- 1Division of Cardiology, Department of Medical Biotechnologies, University of Siena, Siena, Italy
- 2Department of Cardiac Surgery, University of Siena, Siena, Italy
- 3Cardiac Surgery Unit, Department of Cardiac, Thoracic, Vascular Sciences, and Public Health, University of Padua, Padua, Italy
The implantation of left ventricular assist devices (LVADs) has been increasing, with good long-term results, in parallel with a growing population with advanced heart failure (HF). However, in some European countries, LVADs are still underused, with one of the main issues being the patient's late referral. On the contrary, the use of transcatheter edge-to-edge mitral valve repair (TEER) has exponentially increased over the past decade, expanding its potential use even in patients on the heart transplantation waiting list. Even though the study populations of the main trials that investigated the prognostic impact of LVAD and TEER are different, in clinical practice a clear distinction might not be so clear. Therefore, patients with refractory HF symptoms and significant mitral regurgitation should be thoroughly evaluated through a multidisciplinary Heart Team meeting with both an advanced HF specialist and interventional cardiologist, to avoid futile procedures and to define the optimal timing for advanced HF therapies, when they are indicated. We analyzed the main available studies and registries on both TEERs and LVADs and we compared their populations and outcomes, to provide the current evidence on the use of LVAD and TEER in the HF population, especially in the light of the recently released 5-year follow-up results, giving some insights on the Italian situation, and finally to stress the importance of a solid HF network between hospitals, aiming for advanced HF patients’ timely referral for LVAD or heart transplants.
Introduction
Heart failure (HF) is one of the major causes of morbidity and mortality (1–3) and due to advances in both diagnostic and treatment options, there is a growing portion of HF patients that eventually progresses to more advanced stages of the disease. As a matter of fact, patients with advanced HF (aHF) are estimated to represent between 1% and 10% of the overall HF population (4–6), even though defining its true prevalence remains a challenge, more so because of its evolving definitions. In fact, over the years, the effort of scientific societies was focused on the avoidance of delays in referral through an easier identification of patients with or at risk of developing aHF (7–9), and with that aim in mind, the recent mnemonic I-NEED-HELP was defined (9). The criteria for defining aHF are different, based on the adopted classification, such as the one from the Heart Failure Society of America, American College of Cardiology (ACC) and the European Society of Cardiology (ESC), with several overlaps (7, 9). In this scenario, in 2018, the Heart Failure Association of the ESC (HFA-ESC) proposed a new definition of aHF, based on four criteria, such as severe symptoms, severe cardiac dysfunction, hospitalizations/unplanned visits, and exercise impairment. According to the results of the HELP-HF registry, this classification identifies a high-risk HF population with all-cause mortality of 69.3% and HF hospitalization of 46.5% in patients fulfilling all four criteria (3). In aHF patients, all recent evidence underlines the importance of timely referral to HF centers to evaluate advanced strategies, such as heart transplants (HTx) and left ventricular assist devices (LVAD).
The HFA Atlas survey conducted in Europe between 2018 and 2019 attempted to provide data on HF epidemiology and resources for HF management among the 42 participating nations. According to the HFA Atlas, despite Italy being among the top European countries to have hospitals with dedicated HF centers, cardiopulmonary exercise testing, and HF rehabilitation programs (7.40, 4.50, and 4.50 per million people, respectively), it is one of the European nations with the lowest rate of LVAD implants (2.1 per million people) (11). Even though the data on the number of hospitals implanting LVAD in Italy were not available, it is the country with the highest number of cardiology departments performing MitraClip (1.83 per million people) treatment, followed by Germany, which however was the first European country for number of LVAD implantations (13.9 per million people) (11). However, data from ITAMACS Registry, which is the registry of Italian centers implanting mechanical circulatory support, show a growing number of LVAD implants throughout the years, in line with other European countries. In particular, between 2010 and 2021, 1,061 adult patients were supported by a long-term LVAD.
This type of information gives rise to several considerations, the first and foremost is why is the number of LVAD implants in countries such as Italy relatively low compared with other European countries while the use of the MitraClip system is fairly high? Is it possible that performing transcatheter edge-to-edge mitral valve repair (TEER) could be responsible for delaying or worst for preventing aHF patients from timely and appropriate referral for HTx/LVAD? How many patients that undergo TEER can actually be classified as aHF patients?
To answer these questions, it is essential to closely analyze the three most relevant trials related to the matter, which are the MOMENTUM 3 (Multicenter Study of MagLev Technology in Patients Undergoing MCS Therapy with HeartMate 3) trial, MITRA-FR trial (Percutaneous Repair with the MitraClip Device for Severe Functional/Secondary Mitral Regurgitation), and the COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation) trial, including the recently published extended results from 5-year follow-ups from both the MOMENTUM 3 and COAPT trials (12–16).
Comparison between study populations
Both the MOMENTUM 3 investigation and COAPT trial centered their focus on HF patients with refractory symptoms despite guideline-directed medical therapy (GDMT), even though several differences must be drawn. Tables 1, 2 summarize the inclusion criteria and the study populations of the two studies, respectively.
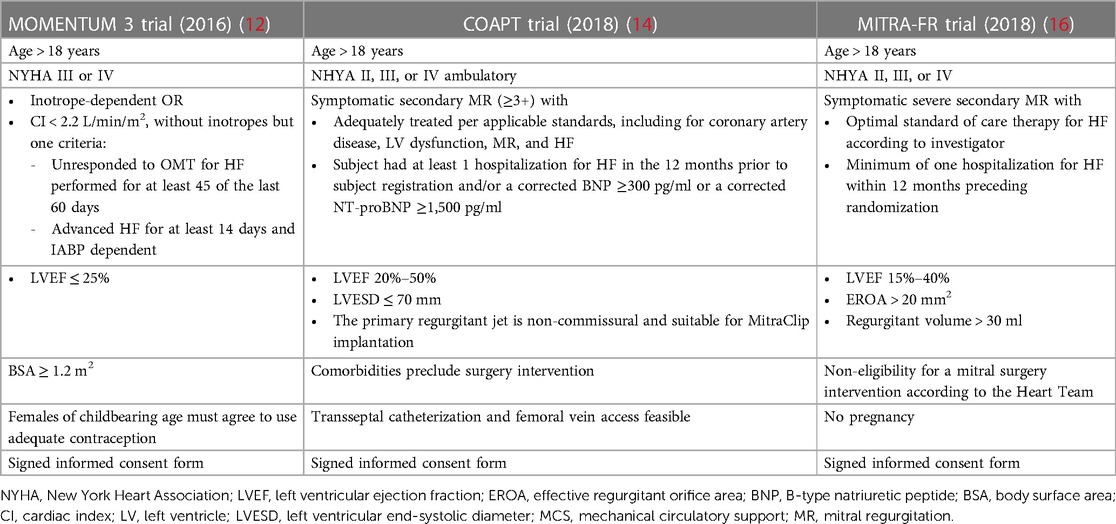
Table 1. Comparison of inclusion criteria between the MOMENTUM 3 investigation, the COAPT study, and the MITRA-FR trial.
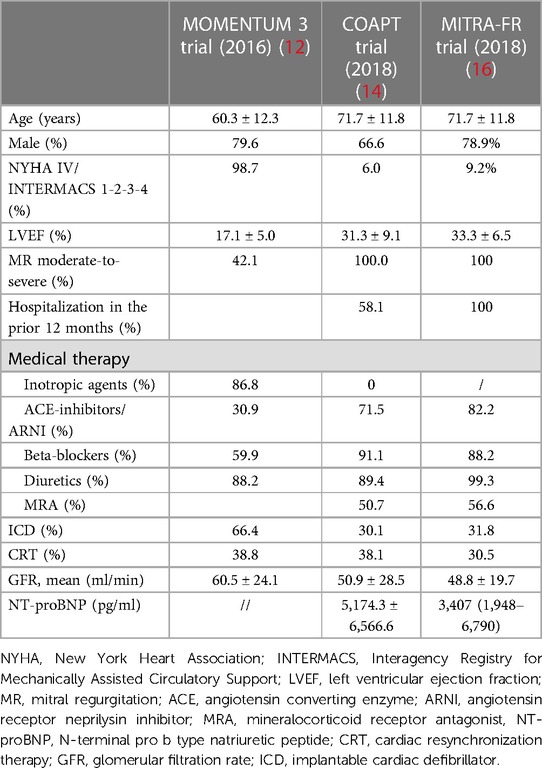
Table 2. Comparison of study populations between MOMENTUM 3 investigation, COAPT study, and the MITRA-FR trial.
The MOMENTUM 3 investigation is a prospective, multicenter, randomized pivotal trial that aimed to evaluate the safety and effectiveness of the HeartMate3 (HM3) LVAD compared with the HeartMate II LVAD in patients with advanced and refractory left ventricular HF (17). The study population was based on HF patients with the New York Heart Association (NYHA) classification III or IV, left ventricular ejection fraction (LVEF) below 25%, severe cardiac index reduction, or inotrope- or intra-aortic balloon pump-dependency (IABP) (17). Regarding the patients’ characteristics in the HeartMate 3 group, patients presented a mean LVEF of 17.1 ± 5.3% and more than half were defined as INTERMACS 3 profile (12), identifying HF patients with an advanced stage of the disease.
The MITRA-FR trial was a multicenter, randomized, open-label trial that aimed to evaluate the clinical efficacy and safety of TEER on top of optimal medical therapy (OMT) in HF patients and secondary mitral regurgitation (SMR) (16). The final study population comprised 307 patients symptomatic for HF, ranging from NYHA functional class II to IV, with severe SMR and LVEF between 15% and 40%. Each of the enrolled patients had to be hospitalized at least one time within the 12 months prior to the randomization.
The COAPT trial is a prospective, randomized and multicenter study that aimed to assess the safety and effectiveness of the MitraClip for treatment significant SMR in symptomatic HF patients despite both medical and device therapy when appropriate, such as resynchronization therapy and myocardial revascularization (18). The study population was based on HF patients with moderate-to-severe and severe SMR, range of LVEF between 20% and 50%, a NYHA functional class between II and IV ambulatory, and a previous HF hospitalization or elevated natriuretic peptides. However, among the exclusion criteria, besides moderate or severe right ventricular dysfunction and irreversible severe pulmonary hypertension, ACC/AHA stage D HF was stated. ACC/AHA stage D HF identifies patients with refractory HF symptoms despite maximum tolerated GMDT or device therapy, with frequent hospitalizations and exercise intolerance (19–21). Therefore, a clear distinction between these patients and the ones finally enrolled in the COAPT trial and MITRA-FR trial could have been challenging, and this issue is further augmented in clinical practice. In fact, on analyzing the patients’ characteristics in the device group at baseline, it was noted that 82.2% had an LVEF below 40%, 58% had experienced a HF-related hospitalization within the previous year, 54% were in NYHA class III, and the mean level of N-terminal pro-B-type natriuretic peptide (NT-proBNP) was 5,174.3 ± 6,566.6 pg/ml (18). In the intervention group of MITRA-FR trial, all patients had an LVEF below 40% and had at least one hospitalization for HF in the previous 12 months, as stated in the inclusion criteria, and 53.9% and 9.2% of them were NYHA class III and IV, respectively (16). Based on these data, it is evident that a fairly high proportion of the patients enrolled in these two investigations might fit the definition of aHF.
The EXPAND study (The MitraClip EXPAND Study of the Next Generation of MitraClip Devices) was a prospective, multicenter observational study that aimed to evaluate outcomes with the third-generation MitraClip NTR or XTR devices in patients with primary MR or SMR (22). Out of 1,041 enrolled patients, 413 had SMR, among whom 83.1% presented with NYHA class III/IV symptoms and 64.8% had at least one HF hospitalization in the previous year with the mean LVEF of 39.4 ± 13.5%.
The multicenter observational MitraBridge Registry tried to enroll a population with advanced stages of HF, including all patients with aHF, defined as NYHA class III or IV and/or with LVEF below 30%, with moderate-to-severe and severe SMR that were potential HTx candidates (23). Among the overall population of 119 patients, at the time of the MitraClip procedure, 54 patients (45.5%) were waiting for the final decision to be listed, 34 patients (28.5%) could not be listed yet (bridge to candidacy group), and finally 31 patients (26%) were on the active HTx list (23). On analysis of the patients’ characteristics, in the overall study population, it was found that 61.5% reported at least one HF hospitalization in the previous 6 months, 43.5% of the them were defined as INTERMACS profiles 5–6, and 37% as INTERMACS profiles 3–4. Compared with the COAPT study, the patients included in the MitraBridge Registry did not have to respect the echocardiographic COAPT criteria to undergo the procedure.
MitraClip vs. LVAD: comparison between outcomes
The MOMENTUM 3 investigation showed that the centrifugal-flow HM3 LVAD was superior to the axial-flow HeartMate II LVAD with respect to survival free of disabling stroke or reoperation to replace or remove a malfunctioning device (12). Among the total study population of 1,020 patients, 515 patients were implanted with HM3 LVAD and 88.4% of them were alive after 2 years. In addition, 76.9% of them remained alive and free of disabling stroke or reoperation to replace or remove a malfunctioning device at 2 years follow-up. An excellent 2-year survival rate after HeartMate 3 LVAD implant was also confirmed by the ELEVATE Registry, which was a prospective, observational, and multicenter registry that included 540 patients, of which 463 patients received the HM3 as primary implant (24). At the 2-year follow-up, the overall survival rate of patients who received the HM3 as primary implant was 83.4% (24). This real-world population registry confirmed a significant improvement in both functional capacity and quality of life, which was sustained through the duration of the study (24). It finally confirmed the relatively low incidence of adverse events such as strokes (10.2%) and pump thrombosis (1.5%) (24). In 2021, Mehra et al. published the results of long-term outcomes in the MOMENTUM 3 pivotal trial and the continued access protocol (CAP) study phase, which overall enrolled 2,200 patients implanted with the HM3 LVAD (515 pivotal trial and 1,685 CAP) (25). The 2-year Kaplan–Meier estimates of survival free of disabling stroke or reoperation to replace or remove a malfunctioning pump were similar, attested at 76.7% in the CAP and 74.8% in the pivotal trial. The overall 2-year survival was 79% in the pivotal trial cohort and 81.2% in the CAP cohort, despite a sicker population among the latter. Finally, they reported that the survival rate of ineligible HTx patients was comparable with the one reported after HTx (25).
The investigators of MOMENTUM 3 recently published the results of the extended 5-year follow-up of the study. At the 5-year follow-up, 141 of the 515 patients (27.4%) who received the centrifugal-flow pump remained receiving LVAD support and a total of 156 underwent HTx. The extended 5-year follow-up results attested an overall Kaplan–Meier survival in the centrifugal-flow group of 58.4% (13). This overall survival rate was confirmed also in a post hoc analysis in the destination therapy-subgroup with centrifugal-flow pump (13). Furthermore, the 5-year Kaplan–Meier estimate of survival to transplant, recovery, or LVAD support free of debilitating stroke or reoperation to replace the pump was 54% in the HM3 group (13). Regarding cause mortality in this group, the leading causes for adverse events and deaths were right HF and infection (13).
In Italy, LVAD survival rates come from the ITAMACS Registry, attesting a 1-year survival rate of 73.5% whereas the 5-year survival rate was of 42.1%. However, it has to be underlined that these data come from a broad period of time, from 2010 to 2021, and only 34.2% of the patients were implanted with HM3, whereas 38% with HVAD, 14.2% with HeartMate II, and 13.3% with JARVIK 2000. It could be useful to further stratify survival rates according to LVAD type and time of implant, to have data that could be more easily compared with the American ones. In fact, according to the recent European PCHF-VAD Registry, the LVAD survival rate significantly increased between the years 2013 and 2020 compared with the period of 2006–2012, even though the candidate patients are much older and with multiple comorbidities (26). These data can be interpreted in light of better expertise, better patient selection, as well as improved technologies.
The COAPT Study showed that TEER using the MitraClip device in HF patients with refractory symptoms and moderate-to-severe or severe SMR improved the 2-year survival rate and reduced HF hospitalization rate compared with medical therapy alone (14). Analyzing the device group, the 2-year survival rate was 70.9%. In fact, after 2 years from the procedure, 80 patients out of 302 (29.1%) died from any cause, among which 61 patients (23.5%) died because of cardiovascular causes. The annualized rate of HF hospitalization within 2 years was 35.8% in the device group. Furthermore, 9 patients (4.4%) in the device group underwent HTx or LVAD implant compared with 22 patients (9.5%) in the control group (14).
The outcome results from a 5-year follow-up were recently published by the COAPT Investigators, confirming the superiority of MitraClip compared with medical therapy alone in reducing HF hospitalization and all-cause mortality (15). In particular, in the device group, the 5-year survival rate was 42.7% whereas the annualized rate of HF hospitalization through 5 years was 33.1% (15).
The EXPAND study showed a significant improvement in both NYHA functional class and quality of life as well as reduced HF hospitalization after TEER in both primary MR and SMR (22). However, even though the 1-year mortality rate for primary MR was significantly lower compared with the previous studies, it remained above 17% for SMR, comparable to COAPT (22). Furthermore, in NYHA class IV patients, the 1-year mortality rate was higher, around 29% (27).
The 1-year outcomes from the MitraBridge Registry were recently published, attesting that the 1-year Kaplan–Meier estimates of freedom from composite of all-cause death, urgent HTx, or LVAD implantation and first HF rehospitalization was 64% (23). Mortality rate after the procedure was 11%, mainly due to cardiac causes (HF and sudden death), urgent HTx was necessary in 7 patients (6%), and LVAD implantation, in 21 patients (18%). Elective HTx was performed in 17 patients (15%) and 23.5% no longer needed to be on the HTx list due to clinical improvement.
Conversely, in contrast to the results of the COAPT trial, the MITRA-FR investigation showed no reduction in both all-cause mortality and HF hospitalizations with TEER on top of OMT vs. OMT alone (16). The 1-year survival rate in the device group was 76%, cardiovascular death being the main determinant (21.7% of all deaths). Furthermore, almost half of the patients experienced at least one HF hospitalization after 1 year (16).
Analyzing closely the intervention groups and the control groups of both the MITRA-FR and COAPT trials, several considerations should be made. First of all, a higher proportion of the overall population of the MITRA-FR trial was receiving ACEi/ARB or angiotensin receptor-neprilysin inhibitors (ARNI) compared with the overall population of the COAPT trial (83.9% vs. 67.10%, respectively), while a similar proportion was on beta-blockers. Furthermore, patients in the medical group of both trials significantly differed with regards to ACE-ARNI use, with 85.5% in MITRA-FR vs. 62.8% in COAPT (14, 16). Although these data cannot be directly compared, this aspect might have a significant impact on the overall survival, considering the worldwide-proven benefit of these medications, limiting the benefit of TEER in the MITRA-FR trial. Even though in the COAPT trial medical therapy needed to be titrated to maximally tolerated doses, this was not specifically required in the MITRA-FR investigation and no data are available on the dosage of the drugs used in both trials.
The concept of proportionate MR vs. disproportionate MR was formulated as a possible explanation for the discordant results of these investigations (28). In fact, in the MITRA-FR trial, patients had greater left ventricular dimensions compared with the degree of MR, which defines the concept of proportionate MR in contrast to COAPT patients in whom a less severe left ventricular dilatation was found compared with more severe MR (14, 16).
Discussion
The proportion of aHF patients has been steadily increasing due to the implementation of evidence-based therapies and consequent prolonged survival. Therefore, HF in its advanced stages has become a relevant socio-economic matter since it is associated with a high morbidity and mortality rate (2). The only two available therapies that are able to offer a significantly improved quality of life as well as higher survival are HTx and LVADs. The rate of LVAD implants in the last few decades has risen both due to the improvement of the safety profile of the devices and due to heart donor shortage, which is making HTx less and less available despite a growing aHF population. Based on the results of the analyzed studies, HF patients with SMR still have high mortality rates, especially when the burden of symptoms increases. The therapeutic goal should be to reduce this mortality rate as well as ameliorate symptoms and quality of life. In this population, TEER has been proven to significantly reduce NYHA functional class, even according to the latest trials, compared with OMT (27), while the mortality rate still remains quite high (22, 27).
As mentioned previously, the overall 2-year survival rate after centrifugal-flow LVAD is proven to be excellent, attested at 81.2% and a 2-year survival free of serious adverse event at 76.7% (25). Even though the survival rate decreases, as expected, during a 5-year follow-up post-LVAD, it still remains above 50% (13). Conversely, the 2- and 5-year survival rate after MitraClip was 70.9% and 42.7%, respectively, according to the results of the COAPT study (14, 15). Based on these results, despite a sicker population enrolled in the MOMENTUM 3 trial, the survival rate after LVAD was higher if compared with the one after the MitraClip procedure (Figure 1).
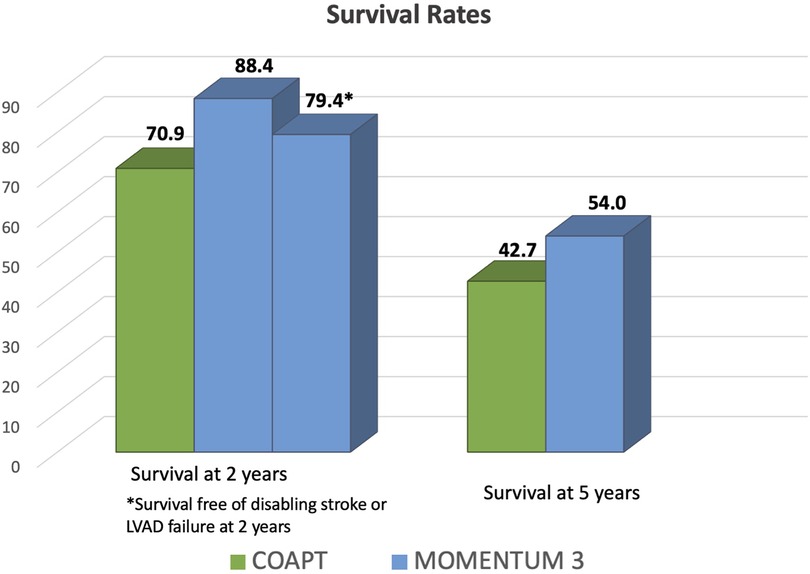
Figure 1. Comparison of survival rates after left ventricular assist device implant in MOMENTUM 3 investigation and after transcatheter edge-to-edge mitral valve repair in the COAPT trial. During a 2- and 5-year follow-up after LVAD implant in the MOMENTUM 3 investigation, the survival rates were 88.4% and 54%, respectively (12, 13), which is higher compared with the one from the COAPT trial. In fact, in the latter trial, the 2- and 5-year survival rates were 70.9% and 42.7%, respectively (14, 15).
For instance, despite this evidence, Italy still remains one of the nations with the fewest implants in Europe while the use of TEER with MitraClip system has widely spread in recent years. In particular, in 2021 a total of 103 LVADs were implanted in Italy (96 of which were HM3) while in 2022 the total number of LVAD implants was 126 (29). Regarding the use of TEER in Italy, 1,157 and 1,243 procedures were performed, respectively, in 2021 and 2022 (30). Table 3 summarizes the current indication for TEER and LVAD implant according to the latest European and American guidelines.
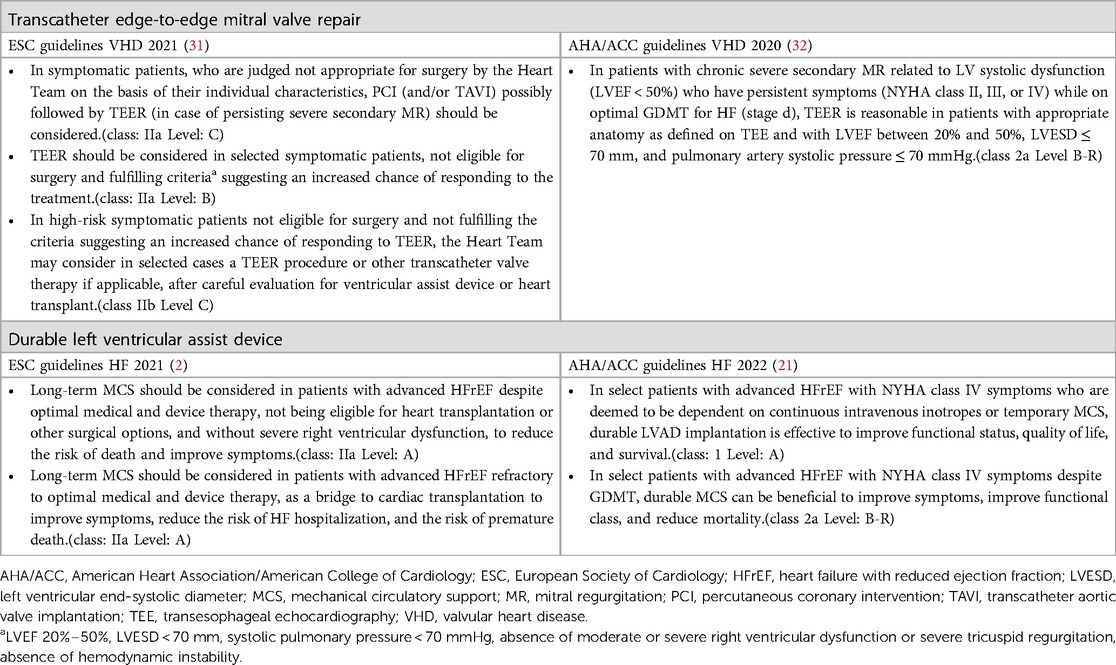
Table 3. Current indication for transcatheter edge-to-edge mitral valve repair and durable left ventricular assist device.
Starting from this analysis in Italy, which could apply to other European countries, several considerations must be made. A first possible explanation could be the significant difference in the costs of these two devices, being higher for LVADs. On this matter, Baron et al. recently published a cost-effectiveness analysis of the use of the MitraClip device in the US, which showed higher cumulative 2 years costs for the MitraClip group ($73,416 vs. $38,345; p < 0.001), due to cost of index procedure, but showed advantages in term of increase of life expectancy by 1.13 years and quality-adjusted life-years by 0.82 years (33). Conversely, Lim et al. reported a cost-effectiveness analysis for LVAD use in HTx ineligible patients in the UK, showing an incremental cost-effectiveness ratio for LVAD vs. GMDT in inotrope-dependent hospitalized patients, whereas the cost-effectiveness ratio is significantly reduced for ambulatory patients that undergo LVAD implantation (34). However, it is worth underlying that the use of HM3 compared with other LVADs has significantly reduced the LVAD-related economic burden, as reported in the CLEAR-LVAD study, due to a significant reduction of LVAD-related complications (35). The cost-effectiveness analysis of both TEER and LVAD should be carried out in each country, owing to the significant differences in the health systems.
A second explanation relies on the significantly higher number of cardiology departments where MitraClip can be performed compared with the relatively low number of hospitals in which LVAD can be implanted. A third explanation relies on the dimension of psychological acceptability of the treatment. If, on the one hand, TEER is significantly more accepted among both clinicians and patients, on the other hand, acceptance of LVAD is still very difficult to come by. Even HF specialists might see LVAD as a therapy that is too aggressive and definitive. In line with this thought, LVAD is considered as a therapeutic option only when the clinician has to face the decision whether or not start palliation, which is generally too late. In fact, in presence of severe right ventricular dysfunction, severely impaired renal or hepatic function, or severe cardiac cachexia, complications that could arise after long-standing advanced HF, LVAD could no longer represent a valid option, owing to the higher rate of post-implant complications that significantly decrease survival. Finally, another possible explanation is the lack of strong HF network between the hub and spoke centers as well as HF centers in which advanced HF strategies are available. In fact, one of the most frequent problems that these latter centers encounter is a late referral of advanced HF patients, which often precludes them from accessing these therapies, and which is probably associated with the scarce acceptability of LVAD as a therapeutic option. However, if the COAPT trial reported that TEER is a valid strategy to pursue in patients with SMR to obtain a clinical and survival benefit, it has to be stressed that in this trial patients with advanced HF were excluded. In the device group of both MITRA-FR trial and COAPT study, around 4% of patients had to undergo mechanical circulatory or Htx. On the other hand if we analyze the MitraBridge Registry, it appears that although it is true that TEER is safe in a sicker population such as patients on the HTx waiting list, almost 24% of the patients required urgent HTx or LVAD implant after the procedure. This consideration should underline the importance of a careful and thorough evaluation in patients presenting with refractory HF symptoms and significant MR, since TEER should not preclude them from being evaluated for HTx or LVAD implant but should be complimentary to it in selected cases (Figure 2). In this direction, it would be useful to set a country-specific registry of all patients that undergo TEER for SMR, to identify the subset of patients that might be classified with aHF and how many of them are actually referred to an HTX/LVAD center before or after TEER. Furthermore, future research should focus on the identification of HF patients in which TEER is associated with a high risk of urgent LVAD/HTx to avoid futile and potentially harmful procedures as well as to avoid late referral for advanced therapies. Several case series and small studies have reported that after TEER, LVAD implant is feasible and safe with no perisurgical complications related to the previous procedure (36–38). However, one aspect has to be factored in which is the number of implanted clips since multiple clips implantation might lead to a restrictive physiology that might preclude LVAD implant. Analyzing one case series of six patients, if it is true that no patients had surgical complication during LVAD implant related to the previous MitraClip procedure, it is also true that all patients underwent LVAD implant within almost a year from TEER (38). Furthermore, even though TEER successfully reduced the severity of MR, neither the echocardiographic parameters nor the hemodynamics improved significantly. This evidence points out that in a population of aHF patients, HF progresses despite the degree of MR or TEER procedure. Kreusser et al. showed in a retrospective study that despite the LVAD implant being safe and feasible after TEER, the use of TEER in patients with aHF with severe SMR might only delay LVAD implantation with potentially negative effects on long-term outcomes (39). In fact, the group of patients that underwent LVAD implantation after MitraClip, compared with patients that directly received LVAD, had a higher incidence of right ventricular (RV) failure and the need of RV assist device post-LVAD with worse outcome, even though this difference did not reach statistical significance (39). Finally, based on the recently released results from the MOMENTUM 3 investigation, LVAD implant significantly reduced the incidence as well as the severity of preimplant MR, from an incidence of 43.5%–6.2% after LVAD (40). Furthermore, the presence of MR before LVAD or during follow-up did not have a prognostic impact on this population (40).
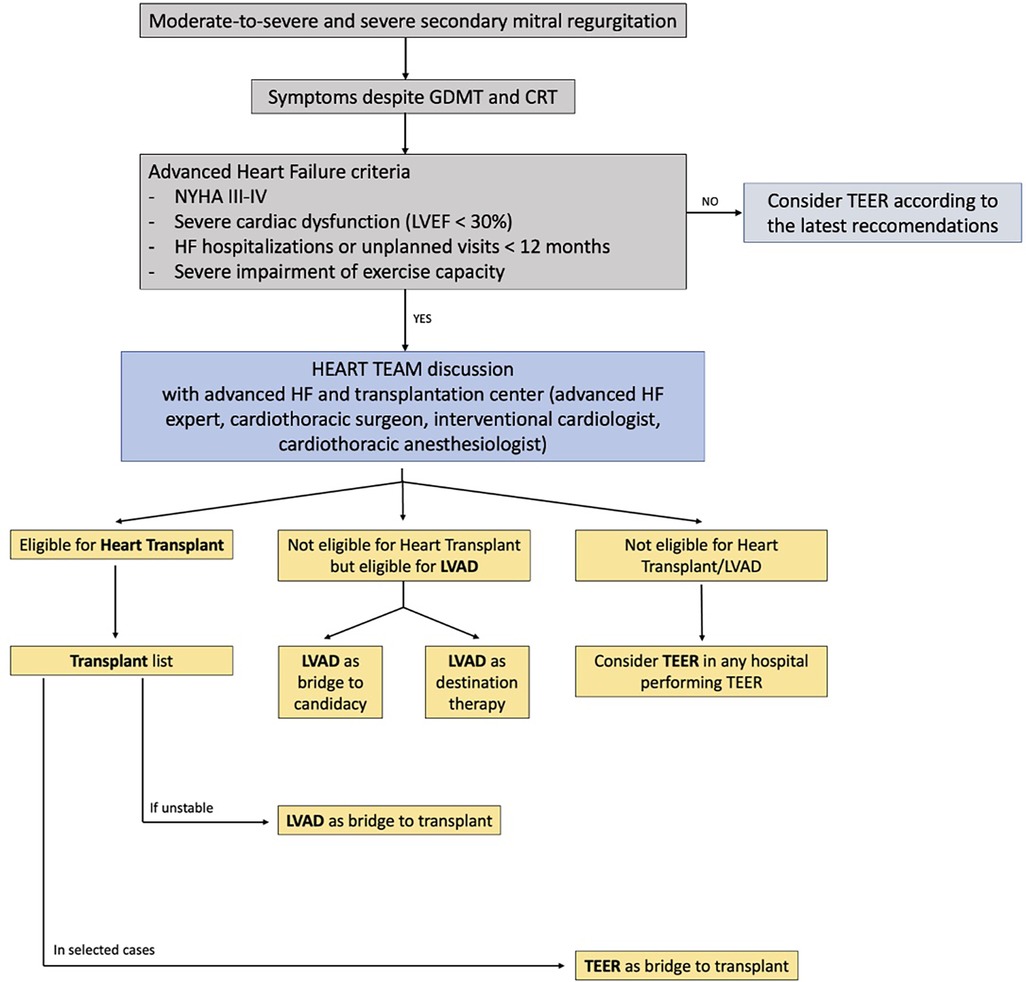
Figure 2. Flow chart for management of patients with moderate-to-severe secondary mitral regurgitation and advanced heart failure. The role of a multidisciplinary Heart Team, composed not only of interventional cardiologists but also of advanced heart failure experts and cardiothoracic surgeons, is essential to assess the optimal timing for interventional advanced heart failure therapy (heart transplantation, LVAD implantation, TEER).
Based on these premises, we believe that TEER might still be a valuable therapeutic option for patients with HF symptoms and SMR, even though it is essential to keep in mind that mortality remains high, especially in NYHA class IV patients. For this reason, patients that might be classified with aHF that are being considered for TEER should be discussed in the Heart Team, not only by the general cardiologist and interventional cardiologist but also a HF specialist in contact with a center in which HTx and/or LVAD are available options (Figure 3). This does not necessarily imply immediate LVAD implantation over TEER, but as stated above, it might improve patients’ selection and sometimes avoid futile procedures. Furthermore, it reduces the chances of losing patients that still underwent TEER at follow-up, since they were already notified to the HTx/LVAD center, facilitating the communications between hospitals and practitioners and therefore immediately intercepting initial signs of worsening HF.
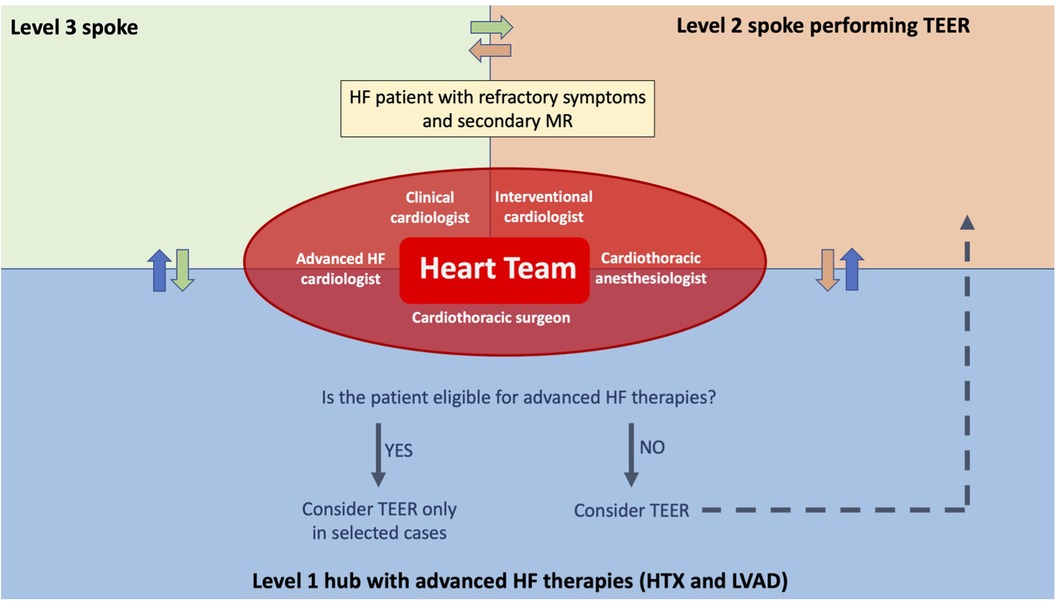
Figure 3. Advanced heart failure network for patients with significant secondary mitral regurgitation. An advanced HF network between hospitals is essential to avoid patients’ late referral for advanced HF therapies and to avoid futile procedures. With that aim, when a patient presents refractory HF symptoms and a significant secondary mitral regurgitation, discussion in the Heart Team, including interventional cardiologists and advanced HF cardiologists, is needed to define the best therapeutic option for that patient. In this way, if the patient is eligible for advanced therapies, such as HTx or LVAD, it is possible to proceed with a preliminary evaluation and, in selected cases, the patient could still undergo TEER if it is not judged futile. This is particularly important, on the one hand, for being able to perform HTx or LVAD implantation in a more urgent setting if TEER gets complicated and, on the other hand, to exclude patients that could hardly benefit from TEER and instead proceed with an LVAD implant and/or HTx listing. Finally, TEER can be performed in the tertiary center or in hospitals performing TEER, according to the patient's perioperative risk and to the interventional cardiologists’ preference.
Regarding the possible limitation of this review, there might have been a possible selection bias for not including smaller studies or registries in the discussion, since we considered the main available trials on LVADs and TEER. Furthermore, there are no studies directly comparing the use of LVAD and TEER, which might have shed further light on this topic, even though they are not practically realizable.
Conclusions
Patients with aHF and moderate-to-severe or severe SMR, who are potentially eligible for HTx or LVAD, should be discussed in multidisciplinary Heart Teams, which should include aHF experts as well as interventional cardiologists, to avoid futile interventional procedures, define optimal timing for more advanced therapies, and carefully follow up with those patients that undergo TEER to avoid their future preclusion from HTx/LVAD resulting from late referral. To achieve this goal, it is essential to create a strong HF network between spoke, hub, and hospitals with HTx and LVAD.
Author contributions
SV: Writing – review & editing, Conceptualization, Validation, Writing – original draft. CS: Writing – original draft. CSD: Writing – review & editing, Conceptualization, Validation. FR: Formal Analysis, Writing – review & editing. MC: Supervision, Writing – review & editing. SB: Formal Analysis, Writing – review & editing. MM: Methodology, Validation, Writing – review & editing. VT: Methodology, Writing – review & editing. GG: Supervision, Validation, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Metra M, Teerlink JR. Heart failure. Lancet. (2017) 390:1981–95. doi: 10.1016/S0140-6736(17)31071-1
2. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2022) 24:4–131. doi: 10.1093/eurheartj/ehab670
3. Pagnesi M, Lombardi CM, Chiarito M, Stolfo D, Baldetti L, Loiacono F, et al. Prognostic impact of the updated 2018 HFA-ESC definition of advanced heart failure: results from the HELP-HF registry. Eur J Heart Fail. (2022) 24(9):1493–503. doi: 10.1002/ejhf.2561
4. Crespo-Leiro MG, Metra M, Lund LH, Milicic D, Costanzo MR, Filippatos G, et al. Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. (2018) 20:1505–35. doi: 10.1002/ejhf.1236
5. Xanthakis V, Enserro DM, Larson MG, Wollert KC, Januzzi JL, Levy D, et al. Prevalence, neurohormonal correlates, and prognosis of heart failure stages in the community. JACC Heart Fail. (2016) 4:808–15. doi: 10.1016/j.jchf.2016.05.001
6. Bjork JB, Alton KK, Georgiopoulou VV, Butler J, Kalogeropoulos AP. Defining advanced heart failure: a systematic review of criteria used in clinical trials. J Card Fail. (2016) 22:569–77. doi: 10.1016/j.cardfail.2016.03.003
7. Truby LK, Rogers JG. Advanced heart failure: epidemiology, diagnosis, and therapeutic approaches. JACC Heart Fail. (2020) 8(7):523–36. doi: 10.1016/j.jchf.2020.01.014
8. Morris AA, Khazanie P, Drazner MH, Albert NM, Breathett K, Cooper LB, et al. Guidance for timely and appropriate referral of patients with advanced heart failure: a scientific statement from the American Heart Association. Circulation. (2021) 144(15):e238–50. doi: 10.1161/CIR.0000000000001016
9. Tedford RJ, Leacche M, Lorts A, Drakos SG, Pagani FD, Cowger J. Durable mechanical circulatory support: JACC scientific statement. J Am Coll Cardiol. (2023) 82(14):1464–81. doi: 10.1016/j.jacc.2023.07.019
10. Baumwol J. “I NEED HELP”: a mnemonic to aid timely referral in advanced heart failure. J Heart Lung Transplant. (2017) 36:593–4. doi: 10.1016/j.healun.2017.02.010
11. Seferović PM, Vardas P, Jankowska EA, Maggioni AP, Timmis A, Milinković I, et al. The Heart Failure Association Atlas: heart failure epidemiology and management statistics 2019. Eur J Heart Fail. (2021) 23(6):906–14. doi: 10.1002/ejhf.2143
12. Mehra MR, Uriel N, Naka Y, Cleveland JC Jr, Yuzefpolskaya M, Salerno CT, et al. A fully magnetically levitated left ventricular assist device: final report. N Engl J Med. (2019) 380(17):1618–27. doi: 10.1056/NEJMoa1900486
13. Mehra MR, Goldstein DJ, Cleveland JC, Cowger JA, Hall S, Salerno CT, et al. Five-year outcomes in patients with fully magnetically levitated vs axial-flow left ventricular assist devices in the MOMENTUM 3 randomized trial. J Am Med Assoc. (2022) 328(12):1233–42. doi: 10.1001/jama.2022.16197
14. Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. (2018) 379(24):2307–18. doi: 10.1056/NEJMoa1806640
15. Stone GW, Abraham WT, Lindenfeld J, Kar S, Grayburn PA, Lim DS, et al. Five-year follow-up after transcatheter repair of secondary mitral regurgitation. N Engl J Med. (2023) 388(22):2037–48. doi: 10.1056/NEJMoa2300213
16. Obadia JF, Messika-Zeitoun D, Leurent G, Iung B, Bonnet G, Piriou N, et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med. (2018) 379(24):2297–306. doi: 10.1056/NEJMoa1805374
17. Heatley G, Sood P, Goldstein D, Uriel N, Cleveland J, Middlebrook D, et al. Clinical trial design and rationale of the multicenter study of MagLev technology in patients undergoing mechanical circulatory support therapy with HeartMate 3 (MOMENTUM 3) investigational device exemption clinical study protocol. J Heart Lung Transplant. (2016) 35(4):528–36. doi: 10.1016/j.healun.2016.01.021
18. Mack MJ, Abraham WT, Lindenfeld J, Bolling SF, Feldman TE, Grayburn PA, et al. Cardiovascular outcomes assessment of the MitraClip in patients with heart failure and secondary mitral regurgitation: design and rationale of the COAPT trial. Am Heart J. (2018) 205:1–11. doi: 10.1016/j.ahj.2018.07.021
19. Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. (2009) 53:e1–e90. doi: 10.1016/j.jacc.2008.11.013
20. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. (2013) 128:1810–52. doi: 10.1161/CIR.0b013e31829e8807
21. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. (2022) 145(18):e876–94. doi: 10.1016/j.jacc.2021.12.012
22. Kar S, von Bardeleben RS, Rottbauer W, Mahoney P, Price MJ, Grasso C, et al. Contemporary outcomes following transcatheter edge-to-edge repair: 1-year results from the EXPAND study. JACC Cardiovasc Interv. (2023) 16(5):589–602. doi: 10.1016/j.jcin.2023.01.010
23. Godino C, Munafò A, Scotti A, Estévez-Loureiro R, Portolés Hernández A, Arzamendi D, et al. MitraClip in secondary mitral regurgitation as a bridge to heart transplantation: 1-year outcomes from the International MitraBridge Registry. J Heart Lung Transplant. (2020) 39(12):1353–62. doi: 10.1016/j.healun.2020.09.005
24. Zimpfer D, Gustafsson F, Potapov E, Pya Y, Schmitto J, Berchtold-Herz M, et al. Two-year outcome after implantation of a full magnetically levitated left ventricular assist device: results from the ELEVATE Registry. Eur Heart J. (2020) 41(39):3801–9. doi: 10.1093/eurheartj/ehaa639
25. Mehra MR, Cleveland JC Jr, Uriel N, Cowger JA, Hall S, Horstmanshof D, et al. Primary results of long-term outcomes in the MOMENTUM 3 pivotal trial and continued access protocol study phase: a study of 2200 HeartMate 3 left ventricular assist device implants. Eur J Heart Fail. (2021) 23(8):1392–400. doi: 10.1002/ejhf.2211
26. Jakus N, Brugts JJ, Claggett B, Timmermans P, Pouleur A, Rubiś P, et al. Improved survival of left ventricular assist device carriers in Europe according to implantation eras: results from the PCHF-VAD registry. Eur J Heart Fail. (2022) 24(7):1305–15. doi: 10.1002/ejhf.2526
27. Shuvy M, von Bardeleben RS, Grasso C, Raake P, Lurz P, Zamorano JL, et al. Safety and efficacy of MitraClip in acutely ill (NYHA class IV) patients with mitral regurgitation: results from the global EXPAND study. ESC Heart Fail. (2023) 10(2):1122–32. doi: 10.1002/ehf2.14273
28. Grayburn PA, Sannino A, Packer M. Proportionate and disproportionate functional mitral regurgitation: a new conceptual framework that reconciles the results of the MITRA-FR and COAPT trials. JACC Cardiovasc Imaging. (2019) 12(2):353–62. doi: 10.1016/j.jcmg.2018.11.006
31. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. (2022) 43(7):561–632. doi: 10.1093/eurheartj/ehab395
32. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. (2021) 143(5):e35–71. doi: 10.1161/CIR.0000000000000932
33. Baron SJ, Wang K, Arnold SV, Magnuson EA, Whisenant B, Brieke A, et al. Cost-effectiveness of transcatheter mitral valve repair versus medical therapy in patients with heart failure and secondary mitral regurgitation: results from the COAPT trial. Circulation. (2019) 140(23):1881–91. doi: 10.1161/CIRCULATIONAHA.119.043275
34. Lim HS, Shaw S, Carter AW, Jayawardana S, Mossialos E, Mehra MR. A clinical and cost-effectiveness analysis of the HeartMate 3 left ventricular assist device for transplant-ineligible patients: a United Kingdom perspective. J Heart Lung Transplant. (2022) 41(2):174–86. doi: 10.1016/j.healun.2021.11.014
35. Pagani FD, Mehra MR, Cowger JA, Horstmanshof DA, Silvestry SC, Atluri P, et al. Clinical outcomes and healthcare expenditures in the real world with left ventricular assist devices—the CLEAR-LVAD study. J Heart Lung Transplant. (2021) 40(5):323–33. doi: 10.1016/j.healun.2021.02.010
36. Ammirati E, Van De Heyning CM, Musca F, Brambatti M, Perna E, Cipriani M, et al. Safety of centrifugal left ventricular assist device in patients previously treated with MitraClip system. Int J Cardiol. (2019) 283:131–3. doi: 10.1016/j.ijcard.2019.02.039
37. Fox H, Gyoten T, Rojas SV, Deutsch M-A, Schramm R, Rudolph V, et al. Safety, mortality, and hemodynamic impact of patients with MitraClip undergoing left ventricular assist device implantation. J Cardiovasc Transl Res. (2022) 15(3):676–86. doi: 10.1007/s12265-021-10178-w
38. Dogan G, Hanke JS, Ricklefs M, Chatterjee A, Feldmann C, Mashaqi B, et al. MitraClip procedure prior to left ventricular assist device implantation. J Thorac Dis. (2018) 10(Suppl 15):S1763–8. doi: 10.21037/jtd.2018.05.36
39. Kreusser MM, Hamed S, Weber A, Schmack B, Volz MJ, Geis NA, et al. MitraClip implantation followed by insertion of a left ventricular assist device in patients with advanced heart failure. ESC Heart Fail. (2020) 7(6):3891–900. doi: 10.1002/ehf2.12982
Keywords: advanced heart failure, transcatheter edge-to-edge mitral valve repair, left ventricular assist device (LVAD), MitraClip, heart failure
Citation: Valente S, Sciaccaluga C, Sorini Dini C, Righini FM, Cameli M, Bernazzali S, Maccherini M, Tarzia V and Gerosa G (2024) Left ventricular assist device and transcatheter edge-to-edge mitral valve repair in advanced heart failure: allies or enemies?. Front. Cardiovasc. Med. 10:1327927. doi: 10.3389/fcvm.2023.1327927
Received: 25 October 2023; Accepted: 29 December 2023;
Published: 26 January 2024.
Edited by:
Francesco Gentile, Sant'Anna School of Advanced Studies, ItalyReviewed by:
Massimo Mapelli, Monzino Cardiology Center (IRCCS), ItalyMauro Gitto, Humanitas Research Hospital, Italy
© 2024 Valente, Sciaccaluga, Sorini Dini, Righini, Cameli, Bernazzali, Maccherini, Tarzia and Gerosa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: C. Sorini Dini Y2FybG90dGEuc29yaW5pZGluaUBnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
 S. Valente1,†
S. Valente1,† C. Sciaccaluga
C. Sciaccaluga C. Sorini Dini
C. Sorini Dini F. M. Righini
F. M. Righini M. Cameli
M. Cameli V. Tarzia
V. Tarzia G. Gerosa
G. Gerosa