- 1Cardiology Department, Affiliated Minda Hospital of Hubei Minzu University, Enshi, China
- 2Cardiac Pacing and Electrophysiological Department, The First Affiliated Hospital of Xinjiang Medical University, Urumqi, China
- 3Xinjiang Key Laboratory of Cardiac Electrophysiology and Cardiac Remodeling, The First Affiliated Hospital of Xinjiang Medical University, Urumqi, China
- 4Cardiology Department, West China Hospital, Sichuan University, Chengdu, China
Objective: To analyse the characteristics and mortality of hypertrophic cardiomyopathy (HCM) patients with different body compositions.
Methods: In this study, 530 consecutive patients with HCM at West China Hospital were studied from November 2008 to May 2016. An equation based on body mass index (BMI) was used to obtain the Percent body fat (BF) and lean mass index (LMI). Patients were divided into five sex-specific BMI, BF and LMI quintiles.
Results: The average BMI, BF and LMI were 23.1 ± 3.2 kg/m2, 28.1 ± 7.3% and 16.5 ± 2.2 kg/m2, respectively. Patients with higher BMI or BF were older and had more symptoms and adverse cardiovascular conditions; those with higher LMI were younger and had less coronary artery disease and lower serum NT-proBNP and creatine. BF correlated positively with resting left ventricular (LV) outflow tract gradient, mitral regurgitation (MR) degree and left atrial diameter but was inversely associated with septal wall thickness (SWT), posterior wall thickness (PWT), LV mass, and E/A ratio; LMI was positively correlated with SWT, LV end diastolic volume and LV mass but was negatively associated with MR degree.48 all-cause deaths occurred during a median follow-up of 33.8 months. Reversed J-shape associations of BMI and LMI with mortality were observed. A lower BMI or LMI was significantly associated with high mortality, especially for low-moderate BMI and LMI. No significant difference in mortality was found across BF quintiles.
Conclusions: The associations of BMI, BF and LMI with baseline characteristics and cardiac remodelling are different in HCM patients. In Chinese HCM patients, low BMI and LMI predicted mortality but not BF.
Introduction
Hypertrophic cardiomyopathy (HCM) is the most common inherited heart disorder with heterogeneity in its natural history. Although HCM-causing mutations are believed to be the main prognostic factor of HCM patients, a gene-based model is still unable to predict outcomes (1). In fact, even classically inherited diseases can be modified by demographic features, physiological challenges and comorbid conditions. Therefore, understanding the relationship between environmental modulators and outcomes can help manage patients with HCM.
Obesity has become a major health concern as a result of the change in modern lifestyles. The population with obesity is more vulnerable to cardiovascular disease. However, in the context of chronic diseases, including chronic heart failure (2), chronic obstructive pulmonary disease (3), and stroke (4), higher body mass index (BMI) closely associated with lower mortality, presenting an “obesity paradox”. With respect to HCM, it has been reported that obesity in adolescence predicts a higher occurrence rate of HCM in midlife (5). However, it is unclear whether there is an obesity paradox in patients with HCM.
BMI, an index universally applied to measure obesity, has been questioned under the background of the obesity paradox in cardiovascular disease (6). Fat mass and lean mass are included in body composition, and BMI does not differentiate between these components. Having a high BMI does not necessarily mean that you have a higher body fat (BF), but may indicate that you have a higher lean mass. Thus, the paradox, resulting from the apply of an inaccurate surrogate for obesity, may be a misleading finding. For example, a recent study from our group showed that among coronary artery disease patients with mild renal insufficiency, there was no association between fat mass and mortality risk, but an inverse association between lean mass and mortality risk (7). However, data on the effect of body composition on survival in HCM patients are limited. In the present study, the objective was to investigate the relationship between body composition and mortality in a Chinese cohort with HCM.
Methods
Subjects
The data of this study were obtained from the hypertrophic cardiomyopathy database of West China Hospital. The database includes data from consecutive patients diagnosed with HCM at West China Hospital of Sichuan University from November 2008 to May 2016, and the collected data includes demographic, clinical, laboratory and treatment data. Patients with the following conditions will be excluded: (1) patients with malignant tumors, (2) patients with severe aortic valve disease and (3) patients with end-stage renal failure. All patients have been informed consent, so that their medical records for research purposes. The study got the West China Hospital of Sichuan University Ethics Committee approval.
Echocardiographic evaluation
All patients were assessed by echocardiography as recommended by the American Society of Echocardiography (8). Two-dimensional echocardiography was used to diagnose HCM based on a left ventricular (LV) wall thickness ≥ 15 mm in adults who did not have any cardiac or systemic etiology that can cause such hypertrophy (9, 10). Resting left ventricular outflow tract (LVOT) obstruction was determined by apical five-chamber view with a peak outflow gradient ≥ 30 mmHg at rest (11). The continuity equation method was used to analyze the regurgitant jet area, vena contracta width and regurgitation quantitation, and then the mitral regurgitation (MR) was divided into no, mild, moderate and severe regurgitation (12). With the following formula to determine LV mass: LV mass = 0.8{1.04[(LV external end-diastolic diameter (LVEDD) + PWTd + SWT)3 − (LVEDD)3]} + 0.6 g.
Body composition assessment
Using standard methods, nurses measured the patients' height and weight, and calculated BMI by dividing the weight (kg) by the square of the height (m2). The Clínica Universidad de Navarr—Body Adiposity Estimator (CUN-BAE) equation was applied to estimate percentage BF: BF = −44.988 + (0.503 × age) + (10.689 × sex) + (3.172 × BMI) − (0.026 × BMI2) + (0.181 × BMI × sex) − (0.02 × BMI × age) − (0.005 × BMI2 × sex) + (0.00021 × BMI2 ×age), where sex is replaced by 0 for males and 1 for females (13).This formula has been verified in previous study (14). The formula (1 − BF%) × BMI kg/m2 was applied to calculate the lean mass index (15). Because there were no reports of reference value in the Chinese population, study patients were divided into five groups based on the quintiles of sex-specific BMI, LMI and BF.
Baseline data collection and laboratory measurement
The baseline anthropometric data were obtained from electronic medical records. The medical history (hypertension, diabetes mellitus, coronary artery disease, stroke and dyslipidaemia), symptoms of HCM, smoking history, and family history of sudden cardiac death were obtained from hospital records and collected by trained research investigators. Cardiologists assess the risk of sudden cardiac death (SCD) according to the HCM Risk-SCD tool (16). Venous blood samples were collected in tubes containing ethylene diamine tetraacetic acid (EDTA) and analysed at the Department of Laboratory Medicine, West China Hospital, accredited by the College of American Pathology.
Events and follow-up
During follow-up, the primary event recorded was all-cause mortality and the secondary event was cardiovascular mortality. Patients themselves and/or their family members were contacted by telephone or through outpatient visits for follow-up information. Most of the patients visited outpatient appointments every 1–6 months. All the data were consistent with the hospital records.
Patient and public involvement
It was inappropriate or impossible to involve patients or the public in the design, or implementation, or reporting, or dissemination plans of our study.
Statistical analysis
Continuous variables with a normally distributed distribution are presented as the mean ± standard deviation, while non-normally distributed variables are presented as the median (interquartile range). Categorical variables are represented as counts (percentages). The χ2 test or Fisher's exact test was applied to compare categorical variables. Data were compared using one-way analysis of variance if they followed a normal distribution; or else, the data were compared using the Kruskal-Wallis test. Single correlations between body composition and echocardiographic characteristics were evaluated using Pearson's correlation test when the values were normally distributed; otherwise, they were analysed using Spearman's rank correlation test. To determine the association between body composition and outcomes, survival across body composition categories was plotted in Kaplan-Meier curves and compared using the log-rank test. Then, multivariate Cox proportional hazard analyses were applied to identify the independent effects of baseline body composition characteristics on time until death. Possible confounding factors of age, sex and medical history (diabetes mellitus, hypertension, atrial fibrillation, coronary artery disease and family history of SCD) were adjusted; septal wall thickness (SWT); LVOT obstruction; LV wall thickness > 30 mm; LV ejection fraction (LVEF); treatment with β blockers, non-dihydropyridine calcium channel blockers, angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers; and alcohol septal ablation. The method described by Grambsch and Therneau was used to assess the assumption of proportional hazards (17). Restricted spline regression analyses, using four knots, were performed to test the potential non-linear relationship of body composition with all-cause mortality. The fitted spline model for all-cause mortality vs. each body composition characteristic was then plotted on the log-hazards scale, with confidence intervals overlaid. All statistical analyses were conducted using Stata/MP 13.0. We considered a two-sided P value <0.05 to be statistically significant.
Results
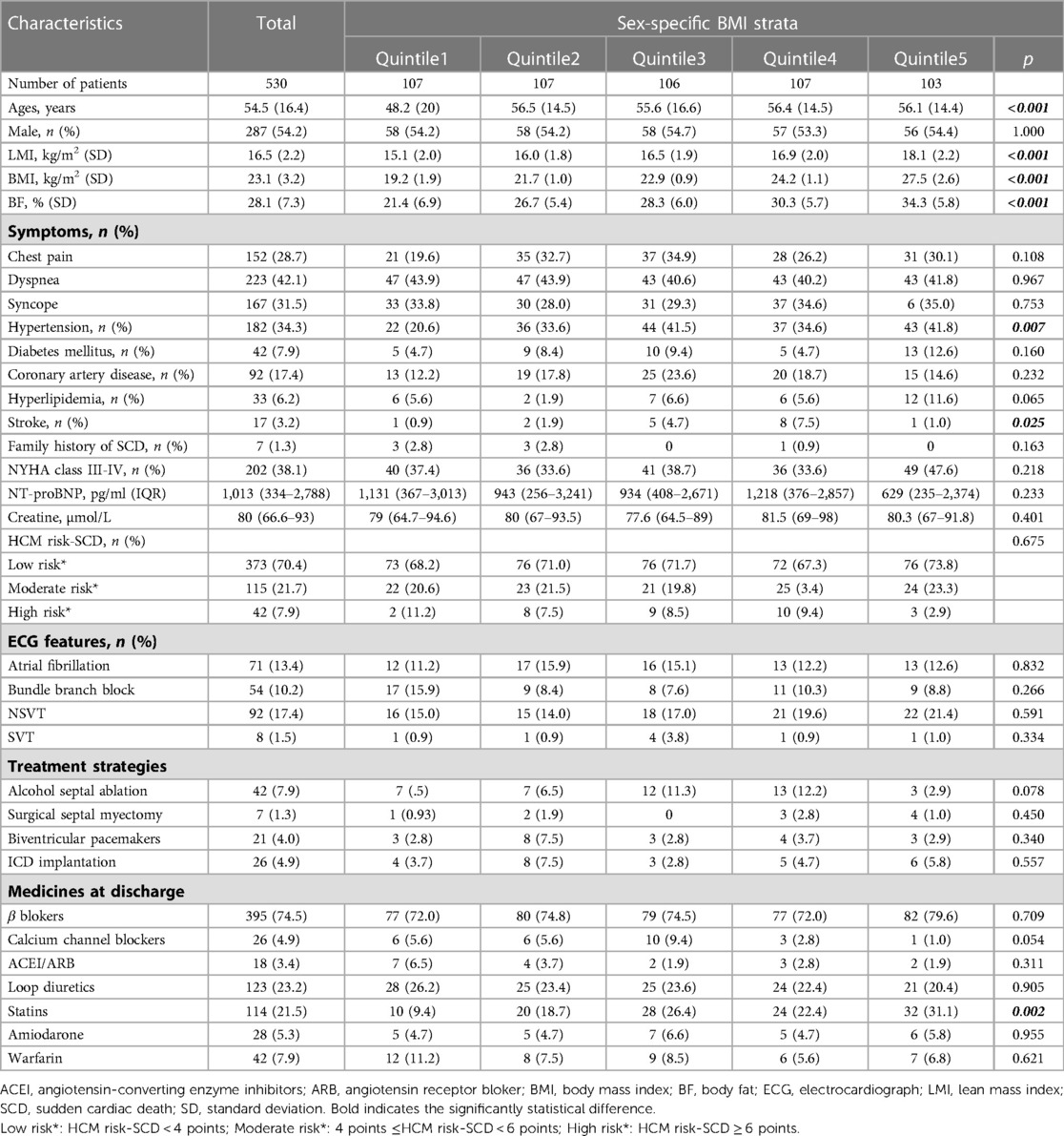
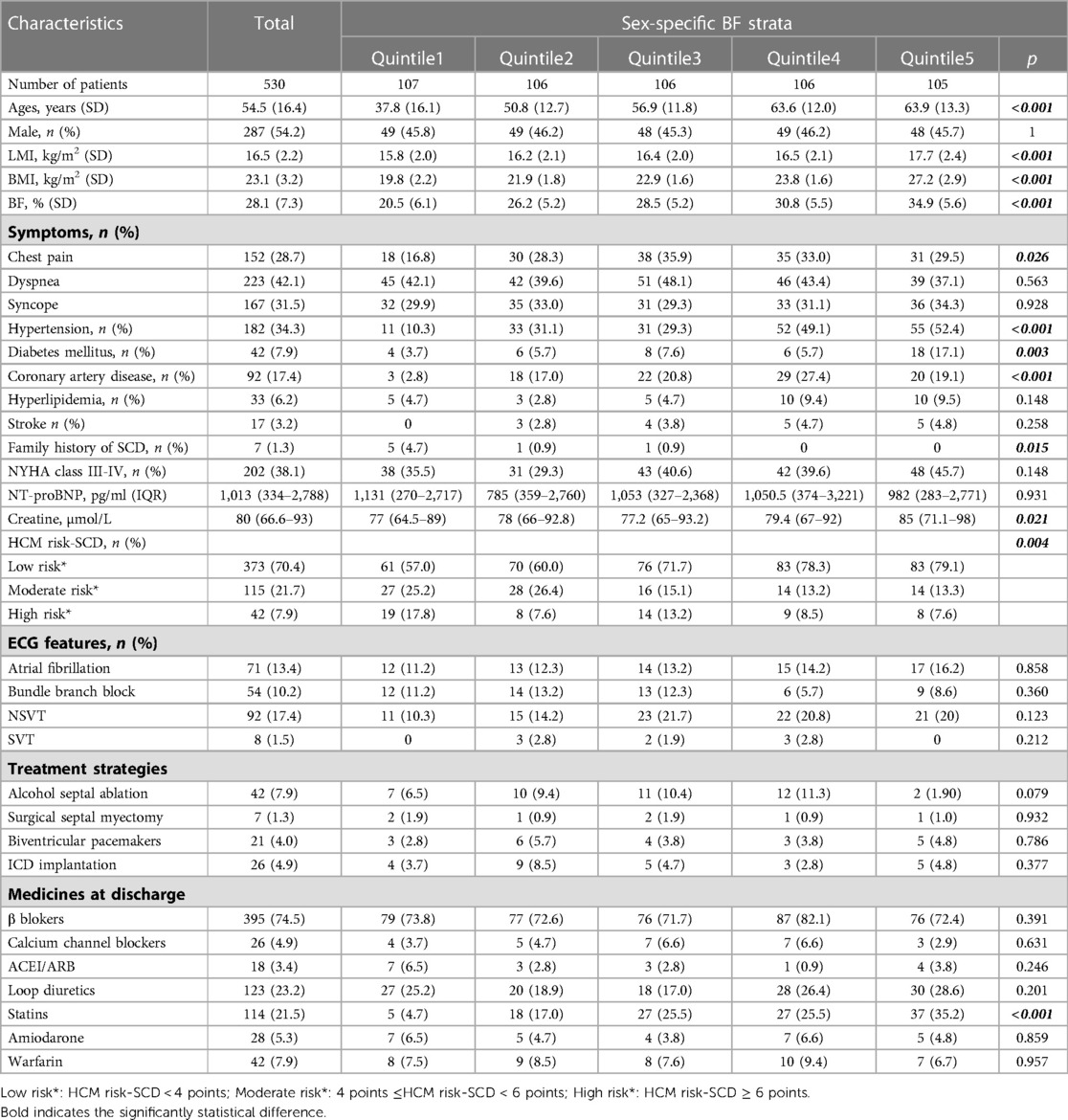
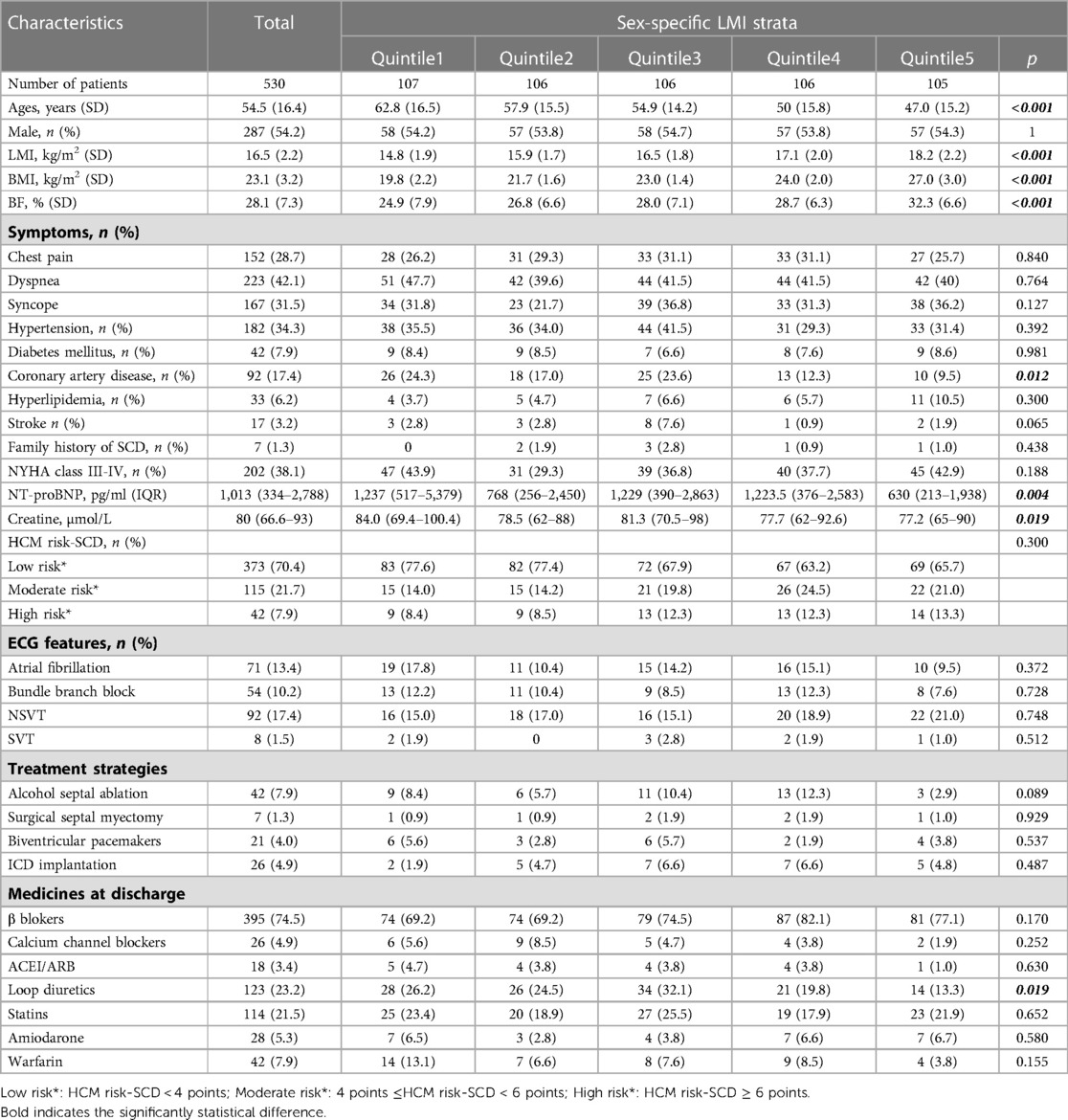
530 patients with complete BMI data from the West China Hospital Hypertrophic Cardiomyopathy database were included in the present study. The average age of these patients was 54.5 ± 16.4 years, and 54.2% of the enrolled patients were male. The average BMI, BF and LMI were 23.1 ± 3.2 kg/m2, 28.1 ± 7.3% and 16.5 ± 2.2 kg/m2, respectively. The distributions of the patients' baseline data across BMI, LMI and BF quintiles are showed in Tables 1–3, respectively. In general, patients with higher BMI or BF were older; had a higher frequency of chest pain symptoms, more comorbidities (hypertension, diabetes, stroke or coronary artery disease), and a higher level of serum creatine; and were more likely to be on statins. More patients with lower BF were classified as having a moderate-high risk of SCD. In contrast, patients with higher LMI had a significantly lower age. A lower proportion of patients with higher LMI had a history of coronary artery disease. Lower serum levels of creatine and NT-proBNP were found among patients with higher LMI. Loop diuretics were prescribed less often in patients with higher LMI.
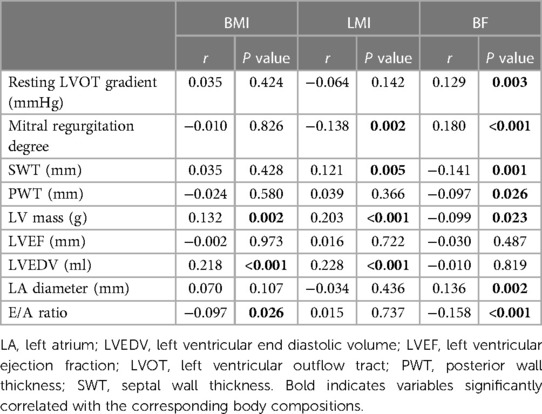
We further explored the potential correlations between body composition and various echocardiographic data (Table 4). BMI was positively correlated with LVEDV but inversely correlated with the E/A ratio. BF was positively associated with the resting LVOT gradient, MR degree and LA diameter but was negatively associated with SWT, posterior wall thickness (PWT), LV mass, and E/A ratio. LMI was positively correlated with SWT, LVEDV and LV mass but was negatively associated with MR degree. No correlation was observed between LVEF and the three body composition measurements.
Over a median follow-up of 33.8 months (interquartile range, 14.8–58.3 months; maximum follow-up, 107 months), a total of 48 [rate 2.74/100 person-year, 95% confidence interval (CI) 2.07–3.64] deaths occurred, and of those deaths, 31 (rate 1.77/100 person-year, 95% CI 1.25–2.51) were ascribed to cardiovascular causes. Kaplan-Meier plots suggested that the risk of all-cause and cardiovascular mortality was higher in the low BMI group (Figures 1A,B). Similar results were also observed in the low LMI group (P for all-cause mortality < 0.001 and P for cardiovascular mortality = 0.012, Figures 1C,D). However, no significant difference in mortality was found across the BF groups (P for all-cause mortality = 0.545 and P for cardiovascular mortality = 0.097, Figures 1E,F).
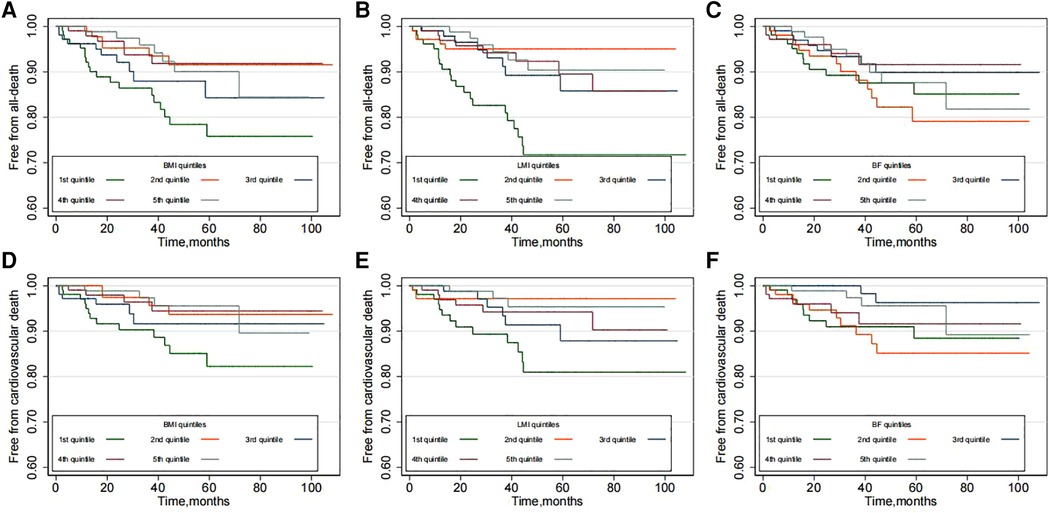
Figure 1. Kaplan-Meier plots according to the quintiles of body composition: (A,B) for BMI; (C,D) for LMI and (E,F) for BF.
The univariate and multivariate HRs for all-cause and cardiovascular mortality by body composition variables are displayed in Supplementary Figure S1. Lower BMI and LMI were associated with higher all-cause mortality. Even after multifactorial correction, BMI (quintile 1: reference; quintile 2: HR 0.31, 95% CI 0.12–0.78; quintile 3: HR 0.60, 95% CI 0.28–1.30; quintile 4: HR 0.33, 95% CI 0.13–0.82; quintile 5: HR 0.37, 85% 0.16–0.90) or LMI (quintile 1: reference; quintile 2: HR 0.21, 95% CI 0.08–0.59; quintile 3: HR 0.37, 95% CI 0.16–0.88; quintile 4: 0.35, 95% CI 0.14–0.87; quintile 5: 0.28, 95% CI 0.10–0.76) were inversely associated with the risk of all-cause mortality (Figure 2A). However, no significant association between BF and mortality could be observed. Similarly, the univariate and multivariate Cox analyses showed that BMI and LMI, but not BF, were inversely relevant to cardiovascular deaths (Figure 2B).
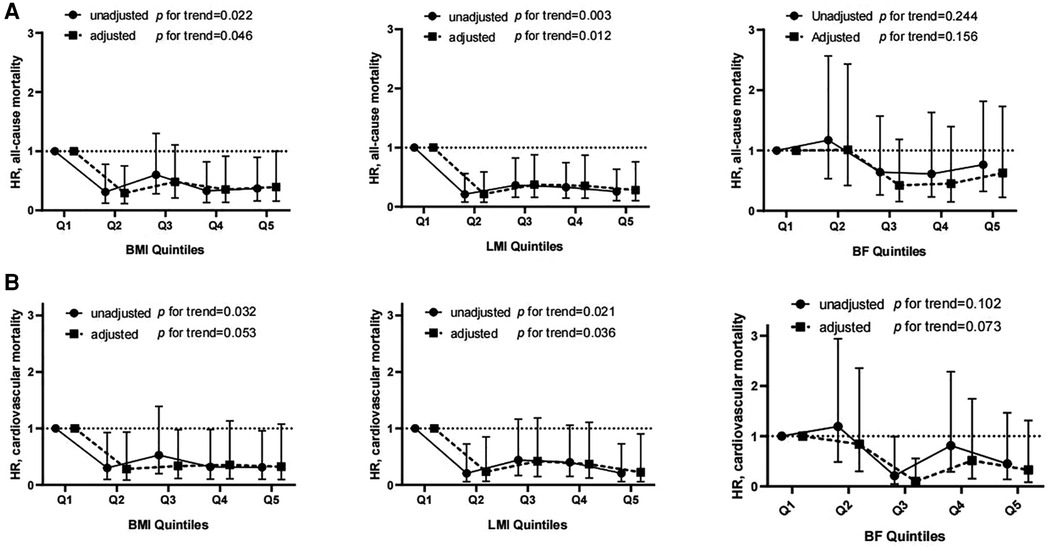
Figure 2. Hrs and 95% CIs for all-cause (A) and cardiovascular mortality (B) associated with different quintiles of body composition. There was no significant violation of the proportional hazard assumption (all-cause death: P = 0.36, 0.16 and 0.46 for BMI-, LMI- and BF-containing models, respectively; cardiovascular death: P = 0.66, 0.59 and 0.73 for BMI-, LMI- and BF-containing models, respectively).
Spline regression curves demonstrating the multivariable-adjusted HRs of BMI, LMI and BF for risk of death are presented in Figure 3. Figure 3A illustrates a reversed J-shape curve, with an inflexion point occurring at BMI ≤ 23 kg/m2, such that a higher BMI was independently correlated with a lower risk of death (Figure 3A). The risk of all-cause mortality was relatively flat when BMI was greater than 23 kg/m2. With respect to LMI, the risk of death decreased in a linear fashion until approximately 18 kg/m2 of LMI and then started to decrease slowly afterwards (Figure 3B). However, Figure 2C shows an overall U-shaped relationship between BF and all-cause mortality, with the inflexion point at BF = 32%.

Figure 3. Splines of the multivariate associations between BMI (A), LMI (B) or BF (C) and all-cause mortality. Adjusted for age; sex; medical history (diabetes mellitus, hypertension, atrial fibrillation, coronary artery disease and family history of SCD); SWT; LVOT obstruction; left ventricular wall thickness > 30 mm; left ventricular ejection fraction; treatment with β blockers, non-dihydropyridine calcium channel blockers, angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers; and alcohol septal ablation. The two dotted lines show the 95% CIs.
Discussion
In this cohort study of 530 Chinese HCM patients, we used a validated anthropometric prediction equation to examine the association of BF and LMI with all-cause and cardiovascular mortality. We found that mortality was inversely related to BMI, especially among normal weight (BMI ≤ 22 kg/m2) HCM patients. The inverse relationship of LMI with mortality seemed to be stronger until approximately 18 kg/m2 of LMI. However, BF was not significantly associated with mortality in HCM patients.
Obesity is emerging as a public health problem and a well-known risk factor for cardiovascular disease. Recently, a Sweden nationwide registry-based prospective cohort study showed that obesity in adolescence resulted in an increased risk of HCM in midlife (5). Moreover, Olivotto et al. reported that obesity may contribute to more rapid clinical progression and worsening of heart failure symptoms in HCM patients (18). In agreement with these findings, Larsen et al. suggested that in patients with HCM, increasing BMI was associated with a higher prevalence of dyspnoea and poorer exercise capacity. However, few studies to date have addressed the effect of obesity on mortality in patients with HCM. Our data demonstrated that the frequency of HCM-related symptoms did not differ across BMI groups, but when BMI was less than 23 kg/m2, higher BMI predicted a lower risk of death, which seems to be contrary to the above reports. Notably, the U- or reversed J-shape relationship between BMI and outcomes of HCM patients has been documented in both Meghji et al.'s study and the present study. Thus, considering the striking distinction of BMI between Caucasian (mean BMI 30 kg/m2) and Chinese (23.1 kg/m2) studies, our data may reflect the exclusive relationship between BMI and outcomes in the Chinese HCM population who have relatively low BMI levels.
The obesity paradox has been documented in patients with coronary artery disease, chronic heart failure and several chronic vascular diseases, but it remains controversial. One of the arguments against the obesity paradox focused on the defect of BMI in measuring body composition (lean mass and fat mass). In fact, Oreoponlous et al. showed that BMI, as a surrogate for obesity, had a better relationship with lean mass than BF. BMI-measured obesity is sometimes associated with excess muscle rather than fat (6). Higher BF reflects lower physical activity, hyperinsulinaemia, and more risk factors, while higher LMI may reflect a healthy metabolic state. Thus, people with the same BMI may have entirely different cardiovascular risk profiles. As expected, in our study, patients with higher BF were concomitant with clustered cardiovascular conditions, such as hypertension, diabetes mellitus, coronary artery disease and higher prescriptions of statins (Table 2). Conversely, higher LMI was associated with relatively benign cardiovascular profiles involving a lower frequency of coronary artery disease and diuretic use and lower levels of serum NT-proBNP and creatine (Table 3).
Obesity has been shown to result in abnormal cardiac remodelling and function mediated by fat-derived inflammatory cytokines. Olivotto et al. demonstrated that obesity indexed by BMI was associated with increased LV mass and LA diameter, LVEDD and greater LVOT obstruction (15). When considering body composition, we found that higher BF, but not LMI, was associated with diastolic dysfunction and more severe LVOT obstruction, which can be seen from the positive association between BF and LA diameter, MR degree and resting LVOT gradient (Table 4). The increased levels of inflammatory factors and hyperinsulinaemia that usually occur in the presence of increased BF might induce myocardial fibrosis, thereby decreasing ventricular compliance (19). Moreover, our data indicated that LMI appears to be associated with eccentric hypertrophy in HCM patients, which can be seen from the positive correlation between LMI and LVEDV and LV mass. These findings in the context of HCM were similar to those in the general population. Previous studies suggested that athletes with greater lean mass have higher LV mass and LVEDV (20). In fact, an increase in LV mass and LVEDV in patients with high LMI may be understood as a result of an increased circulating blood volume and cardiac output necessary to supply the metabolic demand of lean mass (21). Additionally, genetic influences in regard to HCM may concomitantly affect skeletal muscle. However, even though we observed that individuals with higher LMI have greater LV mass, Miyaji et al. showed that the baseline LV mass was not associated with the clinical outcomes of HCM patients (22).
As far as we know, this is the first study to investigate the relationship between body composition and the mortality of HCM patients. Our data added a new insight that the reversed J-shaped relationship between BMI and morality in HCM patients can be explained by a combination of the association of LMI and BF with mortality. The decreased mortality associated with high BMI within the range of less than 23 kg/m2 could be attributed to the low risk of death associated with high lean mass. The stabilized association between BMI and mortality in the higher BMI range (>24 kg/m2) was likely due to the counteracting of the opposite influence of BF and LMI on mortality. In fact, the prognostic value of LMI has been documented in a broader population. In a prospective study of an Asian population, Han et al. suggested that higher LMI was associated with better survival; whereas, BF was not associated with mortality (23). Kokkinos et al. suggested that a high mortality rate related with low BMI levels was observed only in patients without high fitness, indicating that the insufficiency of lean mass might be partly responsible for the obesity paradox in individuals with low BMI levels (24). Therefore, our study suggested that more attention should be paid to nutritional supplements in the management of HCM patients, with the hope of increasing their lean mass.
There are some limitations in our research that should be noted. Firstly, although this study looked at the association between body composition and mortality, BF and lean mass were obtained from formulas and not directly measured. Therefore, direct measurement of lean mass and BF using methods such as dual-energy x-ray absorption and bioelectrical impedance requires further investigation. However, in terms of clinical application, the direct measurement of BF and lean mass is unpractical due to its cost. The CUN-BAE equation was obtained by analyzing a large population and subsequently validated in several other studies. In a sense, the assessment of lean mass derived from BMI via the CUN-BAE equation can be easier to accept by clinicians. Secondly, this is a retrospective observational study that was conducted in a single tertiary referral centre, with inherent biases compared to real-world studies. Third, the study recruited only Chinese HCM patients with a mean BMI of 23.1 kg/m2. Therefore, the extrapolation of these results to other race/ethnicities, especially Caucasians who have much higher BMIs, must be performed cautiously.
Conclusion
In a cohort of the Chinese population with HCM, we provided evidence showing that when BMI is divided into fat and lean mass components, the associations of LMI or BF with baseline cardiovascular factors and cardiac remodelling are entirely different. Patients with higher LMI had a benign cardiovascular profile, lower MR degree and increased LV mass and LVEDV, while patients with higher BF had more concomitant clustered cardiovascular conditions and an increased LVOT gradient, MR degree and diastolic dysfunction. With respect to outcomes, reversed J-shape relationships of BMI and LMI with mortality were observed. Patients with higher BMI or LMI were related to a lower risk of mortality, especially in the low range of BMI and LMI. BF was not significantly associated with mortality in HCM patients.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
KZ, JX: design, methodology, software application, data curation, manuscript writing. GL, XP: visualization, investigation, validation. LZ: supervision writing – reviewing and editing. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by the National Natural Science Foundation of China (grant numbers: 81900258 and 81970325, Beijing, China), the Project of Enshi Science and Technology Bureau (grant numbers: 20181905, Hubei, China), the Health Commission of Hubei Province (grant number: WJ2019F149, Hubei, China), Hubei Provincial Key Laboratory of Occurrence and Intervention of Rheumatic diseases (grant number: OIR19011A, Hubei, China).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.988274/full#supplementary-material.
References
1. Finocchiaro G, Magavern E, Sinagra G, Ashley E, Papadakis M, Tome-Esteban M, et al. Impact of demographic features, lifestyle, and comorbidities on the clinical expression of hypertrophic cardiomyopathy. J Am Heart Assoc. (2017) 6(12):e007161. doi: 10.1161/JAHA.117.007161
2. Horwich TB, Fonarow GC, Clark AL. Obesity and the obesity paradox in heart failure. Prog Cardiovasc Dis. (2018) 61(2):151–6. doi: 10.1016/j.pcad.2018.05.005
3. Spelta F, Fratta Pasini AM, Cazzoletti L, Ferrari M. Body weight and mortality in COPD: focus on the obesity paradox. Eat Weight Disord. (2018) 23(1):15–22. doi: 10.1007/s40519-017-0456-z
4. Forlivesi S, Cappellari M, Bonetti B. Obesity paradox and stroke: a narrative review. Eat Weight Disord. (2021) 26(2):417–23. doi: 10.1007/s40519-020-00876-w
5. Robertson J, Schaufelberger M, Lindgren M, Adiels M, Schiöler L, Torén K, et al. Higher body mass Index in adolescence predicts cardiomyopathy risk in midlife. Circulation. (2019) 140:117–25. doi: 10.1161/CIRCULATIONAHA.118.039132
6. Oreopoulos A, Ezekowitz JA, McAlister FA, Kalantar-Zadeh K, Fonarow GC, Norris CM, et al. Association between direct measures of body composition and prognostic factors in chronic heart failure. Mayo Clin Proc. (2010) 85:609–17. doi: 10.4065/mcp.2010.0103
7. Peng Y, Chen F, Huang FY, Xia TL, Huang BT, Chai H, et al. Body composition and mortality in coronary artery disease with mild renal insufficiency in Chinese patients. J Ren Nutr. (2017) 27:187–93. doi: 10.1053/j.jrn.2017.01.018
8. Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American college of cardiology/American heart association task force on practice guidelines (ACC/AHA/ASE committee to update the 1997 guidelines for the clinical application of echocardiography). Circulation. (2003) 108:1146–62. doi: 10.1161/01.CIR.0000073597.57414.A9
9. Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. (2002) 287:1308–20. doi: 10.1001/jama.287.10.1308
10. Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, et al. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. (2003) 348:295–303. doi: 10.1056/NEJMoa021332
11. Panza JA, Petrone RK, Fananapazir L, Maron BJ, et al. Utility of continuous wave Doppler echocardiography in the noninvasive assessment of left ventricular outflow tract pressure gradient in patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. (1992) 19:91–9. doi: 10.1016/0735-1097(92)90057-T
12. Lancellotti P, Moura L, Pierard LA, Agricola E, Popescu BA, Tribouilloy C, et al. European Association of echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr. (2010) 11:307–32. doi: 10.1093/ejechocard/jeq031
13. Huang FY, Wang H, Huang BT, Liu W, Peng Y, Zhang C, et al. The influence of body composition on the N-terminal pro-B-type natriuretic peptide level and its prognostic performance in patients with acute coronary syndrome: a cohort study. Cardiovasc Diabetol. (2016) 15:58. doi: 10.1186/s12933-016-0370-0
14. Gomez-Ambrosi J, Silva C, Galofre JC, Escalada J, Santos S, Millán D, et al. Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int J Obes (Lond). (2012) 36:286–94. doi: 10.1038/ijo.2011.100
15. Lavie CJ, De Schutter A, Patel DA, Romero-Corral A, Artham SM, Milani RV, et al. Body composition and survival in stable coronary heart disease: impact of lean mass index and body fat in the “obesity paradox”. J Am Coll Cardiol. (2012) 60:1374–80. doi: 10.1016/j.jacc.2012.05.037
16. O'Mahony C, Jichi F, Pavlou M, Monserrat L, Anastasakis A, Rapezzi C, et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur Heart J. (2014) 35:2010–20. doi: 10.1093/eurheartj/eht439
17. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. (1994) 81:515–26. doi: 10.1093/biomet/81.3.515
18. Olivotto I, Maron BJ, Tomberli B, Appelbaum E, Salton C, Haas TS, et al. Obesity and its association to phenotype and clinical course in hypertrophic cardiomyopathy. J Am Coll Cardiol. (2013) 62:449–57. doi: 10.1016/j.jacc.2013.03.062
19. Toczylowski K, Hirnle T, Harasiuk D, Zabielski P, Lewczuk A, Dmitruk I, et al. Plasma concentration and expression of adipokines in epicardial and subcutaneous adipose tissue are associated with impaired left ventricular filling pattern. J Transl Med. (2019) 17:310. doi: 10.1186/s12967-019-2060-7
20. Pressler A, Haller B, Scherr J, Heitkamp D, Esefeld K, Boscheri A, et al. Association of body composition and left ventricular dimensions in elite athletes. Eur J Prev Cardiol. (2012) 19:1194–204. doi: 10.1177/1741826711422455
21. Alpert MA, Omran J, Mehra A, Ardhanari S, et al. Impact of obesity and weight loss on cardiac performance and morphology in adults. Prog Cardiovasc Dis. (2014) 56:391–400. doi: 10.1016/j.pcad.2013.09.003
22. Miyaji Y, Iwanaga Y, Nakamura T, Yasuda M, Kawamura T, Miyazaki S, et al. Interrelationship between the myocardial mass, fibrosis, BNP, and clinical outcomes in hypertrophic cardiomyopathy. Intern Med. (2016) 55:1261–8. doi: 10.2169/internalmedicine.55.6480
23. Han SS, Kim KW, Kim KI, Na KY, Chae DW, Kim S, et al. Lean mass index: a better predictor of mortality than body mass index in elderly asians. J Am Geriatr Soc. (2010) 58:312–7. doi: 10.1111/j.1532-5415.2009.02672.x
Keywords: hypertrophic cardiomyopathy, obesity paradox, body mass index, LEAN MASS, body fat
Citation: Zhou K, Xiang J, Li G, Pu X and Zhang L (2023) Body composition and mortality in a cohort study of Chinese patients with hypertrophic cardiomyopathy. Front. Cardiovasc. Med. 10:988274. doi: 10.3389/fcvm.2023.988274
Received: 11 July 2022; Accepted: 27 February 2023;
Published: 15 March 2023.
Edited by:
Emanuele Monda, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Xiaoyue Zhou, MR Collaboration, Siemens Healthineers Ltd., ChinaOwais Bhat, Virginia Commonwealth University, United States
© 2023 Zhou, Xiang, Li, Pu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Zhang bWR5eXpoYW5nbGlAMTI2LmNvbQ==
†These authors have contributed equally to this work
Specialty Section: This article was submitted to General Cardiovascular Medicine, a section of the journal Frontiers in Cardiovascular Medicine
 Ke Zhou1,†
Ke Zhou1,† Jie Xiang
Jie Xiang Guo-yong Li
Guo-yong Li Li Zhang
Li Zhang