- Department of Cardiology, Guangzhou Key Laboratory of Molecular Mechanism and Translation in Major Cardiovascular Disease, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China
Aims: To investigate the correlation and predictive value of left atrial diameter and blood uric acid levels with the occurrence of left atrial thrombus or dense spontaneous echo contrast in atrial fibrillation patients with low to moderate CHA2DS2-VASc scores.
Methods and results: A total of 849 inpatients diagnosed with atrial fibrillation who had low to moderate CHA2DS2-VASc scores and complete transesophageal echocardiography were included in this study. Among them, 66 patients had left atrial thrombus or dense spontaneous echo contrast. When different models were used to correct other known risk factors, acid levels and abnormal left atrial diameter were identified as additional risk factors for left atrial thrombus or dense spontaneous echo contrast. The incidence of left atrial thrombus or dense spontaneous echo contrast was higher in patients with abnormal serum uric acid levels than in the control group (12.4% vs. 5.6%, p < 0.05), and this difference persisted after correcting the baseline data with propensity score matching (10.6% vs. 4.1%, p < 0.05). Abnormal left atrial diameter was another risk factor suggested by regression analysis, with an increased incidence of left atrial thrombus or dense spontaneous echo contrast in the abnormal left atrial diameter group compared to the control group, both before (18.0% vs. 3.5%, p < 0.05) and after (15.5% vs. 5.2%, p < 0.05) propensity score matching. The best predictive value was obtained by adding both abnormal serum uric acid levels and abnormal left atrial diameter.
Conclusion: Left atrial enlargement and high uric acid levels increase the risk of left atrial thrombus or dense spontaneous echo contrast in atrial fibrillation patients with low to moderate CHA2DS2-VASc scores.
Introduction
Atrial fibrillation (AF) is a common type of rapid supraventricular arrhythmia characterized by uncoordinated atrial excitation and consequently ineffective atrial contraction. Ischemic stroke and embolism of circulatory arteries are major complications in patients with AF, and the risk of ischemic stroke is 5 times higher among these patients. If cardioembolic stroke occurs, it may be followed by high mortality rates (up to 20%) and disability rates (60%) (1–4).
A left atrial thrombus (LAT) may occur if the duration of AF exceeds 48 h. The presence of spontaneous echo contrast (SEC) is an important marker of thrombus formation. Previous studies have shown both dense SEC (Fatkin class 3+ and 4+) and LAT to be prerequisites for thromboembolism in patients with AF, as both have been independently associated with thromboembolic events in these patients (5–9).
Previous studies have shown that hypertension, diabetes, female sex, congestive heart failure, advanced age, left atrial enlargement, renal insufficiency, N-terminal brain natriuretic peptide precursor, left atrial appendage morphology, and blood emptying velocity are independent risk factors for stroke in patients with AF (10–12). Serum uric acid (SUA) level has also been reported to be associated with LAT (13). In addition, endothelial dysfunction (decreased endothelial nitric oxide synthase activity and expression) and changes in blood components (platelet activation, inflammatory response) have been associated with thrombosis in patients with AF (14, 15).
The CHA2DS2-VASc score is currently guideline-recommended for stratifying the risk of thromboembolism in patients with AF. Men with a CHA2DS2-VASc scor ≥2 and women with a CHA2DS2-VASc score ≥3 are considered high-risk patients for whom oral anticoagulant therapy is recommended, and patients with a CHA2DS2-VASc score of 0 or 1 (including women with a score of 2) are considered to be at low to moderate risk of stroke. However, previous studies have shown that a certain percentage of patients with AF who were deemed to be at low to moderate risk still developed thromboembolic stroke, suggesting that unknown factors were contributing to the development of LAT and SEC besides the known risk factors included within the CHA2DS2-VASc score. There are few studies about the risk factors of stroke in patients with AF who are otherwise classified as low risk. In one such study, Yan et al. proposed that a high Lp(a) plasma level and left atrial dilatation might be independent risk factors of thrombotic events for AF patients with a low CHA2DS2-VASc score (16). In a different study, Yao et al. found that an elevated plasma homocysteine level increases the risk of LA/LAA thrombus in AF patients with a low CHA2DS2-VASc score (17). In addition, LAA anatomy could be a risk factor (18). Recently, an increasing amount of research has focused on the relationship between SUA and thromboembolism risk. However, it remains unclear whether SUA can offer sufficient predictive value for LAT/dense SEC in AF patients with a low to moderate CHA2DS2-VASc score.
Identifying patients who are at high risk of LAT or dense SEC from among those who are typically defined as low to moderate risk according to their CHA2DS2-VASc score has important clinical implications. To that end, we retrospectively analyzed the clinical data of 849 AF patients with low to moderate risk CHA2DS2-VASc scores to discern risk factors not included in the CHA2DS2-VASc score that may point to LAT or dense SEC in an effort to help further identify patients at high risk of thromboembolism.
Methods
Study population
Inpatients diagnosed with AF who also had complete transesophageal echocardiography (TEE) performed at Sun Yat-sen Memorial Hospital of Sun Yat-sen University from January 2007 to July 2019 were included in this study. Exclusion criteria were as follows: (1) rheumatic mitral stenosis, post mitral valve repair, or post-mechanical or biological valve replacement; (2) congenital heart disease; (3) severe infectious or autoimmune disease; (4) severe hepatic or renal insufficiency; (5) hyperthyroidism; (6) malignancy; (7) coagulation disorders; (8) those with psychiatric abnormalities; and (9) high CHA2DS2-VASc scores. Clinical data on gender, age, body mass index (BMI), anteroposterior LA diameter (LAD), left ventricular ejection fraction (LVEF), serum uric acid (SUA), hypertension, diabetes mellitus, stroke, peripheral vascular disease, heart failure, and history of gout were collected. Anteroposterior LAD ≥4 cm was defined as LA enlargement (abLAD). Serum uric acid >420 μmol/L was defined as hyperuricemia (abSUA). Patients were divided into a LAT/dense SEC group and a control non-LAT/dense SEC group according to the presence of both LAT and dense SEC.
Assessment of CHA2DS2-VASc score
The CHA2DS2-VASc score was assessed for congestive heart failure (CHF), hypertension, diabetes mellitus, vascular disease (including peripheral vascular disease and myocardial infarction), age (1 point for ages 65–74 years and 2 points for ages ≥75 years), gender (1 point for women), and history of previous stroke/TIA. A score equal to 0 for men or 1 for women was considered low risk, 1 for men or 2 for women was considered moderate risk, and ≥2 for men or ≥3 for women was considered high risk. A total of 849 patients with low- to moderate-risk CHA2DS2-VASc scores were included in this study.
Transesophageal echocardiography (TEE)
All patients with AF routinely undergo TEE 48h before ablation or cardioversion. LAT was defined as an echogenic image in the left atrium that is well-defined, homogeneous in density, and different from the density of adjacent myocardial tissue. SEC was defined as a TEE finding that shows cloudy, swirling, or pre-thrombotic echogenicity in the left atrium. Dense SEC was defined by a Fatkin classification of 3+ and 4+ according to the Fatkin classification of 5 levels of severity (5).
Statistical analysis
Continuous variables were expressed as mean ± standard deviation, and independent sample t-tests were used for comparison between groups. Categorical variables were expressed as frequencies and percentages. Chi-square tests or Fisher's exact method tests were used for comparison between groups. Binary logistic regression analysis was used to screen for risk factors. All statistical tests were performed using SPSS 25.0.
Imbalanced baseline information data were adjusted by using a 1:1 propensity score match (PSM) without replacement. Propensity score matching was used to achieve balance in the baseline exposure group. Propensity scores were obtained using a logistic regression model. The variables used for matching were specifically reported in corresponding tables. The width of the caliper value used was equal to the log standard deviation of a propensity score of 0.02. The degree of balance between the baseline variables was assessed by standardized differences, with a standard deviation of ≤0.1indicating a high level of balance. PSM was performed using R3.6.3.
Receiver operating characteristic (ROC) analysis was used to evaluate the predictive effect of each variable on LAT/dense SEC. ROC analysis was performed using SPSS 25.0. A p-value <0.05 was considered statistically significant.
Baseline information
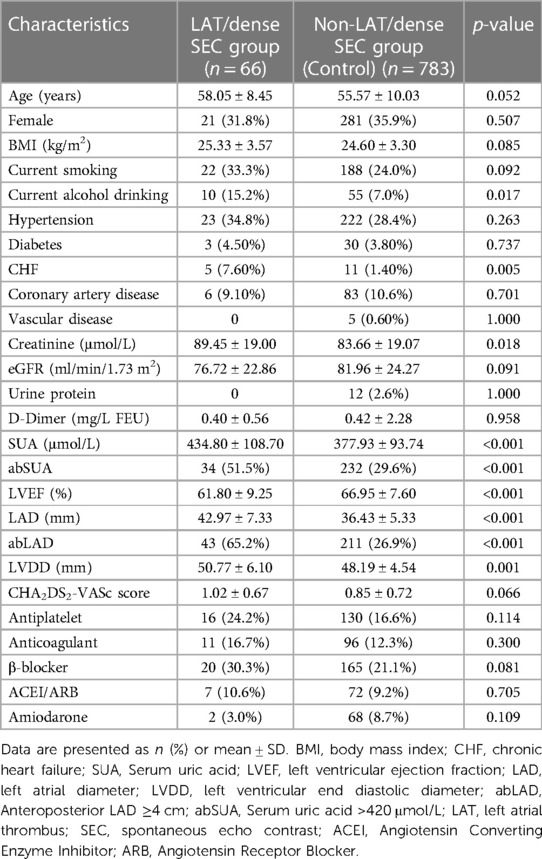
A total of 849 patients were included in this study, including 31 with LAT and 35 with dense SEC. The LAT/dense SEC group had a lower LVEF; higher creatinine, SUA, LAD, and LVDD values; and a higher proportion of abSUA, abLAD, CHF, and alcohol consumption. The difference in CHA2DS2-VASc scores between the two groups was not statistically significant. The clinical and demographic characteristics of the patients are presented in Table 1.
Binary logistic regression analysis of risk factors
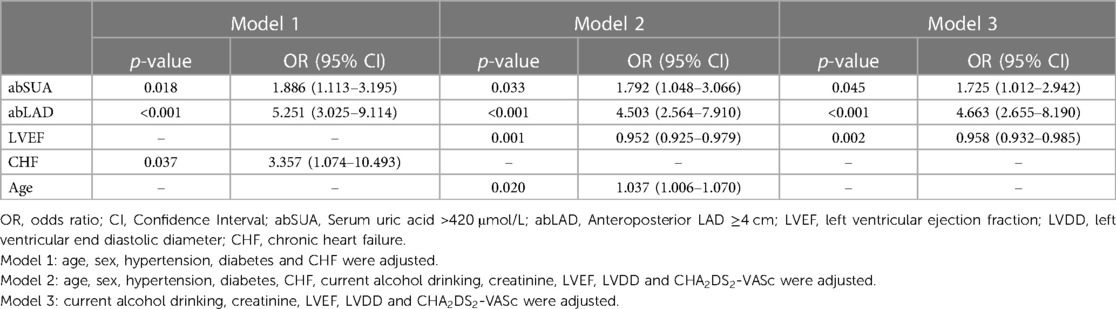
Binary logistic regression analysis was used to screen for risk factors other than those included within the CHA2DS2-VASc score (Table 2). Of the included variables, abSUA, abLAD, current alcohol consumption, and LVDD were not included in the final score. Different models were used to correct other known risk factors. In model 1, after correction for age, sex, hypertension, diabetes, and CHF, we found that abSUA and abLAD were risk factors for LAT/dense SEC. In model 2, abSUA and abLAD were identified as risk factors for LAT/dense SEC after correction for age, sex, hypertension, diabetes, CHF, current alcohol consumption, creatinine, LVEF, LVDD, and CHA2DS2-VASc. In model 3, abSUA and abLAD were found to be risk markers for LAT/dense SEC after adjusting for current alcohol consumption, creatinine, LVEF, LVDD, and CHA2DS2-VASc. Overall, abSUA and abLAD were both found to be risk factors for LAT/dense SEC, so further analysis was done to corroborate these findings.
abSUA is a risk factor for LAT/dense SEC
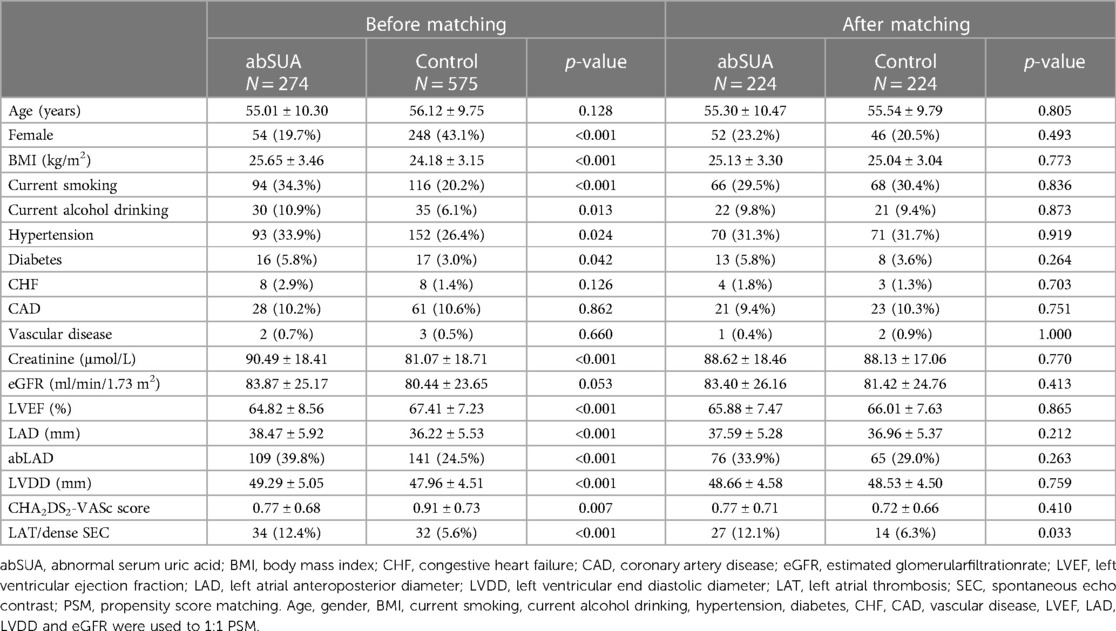
Because regression analysis suggested abSUA as a risk factor for LAT/dense SEC, further analysis was performed. The incidence of LAT/dense SEC was found to be higher in patients with abSUA than in the control group (12.4% vs. 5.6%, p < 0.05), and this difference persisted after correcting the baseline data with PSM (10.6% vs. 4.1%, p < 0.05) (Table 3).
abLAD is a risk factor for LAT/dense SEC
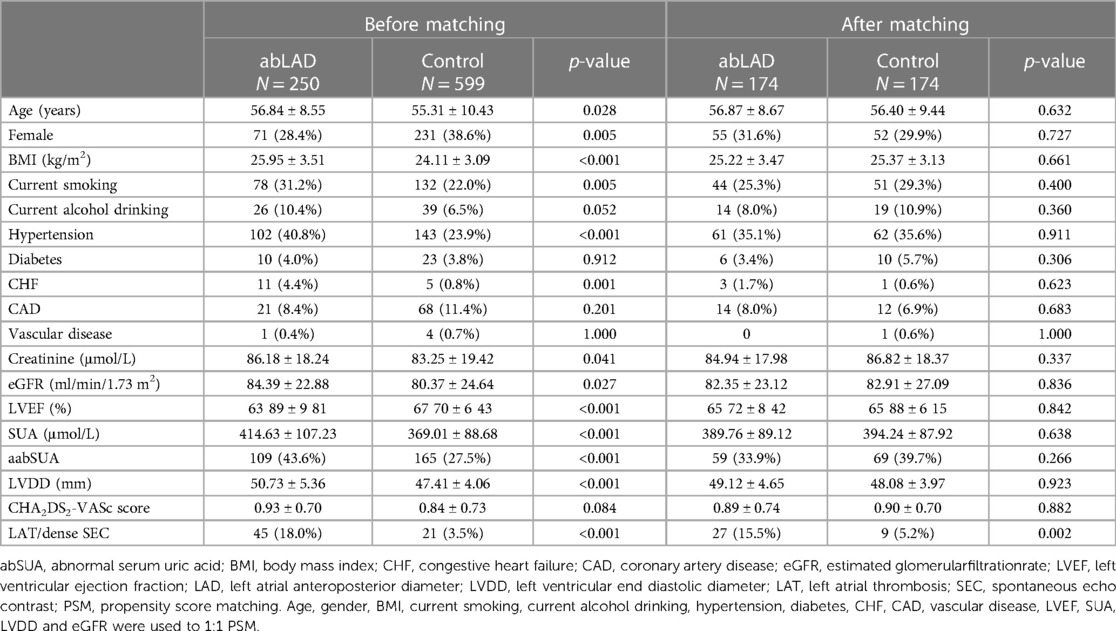
abLAD was another risk factor suggested by the regression analysis, with an increased incidence of LAT/dense SEC in the abLAD group compared to the control group, both before (18.0% vs. 3.5%, p < 0.05) and after (15.5% vs. 5.2%, p < 0.05) PSM was performed (Table 4). In conclusion, abSUA and abLAD were both found to be associated risk factors for LAT/dense SEC.
New predictive models
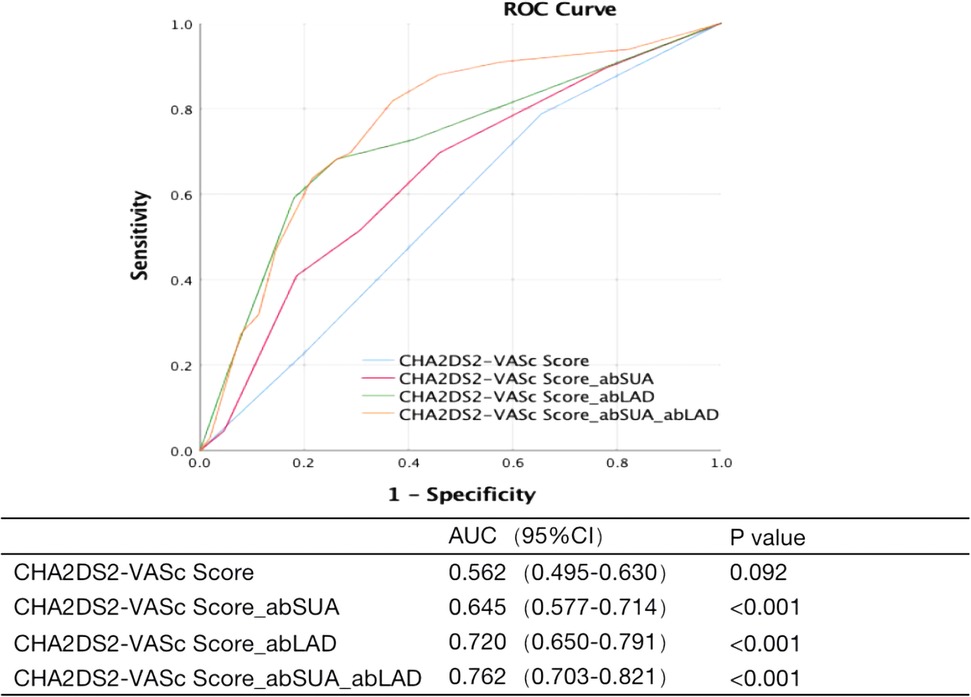
Based on the above results, we considered abSUA and abLAD as independent risk factors for LAT/dense SEC. After adding these new risk factors to the patients' existing CHA2DS2-VASc scores, the area under the ROC curve was used to clarify the predictive effect of the model for LAT/dense SEC in patients with AF. The results showed that the CHA2DS2-VASc score had no predictive value for the occurrence of LAT/dense SEC in AF patients at low to moderate risk; however, the new prediction model had low to moderate predictive power after the respective addition of abSUA and abLAD. The best predictive value was obtained by adding both abSUA and abLAD (Figure 1).
Discussion
By retrospectively analyzing the clinical characteristics, both abSUA and abLAD were identified as risk factors beyond the indicators within CHA2DS2-VASc score for LAT/dense SEC in low to moderate—risk patients with AF. Current guidelines do not explicitly recommend anticoagulants for patients who are deemed low to moderate risk because of their CHA2DS2-VASc score, but a subset of these patients still develop LAT/dense SEC. It is crucial to assess and predict the thromboembolic risk in this group of patients more comprehensively, as well as to provide better dosing guidance and management.
Many previous studies have confirmed the role of uric acid in the development of cardiovascular events. Lazzeroni et al. found that serum uric acid levels could be used to predict mortality and adverse cardiovascular outcomes in patients undergoing myocardial revascularization and/or heart valve surgery, even after correction for age, sex, hypertension, diabetes mellitus, glomerular filtration rate, and medication (19). Several studies have also confirmed uric acid as a risk factor for AF as well as LAT (20). Other research has likewise suggested that uric acid may contribute to the production of LAT/SEC in patients with AF through the following mechanisms. First, uric acid is the end product of purine metabolism by xanthine oxidase, and the oxygen radicals formed during metabolism increase the level of oxidative stress in atrial tissue. At the same time, uric acid inhibits nitric oxide production by endothelial cells and promotes tissue inflammation, leading to endothelial cell damage (15, 21). Secondly, high uric acid levels activate platelets in the body and promote platelet adhesion and aggregation, which leads to thrombosis (14).
To clarify the relationship between abSUA and LAT/dense SEC in patients with AF, we screened patients with AF who had low to moderate—risk CHA2DS2-VASc scores for further analysis and found abSUA to be one of their risk factors. The prevalence of LAT/dense SEC was significantly higher in patients with abSUA than in the control group, and this difference persisted after balancing the baseline data between the two groups using PSM.
The association of LA enlargement with increased risk of stroke and thromboembolism in patients with AF was first proposed in the 1980s (22, 23). Over time, more and more studies have demonstrated that LA enlargement is independently associated with an increased risk of stroke in patients with AF, although the endpoint of some studies has been a surrogate marker of stroke on TEE (10, 24–26). Several mechanisms have been proposed to explain the association of LA enlargement with the risk of thromboembolism in patients with AF. AF can lead to structural and functional changes in the atria that are causally related to LA enlargement. The increased internal diameter of the left atrium reduces its blood flow velocity, while the regular contraction of the atrial muscle is replaced by irregular peristalsis in AF. This makes the blood flow prone to vortex formation and even stasis, leading to the retention of organic fractions in the atria. Severe eddies create a shear-like mechanical force on the atrial wall, which in turn damages the endothelia. The slow blood flow causes the atrial endothelium to become hypoxic and necrotic, depriving it of its ability to synthesize and secrete anticoagulant substances, and produces pro-thrombotic substances such as tissue factor, ultimately leading to thrombosis.
In this study, the prevalence of LAT/dense SEC was significantly higher in patients with abLAD than in the control group, and this difference persisted after balancing the baseline information between the two groups using PSM.
The CHA2DS2-VASc score had no predictive value for the development of LAT/dense SEC in patients with AF who were at low to moderate risk. After the addition of new risk markers using ROC analysis, the results showed that the new model with the respective addition of abSUA and abLAD, especially the combined addition of both abSUA and abLAD, had predictive power for LAT/dense SEC. This finding further suggests that abSUA and abLAD have predictive value for thromboembolic risk in AF patients previously identified as low to moderate risk.
The molecular mechanism of hyperuricemia is a current hot topic of research. Both lifestyle changes and pharmacological interventions can influence uric acid levels. A limitation of this study is that only single measurements of uric acid were included, which provides a somewhat incomplete picture of the long-term uric acid metabolism of patients. In addition, it is unclear whether patients with previous gout or hyperuricemia who were taking uric acid-lowering medication to achieve normal uric acid levels have the same physical impact as patients with naturally-occurring normal uric acid levels. Given the small sample size of this group of patients, a separate analysis could not be performed. The clinical data of this study were obtained from inpatient examination findings of patients. The LAD measured in the hospital was the anterior-posterior LAD, and because of differences in body size and anatomy, the size of the anterior-posterior LAD was often not an accurate assessment of the volumetric size of the left atrium. Some studies have found that the LA volume index better reflects the size of the patient's left atrium (27–29). An association between LAT formation and left auricular morphology and function in AF patients has also previously been found, but given that this study was retrospective, relevant data could not be collected for further analysis (30).
TEE has 100% sensitivity and 99% specificity in detecting LAT as measured by surgical exploration (31), and as such TEE is now considered the gold standard for detecting LAT. In both the present study and previous studies, LAT/dense SEC was present in a certain proportion of patients even at low to moderate risk, and in many centers, TEE is an essential test before invasive treatment in all patients with AF. However, some centers believe that pre-ablation TEE can be reasonably avoided in AF patients without high-risk features. The rate of pre-ablation TEE at Johns Hopkins Hospital was reported to have decreased from 86% in 2010 to 42% in 2015 (32). With the decline in TEE use, risk assessment of LAT in patients with AF becomes critical, especially for patients with AF and a low to moderate—risk score.
In the present study, we found that abSUA and abLAD were risk factors for LAT/dense SEC in AF patients who were previously identified as low to moderate risk according to their CHA2DS2-VASc scores, suggesting the diagnostic and predictive value of these two commonly used clinical parameters. Future studies and evidence are needed to confirm their correlation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not provided for this study on human participants because Retrospective studies do not require an ethics committee review, and the hospital recently requested a summary review, which we have submitted. The patients/participants provided their written informed consent to participate in this study.
Author contributions
S-XZ and C-DT designed this study. C-DT, J-ZL, Y-PZ and Z-jL were in charge of data analysis and data collection. C-DT drafted the article. S-XZ did the critical revision of article. C-DT and J-ZL conducted the data collection. All authors contributed to the article and approved the submitted version.
Funding
The author(s) disclose receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Guangdong Provincial Science and Technology Project (No. 2017A020215175); the National Natural Science Foundation of China (Project No. 81870294); and Guangzhou Municipal School (college) Co-funded project (input from the co-construction unit) (No. 202102010299).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke. (1991) 22(8):983–8. doi: 10.1161/01.STR.22.8.983
2. Kotalczyk A, Lip GY, Calkins H. The 2020 ESC guidelines on the diagnosis and management of atrial fibrillation. Arrhythm Electrophysiol Rev. (2021) 10(2):65–7. doi: 10.15420/aer.2021.07
3. Gladstone DJ, Bui E, Fang J, Laupacis A, Lindsay MP, Tu JV, et al. Potentially preventable strokes in high-risk patients with atrial fibrillation who are not adequately anticoagulated. Stroke. (2009) 40(1):235–40. doi: 10.1161/STROKEAHA.108.516344
4. Perera KS, Vanassche T, Bosch J, Swaminathan B, Mundl H, Giruparajah M, et al. Global survey of the frequency of atrial fibrillation-associated stroke: embolic stroke of undetermined source global registry. Stroke. (2016) 47(9):2197–202. doi: 10.1161/STROKEAHA.116.013378
5. Fatkin D, Kelly RP, Feneley MP. Relations between left atrial appendage blood flow velocity, spontaneous echocardiographic contrast and thromboembolic risk in vivo. J Am Coll Cardiol. (1994) 23(4):961–9. doi: 10.1016/0735-1097(94)90644-0
6. Bernhardt P, Schmidt H, Hammerstingl C, Lüderitz B, Omran H Patients with atrial fibrillation and dense spontaneous echo contrast at high risk a prospective and serial follow-up over 12 months with transesophageal echocardiography and cerebral magnetic resonance imaging. J Am Coll Cardiol. (2005) 45(11):1807–12. doi: 10.1016/j.jacc.2004.11.071
7. Kim YG, Shim J, Oh SK, Lee KN, Choi JI, Kim YH Risk factors for ischemic stroke in atrial fibrillation patients undergoing radiofrequency catheter ablation. Sci Rep. (2019) 9 (1):7051. doi: 10.1038/s41598-019-43566-z
8. Di Minno MN, Ambrosino P, Dello Russo A, Casella M, Tremoli E, Tondo C Prevalence of left atrial thrombus in patients with non-valvular atrial fibrillation. A systematic review and meta-analysis of the literature. Thromb Haemost. (2016) 115(3):663–77. doi: 10.1160/th15-07-0532
9. Lowe BS, Kusunose K, Motoki H, Varr B, Shrestha K, Whitman C, et al. Prognostic significance of left atrial appendage “sludge” in patients with atrial fibrillation: a new transesophageal echocardiographic thromboembolic risk factor. J Am Soc Echocardiogr. (2014) 27(11):1176–83. doi: 10.1016/j.echo.2014.08.016
10. Hamatani Y, Ogawa H, Takabayashi K, Yamashita Y, Takagi D, Esato M, et al. Left atrial enlargement is an independent predictor of stroke and systemic embolism in patients with non-valvular atrial fibrillation. Sci Rep. (2016) 6:31042. doi: 10.1038/srep31042
11. Di Castelnuovo A, Veronesi G, Costanzo S, Zeller T, Schnabel RB, de Curtis A, et al. NT-proBNP (N-Terminal pro-B-type natriuretic peptide) and the risk of stroke. Stroke. (2019) 50(3):610–7. doi: 10.1161/STROKEAHA.118.023218
12. He H, Guo J, Zhang A. The value of urine albumin in predicting thromboembolic events for patients with non—valvular atrial fibrillation. Int J Cardiol. (2016) 221:827–30. doi: 10.1016/j.ijcard.2016.07.145
13. Ning W, Li Y, Ma C, Qiu L, Yu B The refinement of risk stratification for atrial thrombus or spontaneous echo contrast in non-valvular atrial fibrillation. Int Heart. (2017) 58:885–93. doi: 10.1536/ihj.16-444
14. Mustard JF, Murphy EA, Ogryzlo MA, Smythe HA. Blood coagulation and platelet economy in subjects with primary gout. Can Med Assoc J. (1963) 89:1207–11.14084698
15. Kanabrocki EL, Third JL, Ryan MD, Nemchausky BA, Shirazi P, Scheving LE, et al. Circadian relationship of serum uric acid andnitric oxide. JAMA. (2000) 283:2240–1. doi: 10.1001/jama.283.17.2235
16. Yan S, Li Q, Xia Z, Yan S, Wei Y, Hong K, et al. Risk factors of thromboembolism in non-valvular atrial fibrillation patients with low CHA2DS2-VASc score. Medicine (Baltimore). (2019) 98(8):e14549. doi: 10.1097/MD.0000000000014549
17. Yao Y, Shang MS, Gao LJ, Zhao JH, Yang XH, Liu T, et al. Elevated homocysteine increases the risk of left atrial/left atrial appendage thrombus in non-valvular atrial fibrillation with low CHA2DS2-VASc score. Europace. (2018) 20(7):1093–8. doi: 10.1093/europace/eux189
18. Kimura T, Takatsuki S, Inagawa K, Katsumata Y, Nishiyama T, Nishiyama N, et al. Anatomical characteristics of the left atrial appendage in cardiogenic stroke with low CHADS2 scores. Heart Rhythm. (2013) 10(6):921–5. doi: 10.1016/j.hrthm.2013.01.036
19. Lazzeroni D, Bini M, Camaiora U, Castiglioni P, Moderato L, Bosi D, et al. Serum uric acid level predicts adverse outcomes after myocardial revascularization or cardiac valve surgery. Eur J Prev Cardiol. (2018) 25(2):119–26. doi: 10.1177/2047487317744045
20. Tang RB, Dong JZ, Yan XL, Du X, Kang JP, Wu JH, et al. Serum uric acid and risk of left atrial thrombus in patients with non-valvular atrial fibrillation. Can J Cardiol. (2014) 30(11):1415–21. doi: 10.1016/j.cjca.2014.06.009
21. Korantzopoulos P, Kolettis TM, Galaris D, Goudevenos JA The role of oxidative stress in the pathogenesis and perpetuation of atrial fibrillation. Int J Cardiol. (2007) 115(2):135–43. doi: 10.1016/j.ijcard.2006.04.026
22. Caplan LR, D'Cruz I, Hier DB, Reddy H, Shah S Atrial size, atrial fibrillation, and stroke. Ann Neurol. (1986) 19(2):158–61. doi: 10.1002/ana.410190208
23. Aronow WS, Gutstein H, Hsieh FY. Risk factors for thromboembolic stroke in elderly patients with chronic atrial fibrillation. Am J Cardiol. (1989) 63(5):366–7. doi: 10.1016/0002-9149(89)90349-4
24. Ogata T, Matsuo R, Kiyuna F, Hata J, Ago T, Tsuboi Y, et al. Left atrial size and long-term risk of recurrent stroke after acute ischemic stroke in patients with nonvalvular atrial fibrillation. J Am Heart Assoc. (2017) 6(8):e006402. doi: 10.1161/JAHA.117.006402
25. Providência R, Botelho A, Trigo J, Quintal N, Nascimento J, Mota P, et al. Possible refinement of clinical thromboembolism assessment in patients with atrial fibrillation using echocardiographic parameters. Europace. (2012) 14(1):36–45. doi: 10.1093/europace/eur272
26. Faustino A, Providência R, Barra S, Paiva L, Trigo J, Botelho A, et al. Which method of left atrium size quantification is the most accurate to recognize thromboembolic risk in patients with non-valvular atrial fibrillation? Cardiovasc Ultrasound. (2014) 12:28. doi: 10.1186/1476-7120-12-28
27. Ayirala S, Kumar S, O'Sullivan DM, Silverman DI Echocardiographic predictors of left atrial appendage thrombus formation. J Am Soc Echocardiogr. (2011) 24(5):499–505. doi: 10.1016/j.echo.2011.02.010
28. Doukky R, Khandelwal A, Garcia-Sayan E, Gage H External validation of a novel transthoracic echocardiographic tool in predicting left atrial appendage thrombus formation in patients with non-valvular atrial fibrillation. Eur Heart J Cardiovasc Imaging. (2013) 14(9):876–81. doi: 10.1093/ehjci/jes313
29. Osranek M, Bursi F, Bailey KR, Grossardt BR, Brown RD Jr, Kopecky SL, et al. Left atrial volume predicts cardiovascular events in patients originally diagnosed with lone atrial fibrillation: three-decade follow-up. Eur Heart J. (2005) 26(23):2556–61. doi: 10.1093/eurheartj/ehi483
30. Qamruddin S, Shinbane J, Shriki J, Naqvi TZ. Left atrial appendage: structure, function, imaging modalities and therapeutic options. Expert Rev Cardiovasc Ther. (2010) 8(1):65–75. doi: 10.1586/erc.09.161
31. Manning WJ, Weintraub RM, Waksmonski CA, Haering JM, Rooney PS, Maslow AD, et al. Accuracy of transesophageal echocardiography for identifying left atrial thrombi. A prospective, intraoperative study. Ann Intern Med. (1995) 123(11):817–22. doi: 10.7326/0003-4819-123-11-199512010-00001
32. Balouch M, Gucuk Ipek E, Chrispin J, Bajwa RJ, Zghaib T, Berger RD, et al. Trends in transesophageal echocardiography use, findings, and clinical outcomes in the era of minimally interrupted anticoagulation for atrial fibrillation ablation. JACC Clin Electrophysiol. (2017) 3(4):329–36. doi: 10.1016/j.jacep.2016.09.011
Keywords: atrial fibrillation, uric acid, left atrium, thrombus, dense spontaneous echo contrast, CHA2DS2-VASc score
Citation: Tan C-D, Liu J-Z, Zheng Y-P, Li Z-j and Zhou S-X (2023) Left atrial enlargement and high uric acid level are risk factors for left atrial thrombus or dense spontaneous echo contrast in atrial fibrillation patients with low to moderate embolic risk assessed by CHA2DS2-VASC score. Front. Cardiovasc. Med. 10:937770. doi: 10.3389/fcvm.2023.937770
Received: 6 May 2022; Accepted: 12 June 2023;
Published: 3 July 2023.
Edited by:
Jiangtao Yu, Catholic Medical Center Koblenz-Montabaur, GermanyReviewed by:
Ibrahim Marai, The Baruch Padeh Medical Center, IsraelYulin Liao, Southern Medical University, China
Vassil B. Traykov, Tokuda Hospital, Bulgaria
© 2023 Tan, Liu, Zheng, Li and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu-Xian Zhou emhvdXNodXhAbWFpbC5zeXN1LmVkdS5jbg==
 Chao-Di Tan
Chao-Di Tan Shu-Xian Zhou
Shu-Xian Zhou