- 1Department of Cardiology, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Cardiology, Chengdu Seventh People’s Hospital, Chengdu, Sichuan, China
Objective: It has long been debated whether rhythm control vs. rate control strategies have differing effects on mortality and morbidity for atrial fibrillation (AF). Recently, several randomized controlled studies (RCTs) and observational trials described that an early rhythm management method was linked to a lower likelihood of negative clinical outcomes in individuals with AF. We wanted to see if an early rhythm management method may help patients with AF.
Methods: We performed a systematic search to retrieve studies assessing the outcomes of early rhythm control vs. rate control in AF by using PubMed, Web of Science, Cochrane Library, and Embase published between 01/01/2000 and 15/04/2022.
Results: Finally, two RCTs, one retrospective analysis of RCTs, and four observational studies were identified. Compared with rate control, early rhythm control has been linked to lower all-cause mortality. [risk ratio (RR), 0.76; 95% CI 0.69–0.83; P < 0.00001; I2 = 77%]. The early rhythm control group was also associated with a lower risk of cardiovascular mortality (RR, 0.68; 95% CI 0.63–0.74; P < 0.00001; I2 = 33), stroke (RR, 0.77; 95% CI 0.67–0.87; P < 0.001; I2 = 64), and heart failure hospitalization (RR, 0.74; 95% CI 0.59–0.93; P = 0.0009; I2 = 93%). We found no significant difference in nights spent in hospital per year, acute coronary syndrome, major bleeding, and cardiac arrest/ventricular arrhythmia between the groups.
Conclusion: In this meta-analysis, early rhythm therapy was linked to a lower risk of all-cause mortality, cardiovascular mortality, stroke, and heart failure hospitalization compared with the rate control group.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022333592.
1. Introduction
Atrial fibrillation (AF) is a kind of cardiovascular disease that affects millions of people throughout the world and is associated with an increased risk of mortality and morbidity, with a fivefold increased risk of stroke (1–3). The current two essential aims of AF clinical care are (1) thromboembolism prophylaxis with anticoagulation and (2) maintenance of an appropriate heart rate or sinus rhythm by medications or interventional procedures (4). Rate control is part of AF management and can adequately improve related symptoms. Rhythm control refers to the use of antiarrhythmic drugs, cardioversion, and AF ablation to try to restore and maintain sinus rhythm. It has been argued for a long time whether rhythm vs. rate control strategies have differing effects on mortality and morbidity for AF. The choice of rhythm or rate control in current guidelines relies on several randomized controlled studies (RCTs) (5–7). No significant difference in all-cause mortality, cardiovascular mortality, and other related morbidities was found between rhythm control and rate control in the meta-analyses of the above studies included (8, 9). However, the treatment of AF has changed dramatically since the above RCTs were published. Several studies have recently shown that the incidence of adverse cardiovascular outcomes was reduced by early rhythm control compared with rate control (10–17). To determine whether early rhythm control is better than rate control in patients with AF, we performed a systematic review and meta-analysis.
2. Materials and methods
The meta-analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
2.1. Literature search
From 1 January 2000, to 15 April 2022, a systematic search for RCTs and observational studies was undertaken using PubMed, EMBASE, Web of Science, and Cochrane Library databases, with no language restrictions. A manual search was conducted that included all of the relevant references following the computerized search. The following were among the most important search topics and terms: (1) atrial fibrillation, (2) rate control, and (3) rhythm control. Detailed search strategies are summarized in the Supplementary material. The PRISMA statement was followed when performing this meta-analysis. The review protocol has been registered in PROSPERO (registration number: CRD42022333592).
2.2. Selection and data abstraction
The following criteria were used to choose articles: (1) observational studies or RCTs that included patients with AF based on early rhythm control vs. rate control; (2) based on “Early Treatment of Atrial Fibrillation for Stroke Prevention Trial” (EAST-AFNET 4) study, patients were enrolled within 1 year after the first diagnosis of AF (early AF); (10) (3) the studies’ follow-up time was at least 1 year; and (4) the goal of the study was to examine the effect and prognosis of AF treated with early rhythm vs. rate control. All studies were restricted to those including human subjects who were at least 18 years old. Reviews, case studies, conference papers, comments, and animal trials were all omitted from the study. Two reviewers separately evaluated article titles and abstracts to exclude papers that were not relevant. Disagreements were addressed by consensus and, if needed, the consulting of a third reviewer. The risk of bias was assessed using the Cochrane collaboration tool for RCTs and using the Newcastle-Ottawa scale for non-randomized clinical studies. The following information was gathered from eligible studies: (1) design of the research; (2) primary/secondary outcome; (3) mean follow-up time and baseline characteristics; and (4) anticoagulation therapy, rate, and rhythm protocols. The outcomes of the present analysis were as follows: all-cause mortality, cardiovascular mortality, ischemic stroke, heart failure (HF) hospitalization, nights spent in hospital per year, acute coronary syndrome, major bleeding, and cardiac arrest/ventricular arrhythmia.
2.3. Statistical analysis
Dichotomous variables were investigated using the Mantel-Haenszel method. The risk ratio (RR) and 95% confidence interval (CI) were determined. Continuous variables were described as the mean and standard deviation. To examine heterogeneity, the Cochran Q and I2 statistics were utilized. We defined moderate or high heterogeneity as an I2 of more than 50%. Given the expected between-study heterogeneity, a meta-analysis was conducted using a random effects model for all outcomes. If more than 10 studies were included, a funnel plot was used to measure publication bias. By removing one study at a time, we performed a series of sensitivity analyses to establish the contribution of each study to the pooled estimate. All P values were two-tailed. R programming language (version 4.1.2, R Foundation) was used to perform sensitivity analyses. Review Manager Version 5.3 software (The Nordic Cochrane Centre) was used to conduct an overall effect analysis and subgroup analysis.
3. Results
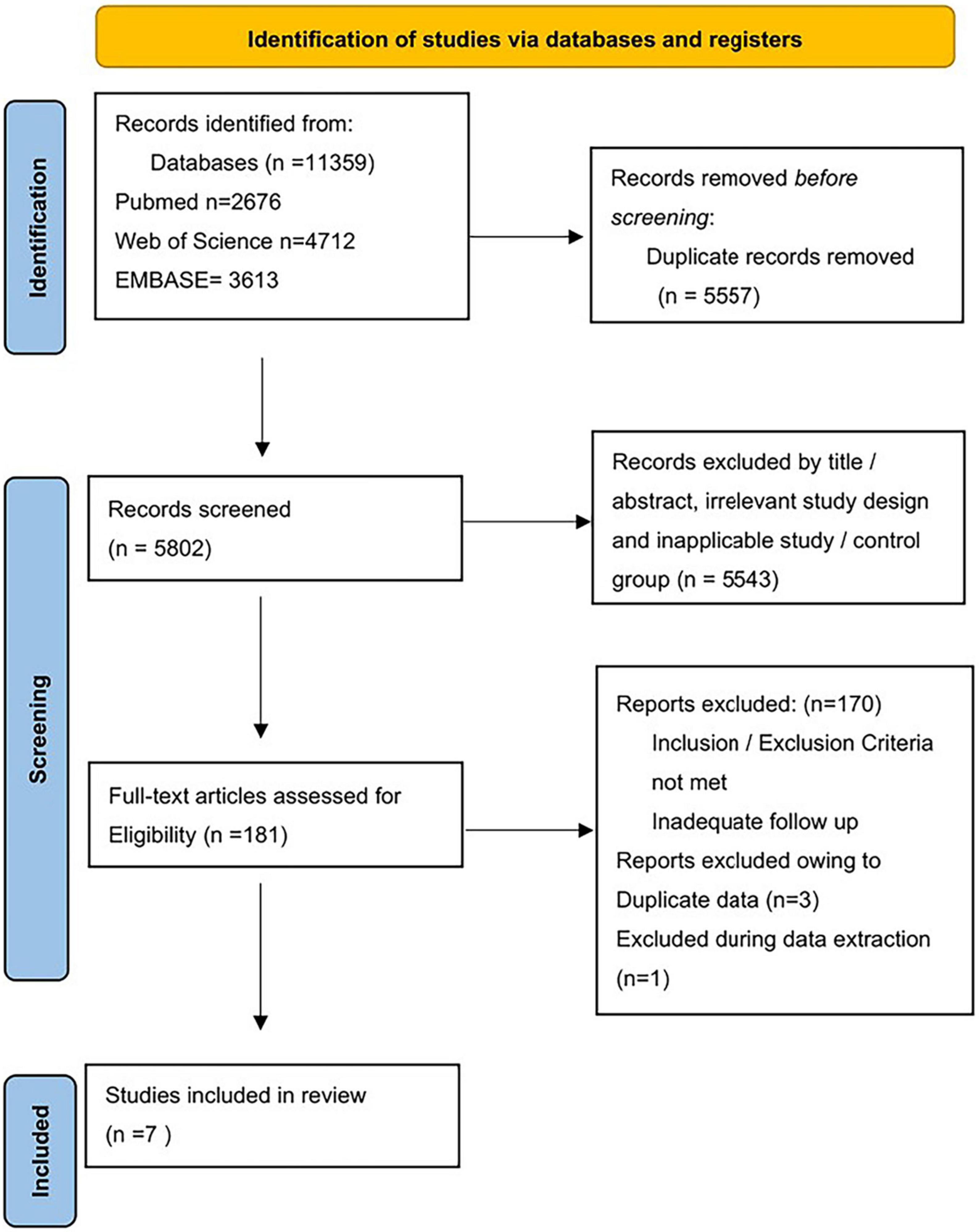
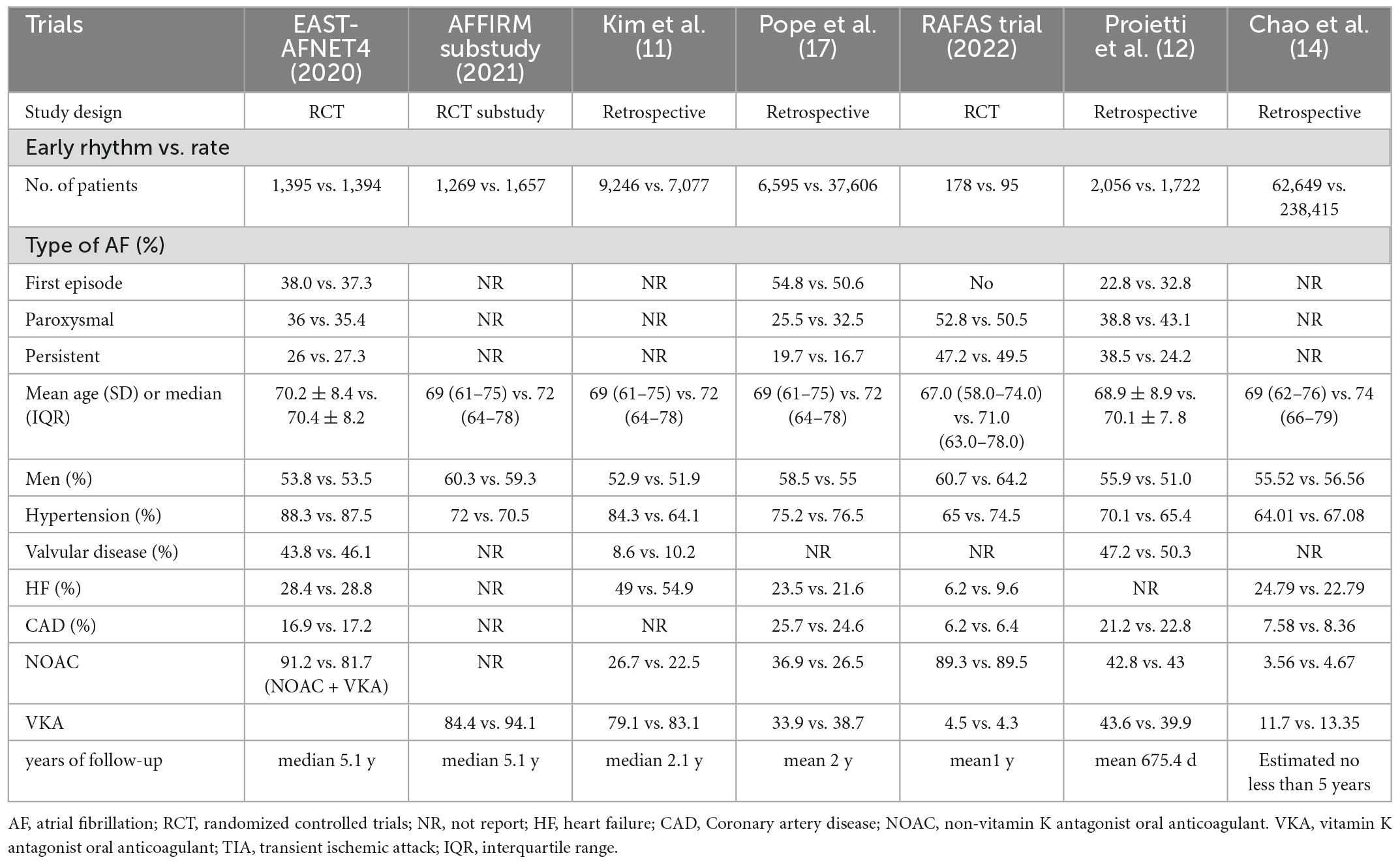
As shown in Figure 1, a total of 11,359 studies were found in the database. 5,557 duplicate records were excluded. A total of 5,543 records were excluded based on title/abstract, animal studies, irrelevant study design, and inapplicable study. We read the full text of 181 studies carefully. Finally, seven studies were included: two RCTs, one retrospective analysis of RCT, and four observational studies. The sample size ranged from 273 to 301,064. The mean follow-up times ranged from 1 to 5 years. Table 1 and Supplementary Table provided the features of our included studies. Table 2 summarizes the quality appraisal for the studies that were included.
3.1. Mortality
Seven studies reported all-cause mortality (10–15, 17, 18). Mortality was as high as 50% in Ionescu-Ittu et al.’s study during a mean follow-up of 3 years. When we included this study, the heterogeneity of our analysis was 97%. Therefore, we excluded this study from the analysis to better interpret our results and reduce heterogeneity. Finally, the pooled analysis showed lower all-cause mortality in the early rhythm group than in the rate control group (RR 0.76; 95% CI 0.69–0.83; P < 0.00001; I2 = 77%; Figure 2A). Analysis of pre-defined subgroups based on the study design also showed significantly lower mortality with RCT (RR, 0.86; 95% CI 0.75–1.00; P = 0.04) or observational studies (RR, 0.73; 95% CI, 0.65–0.81; P < 0.00001; Figure 2A). When the data were pooled based on the time it took for patients to enroll (before 2009 vs. after 2009), similar results were found (Supplementary Figure 1). Three clinical studies reported cardiovascular mortality (10, 11, 14). The incidence of cardiovascular mortality was low in early rhythm control compared to rate control (RR, 0.68; 95% CI 0.63–0.74; P < 0.00001; Figure 2B) without statistically significant heterogeneity (I2 = 33%).
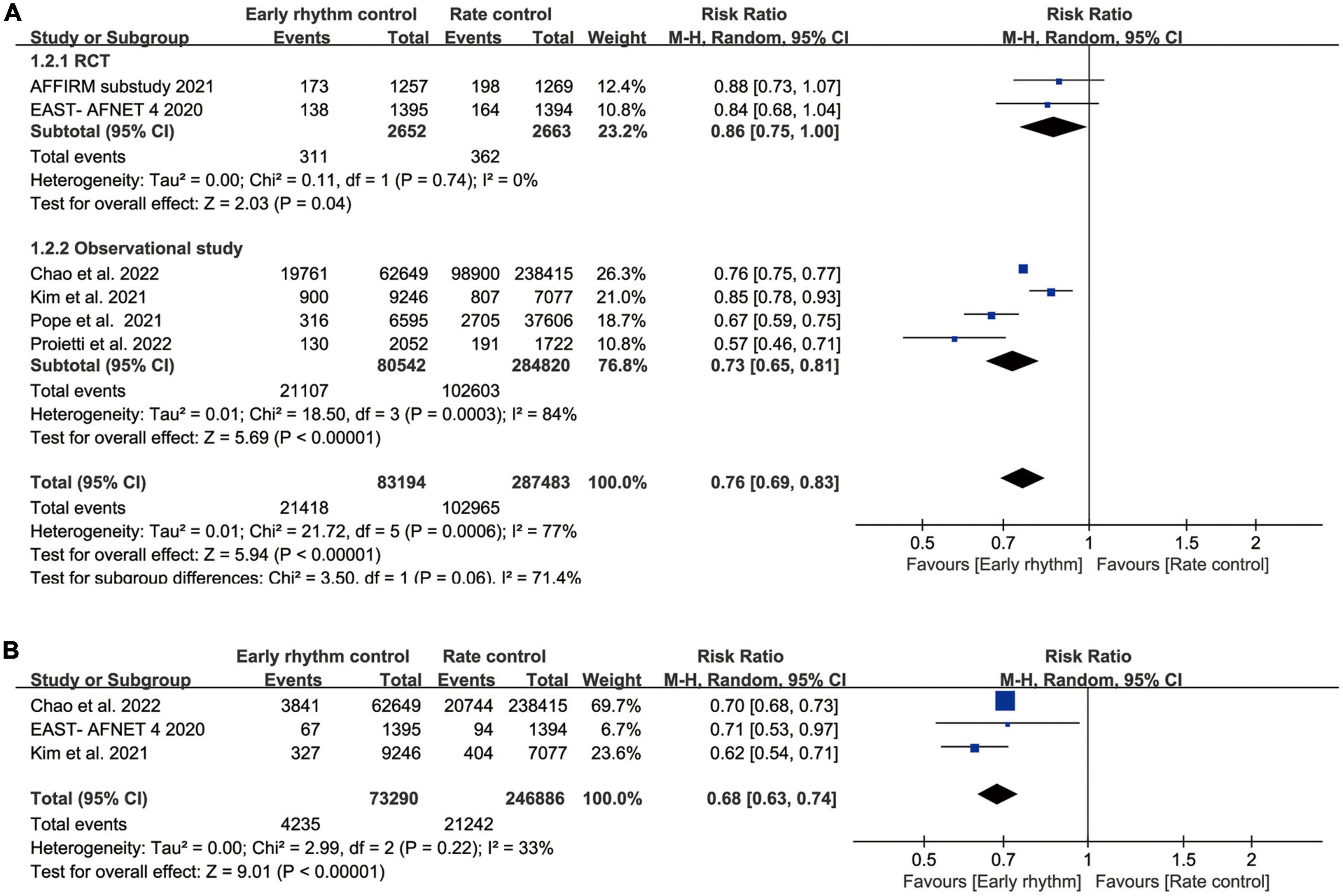
Figure 2. (A) Forest plot showing all-cause mortality between early rhythm group and rate group. (B) Forest plot showing cardiovascular mortality between early rhythm group and rate group.
3.2. Morbidity
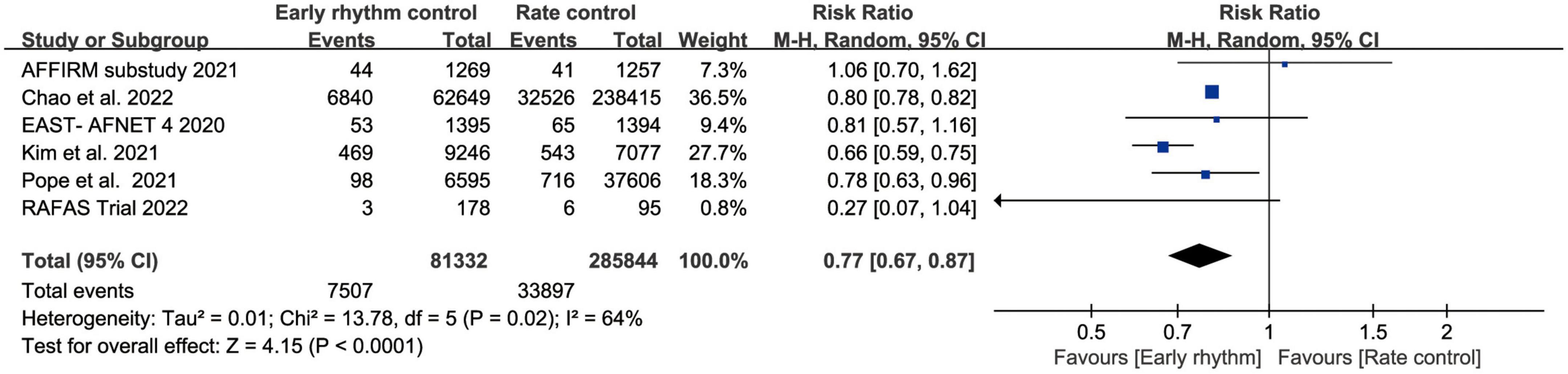
Six studies assessed ischemic stroke (10, 11, 13–15, 17). Early rhythm control was linked to a lower risk of stroke in the patients (RR, 0.77; 95% CI 0.67–0.87; P < 0.0010; I2 = 64%; Figure 3). We also performed subgroup analyses based on the time of patient enrollment, and study design and found no change in the above findings (Supplementary Figure 2). Only four studies included HF hospitalization (10–12, 14). Patients with early rhythm were associated with a reduced relative risk of HF hospitalization. However, there was obvious heterogeneity (RR, 0.74; 95% CI, 0.59–0.93; P = 0.0009; I2 = 93%; Figure 4).
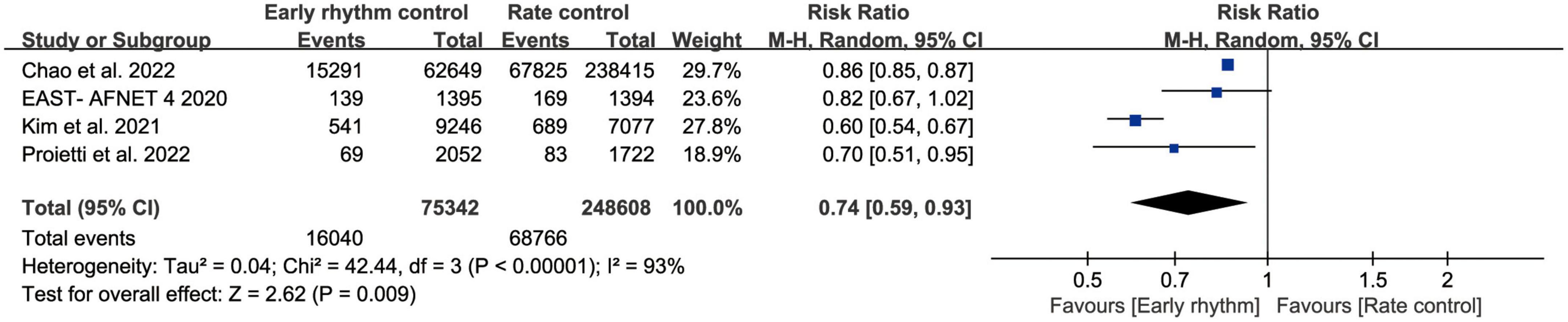
Figure 4. Forest plot showing risk of heart failure hospitalization between early rhythm group and rate group.
Regarding nights spent in the hospital per year, acute coronary syndrome, major bleeding, and cardiac arrest/ventricular arrhythmia, early rhythm control and rate control showed no significant differences. The results were summarized in Table 3 and Supplementary Figure 3.
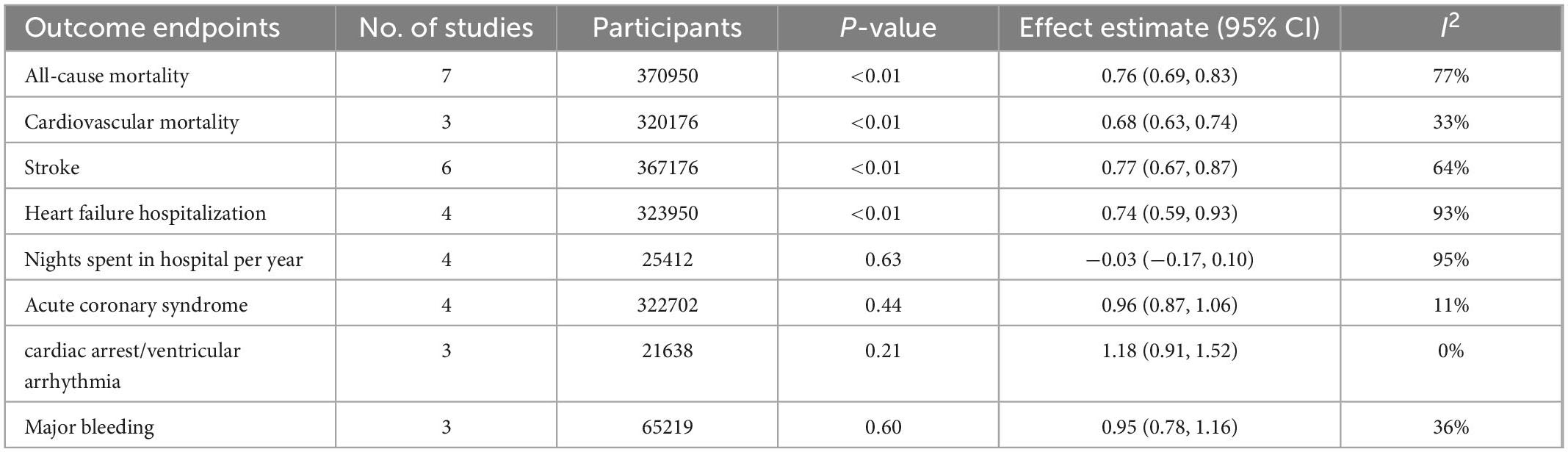
Table 3. Outcome of patients who underwent early rhythm control or rate control for atrial fibrillation.
Two studies reported adverse events related to treatment in early rhythm control vs. rate control. We did not include pooled analysis because of the limited number of studies. We found that syncope, cardiac tamponade, atrioventricular block, and pacemaker implantation were higher in the early rhythm group than rate control group, but none were statistically significant.
3.3. Sensitivity analyses
We conducted a sensitivity analysis by eliminating one study at a time to determine how each one affected the outcomes. The sensitivity analysis findings are summarized in Supplementary Figure 4. Overall, successive exclusion of each study had no meaningful effect on any of the clinical outcomes. Due to the limited number of included studies, we did not perform a publication bias assessment.
4. Discussion
This meta-analysis was performed to compare the benefits of early rhythm control vs. rate control in patients with AF. When compared to rate control, early rhythm control appears to be related to lower all-cause mortality, cardiovascular mortality, stroke, and HF hospitalization. Nevertheless, there was no difference between the two groups for acute coronary syndrome, major bleeding, nights spent in the hospital per year, and cardiac arrest/ventricular arrhythmia. To the best of our knowledge, our systematic review meta-analysis is the first to report on early rhythm control in patients with AF.
4.1. Interpretation of results
Our study concluded that early rhythm control may benefit patients compared with rate control. AF is usually thought to be a progressive disorder, in which arrhythmia begins as paroxysmal form and progresses from persistent to “permanent” AF with electrical and structural remodeling of the atrium (19). AF produces mechanisms for self-perpetuation after it has been established (“AF begets AF’) (20). Structural, electrical, and autonomic remodeling are all affected by arrhythmia and it can exacerbate pre-existing issues, making the patient more susceptible to recurring and chronic AF (21, 22). In addition, the Framingham Heart Study demonstrated that in the first year after AF is identified, the risk of cardiovascular problems increased (2). Amiodarone, the most effective medicine now available for long-term sinus rhythm maintenance, has anti-remodeling effects (23). In patients with AF, catheter ablation is superior to medical therapy for the maintenance and restoration of sinus rhythm (4, 24). Previous research has suggested that catheter ablation can prevent left atrial remodeling (25, 26). A shorter period between the first AF diagnosis and the ablation therapy has also been demonstrated to improve the chances of ablation success (27). Therefore, maintaining sinus rhythm as early in the natural history as feasible would appear to be a rational method to avoid AF development. However, since 2002, rhythm control has been proven to be unlikely to reduce all-cause and cardiovascular mortality in the general population compared to rate control in RCTs (5, 6, 28). This seemingly conflicting outcome might be explained. The poor rate of sinus rhythm restoration and maintenance in most of these experiments is a key issue. Only 39% of patients in the rhythm-control arm of the RACE experiment were in sinus rhythm at the end of the study (28). Patients in the late phases of the illness process were also included in these studies. Patients with chronic AF were enrolled in the STAF, PIAF, and RACE studies (28–30). Likewise, a substudy of the AFFIRM study demonstrated no difference in all-cause mortality, and ischemic stroke when comparing early rhythm control with rate control in patients with AF. Of all the studies we included, this was the only study that early rhythm control showed no benefit compared with rate control. The proportion of anticoagulants used in the early rhythm group was lower than that in the rate group (84.4 vs. 94.1%). We think that this was a key factor leading to the above results. The AFFIRM study recruited patients with dilated left atrium (65%). Even with the use of antiarrhythmic medicines, it is difficult to reverse structural abnormalities and sustain sinus rhythm once AF has structural changes. In addition, over the last 20 years, AF ablation is an important role in the treatment of AF. The AFFIRM study included patients who were not treated with catheter ablation.
4.2. Clinical implications
These findings imply that early rhythm control is superior to rate control. Therefore, patients with AF should receive rhythm control immediately. However, guidelines currently recommend rhythm control therapy to improve symptoms and quality of life in symptomatic patients with AF (IA) (4). In fact, many newly diagnosed AF patients may be asymptomatic (31). A new AF diagnosis is linked to a high risk of stroke (7%), heart failure (14%), and death (49%) (32). Early rhythm management has been proven to have a lower risk of death and stroke in some studies. Nevertheless, there is no recommendation in the current guidelines early rhythm management to reduce severe adverse cardiovascular events such as stroke and mortality. Although early rhythm therapy has some associated side effects, long-term antiarrhythmic drugs-related significant adverse events, and mortality are usually linked to chronic AF and structural heart disease (33). Catheter ablation, an effective strategy for rhythm control, showed no significant increase in adverse events compared with the standard care group (34). Therefore, future guidelines may support the early rhythm control management of AF based on the long-term effects in reduced all-cause mortality, cardiovascular mortality, stroke, and HF hospitalization. Furthermore, patient selection and interaction between patient and operator should not be overlooked.
5. Limitations
First, due to the nature of observational studies, biases cannot be eliminated. Differences in techniques, demographics, and backgrounds inevitably convey unidentified confounders. Despite the random effects method employed in quantitative analysis, heterogeneity of clinical features and interventions among trials is a significant limitation. Second, several studies included patients between 1996 and 2020, causing changes in therapy over time. The difference between the samples included in our study was large, ranging from 273 to 301,064. Therefore, we conducted a sensitivity analysis by eliminating one study at a time to determine how each one affected the outcomes. We did not find a meaningful effect on any of the clinical outcomes. Third, the lack of patient-level data made an extensive evaluation of baseline features regarding clinical outcomes impossible. However, all of the studies’ baseline parameters were well-matched in both groups. Fourth, the studies we included had different definitions of early intervention, but we performed a series of sensitivity analyses and found no significant difference. Finally, most of the studies we included were real-world studies, and only one large-scale prospective RCT in our meta-analysis. In our subgroup analysis, a retrospective analysis of an RCT was also classified as a randomized controlled study.
6. Conclusion
This is the first meta-analysis to conclude that early rhythm control may be more beneficial than rate control in patients with AF. Our study demonstrated that early rhythm control can reduce all-cause mortality, cardiovascular mortality, stroke, and HF hospitalization. However, early rhythm control was not associated with acute coronary syndrome, major bleeding, nights spent in the hospital per year, and cardiac arrest/ventricular arrhythmia. We hope that more research will be done in the future to confirm our findings.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SH and RJ conceived the review. SH drafted and wrote the manuscript. RJ, ZC, SZ, RG, and YB revised and edited all the version of the manuscript. During the revision of the manuscript, MX made great contributions to us, including the correction of the manuscript, statistical analysis, language polishing and literature retrieval. KC revised the sections. All authors contributed to manuscript revision and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.978637/full#supplementary-material
References
1. Mozaffarian D, Benjamin E, Go A, Arnett D, Blaha M, Cushman M, et al. Heart disease and stroke statistics–2015 update: a report from the American heart association. Circulation. (2015) 131:e29–322. doi: 10.1161/cir.0000000000000152
2. Benjamin E, Wolf P, D’Agostino R, Silbershatz H, Kannel W, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham heart study. Circulation. (1998) 98:946–52. doi: 10.1161/01.cir.98.10.946
3. Odutayo A, Wong C, Hsiao A, Hopewell S, Altman D, Emdin C. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ. (2016) 354:i4482. doi: 10.1136/bmj.i4482
4. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with eacts. Eur J Cardio Thor Surg. (2016) 50:e1–88. doi: 10.1093/ejcts/ezw313
5. Wyse D, Waldo A, DiMarco J, Domanski M, Rosenberg Y, Schron E, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. (2002) 347:1825–33. doi: 10.1056/NEJMoa021328
6. Roy D, Talajic M, Nattel S, Wyse D, Dorian P, Lee K, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. (2008) 358:2667–77.
7. Tsadok M, Jackevicius C, Essebag V, Eisenberg M, Rahme E, Humphries K, et al. Rhythm versus rate control therapy and subsequent stroke or transient ischemic attack in patients with atrial fibrillation. Circulation. (2012) 126:2680–7. doi: 10.1161/CIRCULATIONAHA.112.092494
8. Sethi N, Feinberg J, Nielsen E, Safi S, Gluud C, Jakobsen J. The effects of rhythm control strategies versus rate control strategies for atrial fibrillation and atrial flutter: a systematic review with meta-analysis and trial sequential analysis. PLoS One. (2017) 12:e0186856. doi: 10.1371/journal.pone.0186856
9. Caldeira D, David C, Sampaio C. Rate versus rhythm control in atrial fibrillation and clinical outcomes: updated systematic review and meta-analysis of randomized controlled trials. Arch Cardiovasc Dis. (2012) 105:226–38. doi: 10.1016/j.acvd.2011.11.005
10. Kirchhof P, Camm A, Goette A, Brandes A, Eckardt L, Elvan A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. (2020) 383:1305–16. doi: 10.1056/NEJMoa2019422
11. Kim D, Yang P, You S, Sung J, Jang E, Yu H, et al. Treatment timing and the effects of rhythm control strategy in patients with atrial fibrillation: nationwide cohort study. BMJ. (2021) 373:n991. doi: 10.1136/bmj.n991
12. Proietti M, Vitolo M, Harrison S, Lane D, Fauchier L, Marin F, et al. Real-world applicability and impact of early rhythm control for European patients with atrial fibrillation: a report from the esc-ehra eorp-af long-term general registry. Clin Res Cardiol. (2022) 111:70–84. doi: 10.1007/s00392-021-01914-y
13. Park J, Shim J, Lee J, Park J, Heo J, Chang Y, et al. Risks and benefits of early rhythm control in patients with acute strokes and atrial fibrillation: a multicenter, prospective, randomized study (the rafas trial). J Am Heart Assoc. (2022) 11:e023391. doi: 10.1161/jaha.121.023391
14. Chao T, Chan Y, Chiang C, Tuan T, Liao J, Chen T, et al. Early rhythm control and the risks of ischaemic stroke, heart failure, mortality and adverse events when performed early (<3 months). Thromb Haemost. (2022) 122:1899–910. doi: 10.1055/a-1807-0336
15. Yang E, Tang O, Metkus T, Berger R, Spragg D, Calkins H, et al. The role of timing in treatment of atrial fibrillation: an affirm substudy. Heart Rhythm. (2021) 18:674–81. doi: 10.1016/j.hrthm.2020.12.025
16. Kim D, Yang P, You S, Jang E, Yu H, Kim T, et al. Comparative effectiveness of early rhythm control versus rate control for cardiovascular outcomes in patients with atrial fibrillation. J Am Heart Assoc. (2021) 10:e023055. doi: 10.1161/jaha.121.023055
17. Pope M, Hall T, Schirripa V, Radic P, Virdone S, Pieper K, et al. Cardioversion in patients with newly diagnosed non-valvular atrial fibrillation: observational study using prospectively collected registry data. BMJ. (2021) 375:e066450. doi: 10.1136/bmj-2021-066450
18. Ionescu-Ittu R, Abrahamowicz M, Jackevicius C, Essebag V, Eisenberg M, Wynant W, et al. Comparative effectiveness of rhythm control vs rate control drug treatment effect on mortality in patients with atrial fibrillation. Arch Intern Med. (2012) 172:997–1004. doi: 10.1001/archinternmed.2012.2266
19. Jahangir A, Lee V, Friedman P, Trusty J, Hodge D, Kopecky S, et al. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: a 30-year follow-up study. Circulation. (2007) 115:3050–6. doi: 10.1161/circulationaha.106.644484
20. Wijffels M, Kirchhof C, Dorland R, Allessie M. Atrial fibrillation begets atrial fibrillation. a study in awake chronically instrumented goats. Circulation. (1995) 92:1954–68. doi: 10.1161/01.cir.92.7.1954
21. Iwasaki Y, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation. (2011) 124:2264–74. doi: 10.1161/circulationaha.111.019893
22. Li D, Fareh S, Leung T, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. (1999) 100:87–95. doi: 10.1161/01.cir.100.1.87
23. Shinagawa K, Shiroshita-Takeshita A, Schram G, Nattel S. Effects of antiarrhythmic drugs on fibrillation in the remodeled atrium: insights into the mechanism of the superior efficacy of amiodarone. Circulation. (2003) 107:1440–6.
24. Wilber D, Pappone C, Neuzil P, De Paola A, Marchlinski F, Natale A, et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. (2010) 303:333–40. doi: 10.1001/jama.2009.2029
25. Delgado V, Vidal B, Sitges M, Tamborero D, Mont L, Berruezo A, et al. Fate of left atrial function as determined by real-time three-dimensional echocardiography study after radiofrequency catheter ablation for the treatment of atrial fibrillation. Am J Cardiol. (2008) 101:1285–90. doi: 10.1016/j.amjcard.2007.12.028
26. Marsan N, Tops L, Holman E, Van de Veire N, Zeppenfeld K, Boersma E, et al. Comparison of left atrial volumes and function by real-time three-dimensional echocardiography in patients having catheter ablation for atrial fibrillation with persistence of sinus rhythm versus recurrent atrial fibrillation three months later. Am J Cardiol. (2008) 102:847–53. doi: 10.1016/j.amjcard.2008.05.048
27. Chew D, Black-Maier E, Loring Z, Noseworthy P, Packer D, Exner D, et al. Diagnosis-to-ablation time and recurrence of atrial fibrillation following catheter ablation: a systematic review and meta-analysis of observational studies. Circ Arrhythm Electrophysiol. (2020) 13:e008128. doi: 10.1161/circep.119.008128
28. Van Gelder I, Hagens V, Bosker H, Kingma J, Kamp O, Kingma T, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. (2002) 347:1834–40. doi: 10.1056/NEJMoa021375
29. Carlsson J, Miketic S, Windeler J, Cuneo A, Haun S, Micus S, et al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the strategies of treatment of atrial fibrillation (staf) study. J Am Coll Cardiol. (2003) 41:1690–6. doi: 10.1016/s0735-1097(03)00332-2
30. Hohnloser S, Kuck K, Lilienthal J. Rhythm or rate control in atrial fibrillation–pharmacological intervention in atrial fibrillation (piaf): a randomised trial. Lancet. (2000) 356:1789–94. doi: 10.1016/s0140-6736(00)03230-x
31. Siontis K, Gersh B, Killian J, Noseworthy P, McCabe P, Weston S, et al. Typical, atypical, and asymptomatic presentations of new-onset atrial fibrillation in the community: characteristics and prognostic implications. Heart Rhythm. (2016) 13:1418–24. doi: 10.1016/j.hrthm.2016.03.003
32. Piccini J, Hammill B, Sinner M, Hernandez A, Walkey A, Benjamin E, et al. Clinical course of atrial fibrillation in older adults: the importance of cardiovascular events beyond stroke. Eur Heart J. (2014) 35:250–6. doi: 10.1093/eurheartj/eht483
33. Kirchhof P, Andresen D, Bosch R, Borggrefe M, Meinertz T, Parade U, et al. Short-term versus long-term antiarrhythmic drug treatment after cardioversion of atrial fibrillation (flec-sl): a prospective, randomised, open-label, blinded endpoint assessment trial. Lancet. (2012) 380:238–46. doi: 10.1016/s0140-6736(12)60570-4
Keywords: atrial fibrillation, early rhythm control, rate control, meta-analysis, cardiovascular outcome
Citation: Han S, Jia R, Cen Z, Guo R, Zhao S, Bai Y, Xie M and Cui K (2023) Early rhythm control vs. rate control in atrial fibrillation: A systematic review and meta-analysis. Front. Cardiovasc. Med. 10:978637. doi: 10.3389/fcvm.2023.978637
Received: 26 June 2022; Accepted: 19 January 2023;
Published: 06 February 2023.
Edited by:
Daniel M. Johnson, The Open University, United KingdomReviewed by:
Ewa Jȩdrzejczyk-Patej, Silesian Center for Heart Disease, PolandPil-sung Yang, CHA University, Republic of Korea
Copyright © 2023 Han, Jia, Cen, Guo, Zhao, Bai, Xie and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaijun Cui,  Y3Vpa2FpanVuc2N1QDE2My5jb20=; Min Xie,
Y3Vpa2FpanVuc2N1QDE2My5jb20=; Min Xie,  eGllbWluMDEzQDE2My5jb20=
eGllbWluMDEzQDE2My5jb20=
†These authors have contributed equally to this work
 Shaojie Han1†
Shaojie Han1† Ran Guo
Ran Guo Shenyu Zhao
Shenyu Zhao Kaijun Cui
Kaijun Cui