, the TMCA type was developed based on an integrated design, which may not be suitable for all sizes of blood vessels. It functions by exerting pressure on the vascular endothelium to maintain the desired position, although this can potentially result in the formation of blood clots (Figure 8).
2. TMCA is a new type of CEPD that may require further improvement in its design and further enhancement of operator proficiency.
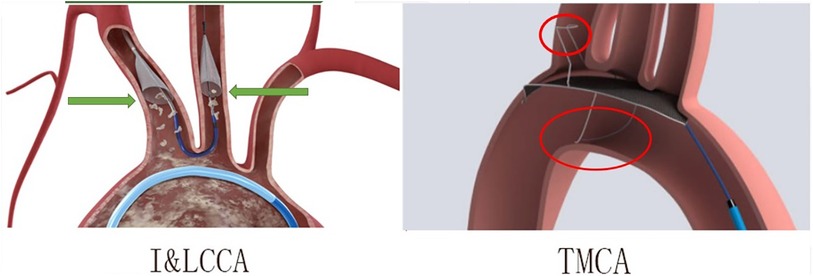
Figure 8. The differences between the I&LCCA type (the innominate–left common carotid artery type) and TMCA type (the three major cerebral arteries type). I&LCCA type: more suitable, less vascular endothelium damage; TMCA type: integrated design, exerting pressure on vascular endothelium.
Given the abundance of research demonstrating the safety of CEPD, safety analysis was not conducted in this study (17, 20–22).
4.1 Limitation
In addition, the outcomes of the included RCTs were influenced by the following factors: (1) Valve type: There were more than five types of valves. By utilizing only one type, the outcome may be improved. (2) Outcome definitions: Both the NIHSS and MACCE were used to assess the occurrence of stroke. It is recommended to use only one. (3) The RCTs conducted by Haussig et al. (14), Kapadia et al. (15), and Van Mieghem et al. (19) were not multicenter studies.
5 Conclusion
In conclusion, our meta-analysis revealed that the I&LCCA-type CEPD could reduce the risk of stroke within 30 days after TAVI, but the TMCA type could not. The efficacy of the TMCA-type CEPD might be demonstrated by the implementation of large-scale research with a substantial sample size, focusing on a single valve type and a single outcome definition. In addition, conducting multicenter studies and ensuring that operators are well trained would contribute to the validity of the findings. A new RCT (NCT05295628) is currently underway to investigate the efficacy of the I&LCCA-type and TMCA-type CEPD. The trial aims to enroll a total of 532 subjects undergoing TAVR at up to 30 investigational sites in the United States. The results of this trial may provide valuable insights into the true efficacy of the TMCA-type CEPD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
CW wrote the main manuscript text, JH and LL prepared the figures and tables, and JQ and YF participated in all discussions. JZ revised and reviewed the article. All authors reviewed the manuscript. CW, JH, and LL contributed equally to this work. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Natural Science Foundation of China (NSFC) [Grant Numbers 82271806] to Junmeng Zheng.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. (2002) 106(24):3006–8. doi: 10.1161/01.CIR.0000047200.36165.B8
2. Pilgrim T, Lee JKT, O’Sullivan CJ, Stortecky S, Ariotti S, Franzone A, et al. Early versus newer generation devices for transcatheter aortic valve implantation in routine clinical practice: a propensity score matched analysis. Open Heart. (2018) 5(1):e000695. doi: 10.1136/openhrt-2017-000695
3. Huded CP, Tuzcu EM, Krishnaswamy A, Mick SL, Kleiman NS, Svensson LG, et al. Association between transcatheter aortic valve replacement and early postprocedural stroke. JAMA. (2019) 321(23):2306–15. doi: 10.1001/jama.2019.7525
4. Ahmad Y, Howard JP. Meta-analysis of usefulness of cerebral embolic protection during transcatheter aortic valve implantation. Am J Cardiol. (2021) 146:69–73. doi: 10.1016/j.amjcard.2021.01.023
5. Ndunda PM, Vindhyal MR, Muutu TM, Fanari Z. Clinical outcomes of sentinel cerebral protection system use during transcatheter aortic valve replacement: a systematic review and meta-analysis. Cardiovasc Revasc Med. (2020) 21(6):717–22. doi: 10.1016/j.carrev.2019.04.023
6. Lind A, Jánosi RA, Totzeck M, Ruhparwar A, Rassaf T, Al-Rashid F. Embolic protection with the TriGuard 3 system in nonagenarian patients undergoing transcatheter aortic valve replacement for severe aortic stenosis. J Clin Med. (2022) 11(7):2003. doi: 10.3390/jcm11072003
7. Voss S, Ernst A, Erlebach M, Ruge H, Sideris K, Bleiziffer S, et al. Effects of a dual-filter-based cerebral embolic protection device in transcatheter aortic valve replacement on cerebral oxygen saturation: a prospective pilot study. J Card Surg. (2021) 36(4):1241–8. doi: 10.1111/jocs.15355
8. Gallo M, Putzu A, Conti M, Pedrazzini G, Demertzis S, Ferrari E. Embolic protection devices for transcatheter aortic valve replacement. Eur J Cardiothorac Surg. (2018) 53(6):1118–26. doi: 10.1093/ejcts/ezx457
9. Nazir S, Zafrullah F, Virk HUH, Sandhu CS, Ameen M, Ahuja KR. Meta-analysis of cerebral embolic protection during transcatheter aortic valve replacement. Am J Cardiol. (2021) 139:138–9. doi: 10.1016/j.amjcard.2020.10.038
10. Radwan Y, Al-Abcha A, Salam MF, Khor SY, Prasad RM, Elshafie A, et al. Meta-analysis of the safety and efficacy of the sentinel cerebral protection system in transcatheter aortic valve implantation. Am J Cardiol. (2021) 152:169–70. doi: 10.1016/j.amjcard.2021.04.006
11. Kapadia SR, Makkar R, Leon M, Abdel-Wahab M, Waggoner T, Massberg S, et al. Cerebral embolic protection during transcatheter aortic-valve replacement. N Engl J Med. (2022) 387(14):1253–63. doi: 10.1056/NEJMoa2204961
12. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
13. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. Br Med J. (2011) 343:d5928. doi: 10.1136/bmj.d5928
14. Haussig S, Mangner N, Dwyer MG, Lehmkuhl L, Lücke C, Woitek F, et al. Effect of a cerebral protection device on brain lesions following transcatheter aortic valve implantation in patients with severe aortic stenosis: the CLEAN-TAVI randomized clinical trial. JAMA. (2016) 316(6):592–601. doi: 10.1001/jama.2016.10302
15. Kapadia SR, Kodali S, Makkar R, Mehran R, Lazar RM, Zivadinov R, et al. Protection against cerebral embolism during transcatheter aortic valve replacement. J Am Coll Cardiol. (2017) 69(4):367–77. doi: 10.1016/j.jacc.2016.10.023
16. Lansky AJ, Schofer J, Tchetche D, Stella P, Pietras CG, Parise H, et al. A prospective randomized evaluation of the TriGuard™ HDH embolic DEFLECTion device during transcatheter aortic valve implantation: results from the DEFLECT III trial. Eur Heart J. (2015) 36(31):2070–8. doi: 10.1093/eurheartj/ehv191
17. Lansky AJ, Makkar R, Nazif T, Messé S, Forrest J, Sharma R, et al. A randomized evaluation of the TriGuard™ HDH cerebral embolic protection device to reduce the impact of cerebral embolic LEsions after TransCatheter aortic valve ImplanTation: the REFLECT I trial. Eur Heart J. (2021) 42(27):2670–9. doi: 10.1093/eurheartj/ehab213
18. Nazif TM, Moses J, Sharma R, Dhoble A, Rovin J, Brown D, et al. Randomized evaluation of TriGuard 3 cerebral embolic protection after transcatheter aortic valve replacement: REFLECT II. JACC Cardiovasc Interv. (2021) 14(5):515–27. doi: 10.1016/j.jcin.2020.11.011
19. Van Mieghem NM, van Gils L, Ahmad H, van Kesteren F, van der Werf HW, Brueren G, et al. Filter-based cerebral embolic protection with transcatheter aortic valve implantation: the randomised MISTRAL-C trial. EuroIntervention. (2016) 12(4):499–507. doi: 10.4244/EIJV12I4A84
20. Langhoff R, Petrov I, Kedev S, Milosevic Z, Schmidt A, Scheinert D, et al. PERFORMANCE 1 study: novel carotid stent system with integrated post-dilation balloon and embolic protection device. Catheter Cardiovasc Interv. (2022) 100(6):1090–9. doi: 10.1002/ccd.30410
21. Jagielak D, Targonski R, Frerker C, Abdel-Wahab M, Wilde J, Werner N, et al. Safety and performance of a novel cerebral embolic protection device for transcatheter aortic valve implantation: the PROTEMBO C trial. EuroIntervention. (2022) 18(7):590–7. doi: 10.4244/EIJ-D-22-00238
Keywords: CEPD, TAVI, TAVR, Stroke, Meta-analysis
Citation: Wang C, Han J, Lu L, Qiu J, Fu Y and Zheng J (2024) The efficacy of different types of cerebral embolic protection device during transcatheter aortic valve implantation: a meta-analysis. Front. Cardiovasc. Med. 11:1205943. doi: 10.3389/fcvm.2024.1205943
Received: 14 April 2023; Accepted: 8 January 2024;
Published: 23 February 2024.
Edited by:
Robert Jeenchen Chen, Stanford University, United StatesReviewed by:
Francesco Giannini, Maria Cecilia Hospital, ItalyAntonino S. Rubino, University of Campania Luigi Vanvitelli, Italy
Massimo Baudo, Lankenau Institute for Medical Research, United States
© 2024 Wang, Han, Lu, Qiu, Fu and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junmeng Zheng emhlbmdqbTI3QG1haWwuc3lzdS5lZHUuY24=
†These authors have contributed equally to this work
Abbreviations TAVI, transcatheter aortic valve implantation; CEPD, cerebral embolic protection device; I&LCCA, filter cover the innominate and the left common carotid artery; TMCA, filter cover the three major cerebral arteries (innominate, left common carotid, and subclavian artery); RCTs, randomized controlled trials; 30-day, 30 days after TAVI; PRISMA, the preferred reporting items for systematic reviews and meta-analyses; CNKI, china national knowledge infrastructure; MeSH, medical subject heading.
 Chao Wang
Chao Wang Jingjun Han2,†
Jingjun Han2,† Junxiong Qiu
Junxiong Qiu Yuan Fu
Yuan Fu Junmeng Zheng
Junmeng Zheng