- 1Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2Cardiovascular Diseases Center, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
Purpose: Atrial fibrosis is the main pathological basis for the pathogenesis and progression of atrial fibrillation (AF). Soluble suppression of tumorigenicity 2 (sST2) is involved in fibrosis. Recent studies have explored its predictive value in AF outcomes. We performed this study to assess whether sST2 is an independent biomarker of AF outcomes and explore the potential mechanism.
Methods: PubMed, Web of Science, EMBASE, and Cochrane Library databases were searched systematically from inception through July 1, 2023, to identify relevant studies. Outcomes of interest included occurrence, recurrence, and major adverse cardiac events (MACEs) of AF. This meta-analysis was reported following the criteria outlined in PRISMA 2020, and the protocol was registered in PROSPERO (number: CRD42023459789). All statistical analyses were performed using the STATA version 16.
Result: Twenty four studies with 14,755 patients were included in the meta-analysis. The meta-analyses found that sST2 was significantly associated with the risk of occurrence [HR:1.04, 95% CI: 1.02–1.07, P < 0.01; I2 = 67.8%], recurrence [HR:1.09, 95% CI: 1.02–1.16, P < 0.01; I2 = 89.5%], and MACEs (HR:1.60, 95% CI: 1.13–2.27, P < 0.01; I2 = 82.0%) of AF. Furthermore, patients with AF showed higher sST2 than controls without AF (SMD: 0.41, 95% CI: 0.27–0.54, P < 0.01; I2 = 0%), and AF patients with recurrence after catheter ablation (CA) showed significantly higher sST2 than those without recurrence (SMD: 0.81, 95% CI: 0.33–1.28, P < 0.01; I2 = 83.9%). Sensitivity analyses showed that the outcomes were stable.
Conclusions: Higher sST2 was association with an increased risk of occurrence, recurrence, and MACEs of AF. Assessing sST2 can be used as a potential screening method to predict AF outcomes.
Systematic Review Registration: PROSPERO (CRD42023459789).
1 Introduction
Atrial fibrillation (AF) is one of the most common clinical arrhythmias, affecting more than 46.3 million individuals worldwide (1). The prevalence of AF is expected to double in the next 30 years to more than 17 million in Europe alone. AF leads to peripheral embolism, stroke, heart failure (HF), and is associated with high mortality and hospitalization rates (2). In addition, AF recurrence is also a challenging problem, with a recurrence rate of up to 30% (3). The pathophysiology of AF is complex and is thought to involve pro-inflammatory responses leading to electrophysiological remodeling, which in turn leads to atrial fibrosis and structural remodeling. The end result is to provide an arrhythmogenic substrate for AF triggers (4–6).
As with other diseases, blood markers have been used for the purpose of risk stratification for AF (7). The suppression of tumorigenicity 2 (ST2) is a member of the interleukin-1 (IL-1) receptor family, which exists in two forms: transmembrane receptor (ST2l) and soluble decoy receptor (sST2) (8). sST2 is relates to markers of hemodynamic load, released from the myocardium and vascular endothelial cells in response to pressure or volume overload, and is involved in fibrosis and remodeling through pathways related to inflammation (8, 9). sST2, mainly a well-known HF biomarker, and is also associated with worsening outcomes after myocardial infarction (MI) (10, 11). Recent studies have identified the predictive value of sST2 in AF (12–14). However, these studies are small and contradictory. Therefore, we aimed to assess whether sST2 is an independent biomarker of AF outcomes and explore the potential mechanism.
2 Methods
2.1 Search strategy
Two researchers performed a systematic literature search using four electronic databases (PubMed, Web of Science, EMBASE, and Cochrane Library) with MESH terms and keywords ((“Atrial fibrillation” OR “AF”) AND (“Biomarkers” OR “Soluble Suppression of Tumorigenicity 2” OR “Soluble ST2” OR “sST2” OR “ST2”). We also conducted a hand-searching of relevant articles. The disagreement was resolved by consulting a senior reviewer (Dazhuo Shi).
2.2 Literature inclusion and exclusion criteria
The inclusion criteria were: (1) study design was observational study (included prospective cohort, retrospective cohort, case-control, and cross-sectional study); (2) target population was AF patients; (3) there were measured sST2 at least two groups in one study; (4) outcomes of interest included occurrence, recurrence, and major adverse cardiac events (MACEs) of AF. The MACEs, which was defined as a composite outcome of fatal or non-fatal cardiovascular events, such as death, MI, HF, stroke, rehospitalization, and revascularization.
The exclusion criteria were: (1) abstracts, editorial, animal experiment, or review; (2) study with inadequate relevance; (3) study with insufficient clinical data.
2.3 Data extraction
The following data were extracted: (1) information on the publication: first author's name, publication year, location; (2) demographic characteristics: sample size, age, gender; (3) study details: study design, follow-up period, measurement methods of sST2, data on the diagnostic analysis (definition of the control group, sample size, mean ± standard deviatio (SDs) or median interquartile ranges (IQR) values, the optimal cut-off value, area under the curve (AUC) for the receiver operating characteristic curve (ROC), sensitivity, and specificity), and data on the prognostic analysis (clinical outcomes, unadjusted and/or multivariable-adjusted HRs/ORs, 95% CIs, the optimal cut-off value, AUC for the ROC, sensitivity, and specificity).
2.4 Bias assessment
The Newcastle-Ottawa Scale (NOS) (15) was used to assessed each study quality. NOS focused on three major aspects: participant selection (0–4 stars), comparability (0–2 stars), and exposure (0–3 stars). Studies were regarded as moderate-to-high quality with the total score ≥6, and <6 for low quality.
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) (16) approach was used to assess the quality of outcome evidence. The quality were categorized into four levels (high, moderate, low, or very low). This meta-analysis only included observational studies, which start with a “low quality”, and other factors may then upgrade or downgrade the quality level.
2.5 Statistical analysis
All data of sST2 were pooled analysis by means ± SDs or HRs/ORs. The Cochrane Q-test and the I2-value were used to assess the statistical heterogeneity, where P < 0.1 or I2 > 50% suggested significant heterogeneity. A random-effect model were selected for this meta-analysis, considering the potential heterogeneity across studies. The publication bias was evaluated by employing the funnel plots and Egger's test, where a P-value higher than 0.1 indicated no significant publication bias. For sensitivity analysis, omitted one study at a time to assess the robustness. STATA version 16 was used for all statistical analyses.
3 Results
3.1 Study search
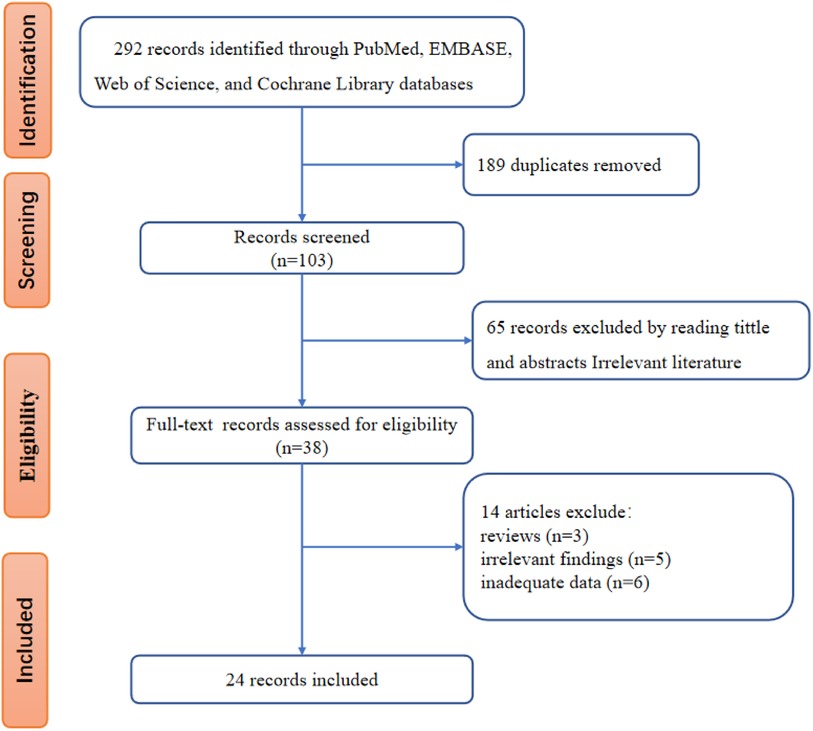
The flowchart of literature screening was shown in Figure 1. The initial database search identified 292 records, 189 of which were duplicates. According to the analysis of titles and abstracts, 65 studies were excluded, and 38 were included. After reading the full text, 14 studies were further excluded because 3 were reviews, 5 were irrelevant findings, and the other 6 were inadequate data. Finally, 24 studies (12–14, 17–37) were included.
3.2 Study characteristics
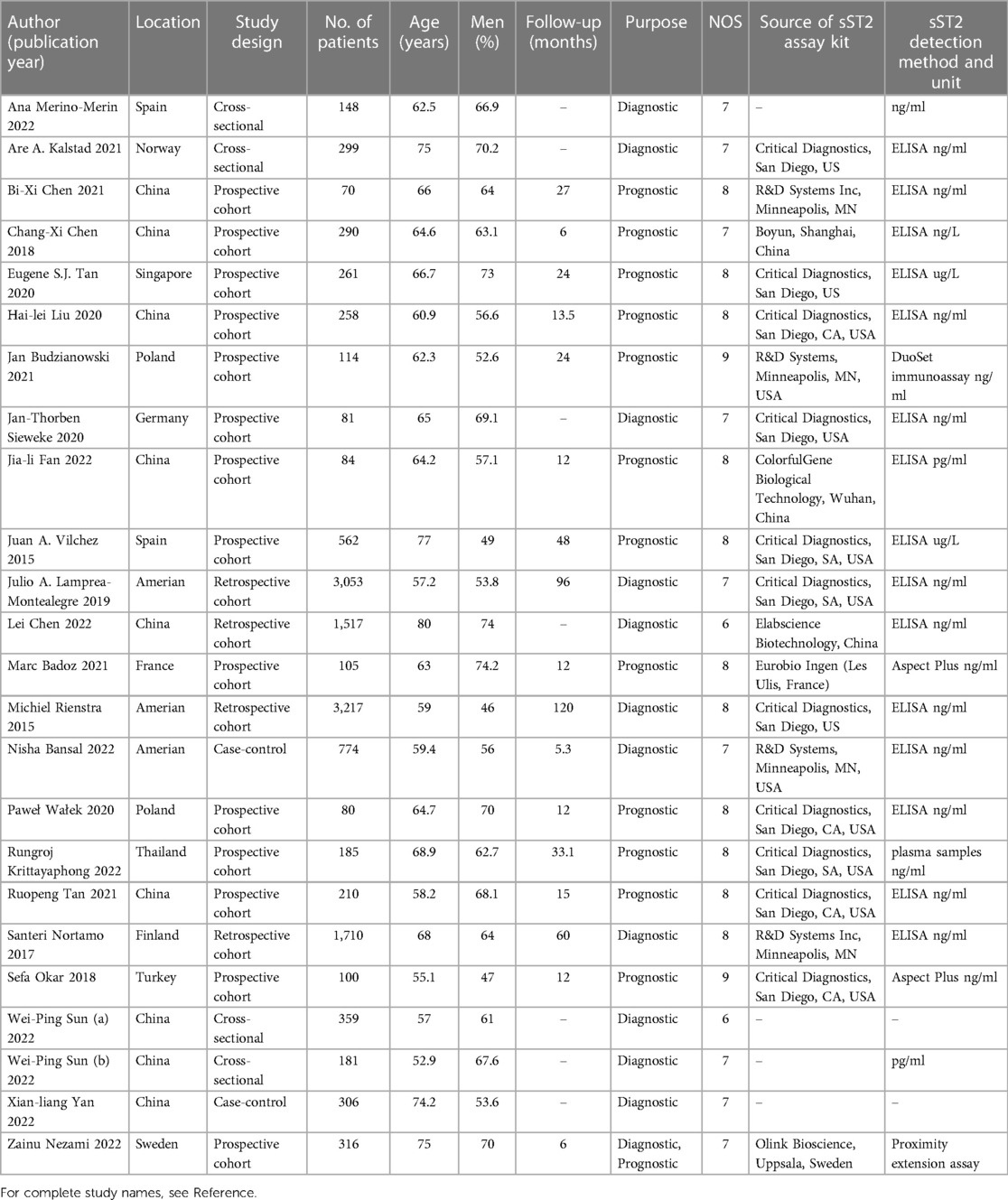
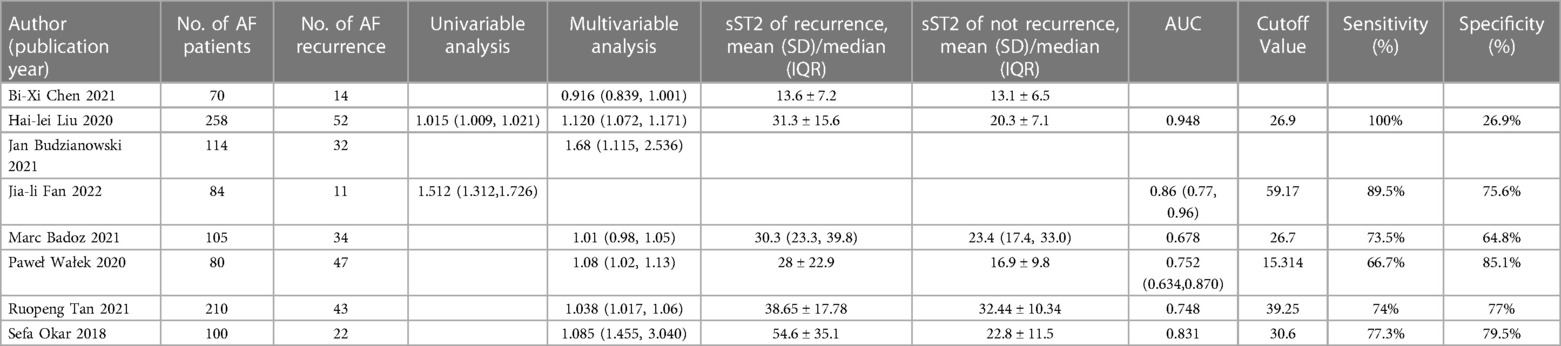
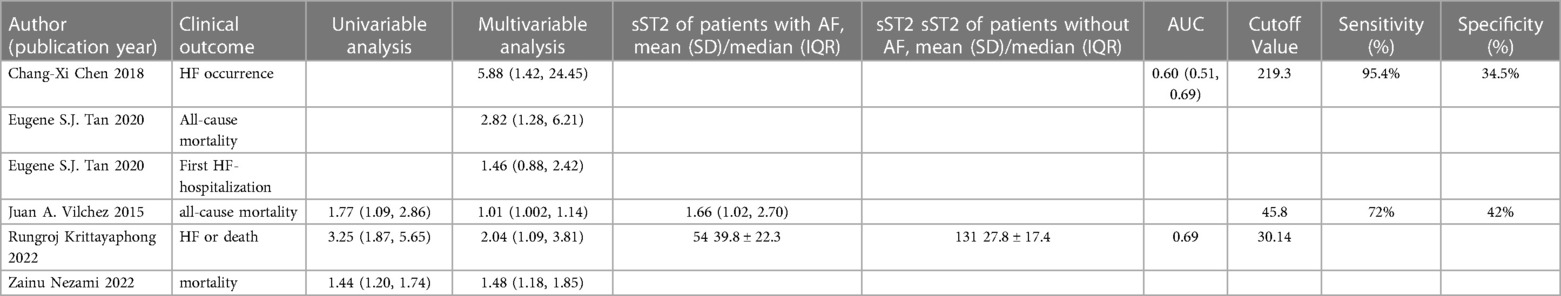
Table 1 displayed the baseline characteristics of 24 included studies from 2015 to 2022, comprising 14 prospective studies (14, 17–23, 26, 29–31, 33, 37), 4 retrospective studies (24, 25, 27, 32), 4 cross-sectional studies (12, 13, 34, 35), and 2 case-control studies (28, 36). Eleven studies were conducted in Asia (China, Thailand, Singapore), 10 studies in Europe (Germany, Poland, Finland, France, Norway, Spain, Sweden, Turkey), and 3 study in America (The united states). A total of 15,118 patients were involved, with an average of 62.07% males and an average age of 64.87 years. The follow-up time was 5.3–120 months. The included studies used various sources of sST2 reagents and adopted diverse detection strategies (e.g., 15 studies used ELISA to detect sST2, 2 used aspect plus assay, 1 used plasma samples assay, and 1 used duoset immunoassay assay). Concerning the purpose of these studies, 12 studies (12, 13, 21, 24, 25, 27, 28, 32, 34–37) evaluated the predictive value of sST2 in occurrence of AF, while 8 studies (14, 19, 20, 22, 26, 29, 31, 33) evaluated AF recurrence, 5 studies (17, 18, 23, 30, 37) evaluated MACEs following AF.
3.3 Study quality
The mean NOS scores was 7.54 (range 6–9), indicating moderate to high quality. The details of the quality assessment were shown in Supplementary Table S1.
The GRADE grade showed that 2 evidences were mediate, 1 was low, and 2 were very low. The results of the GRADE assessment were shown in Supplementary Table S2.
3.4 Results of meta-analysis
3.4.1 Predictive value of sST2 in occurrence of AF
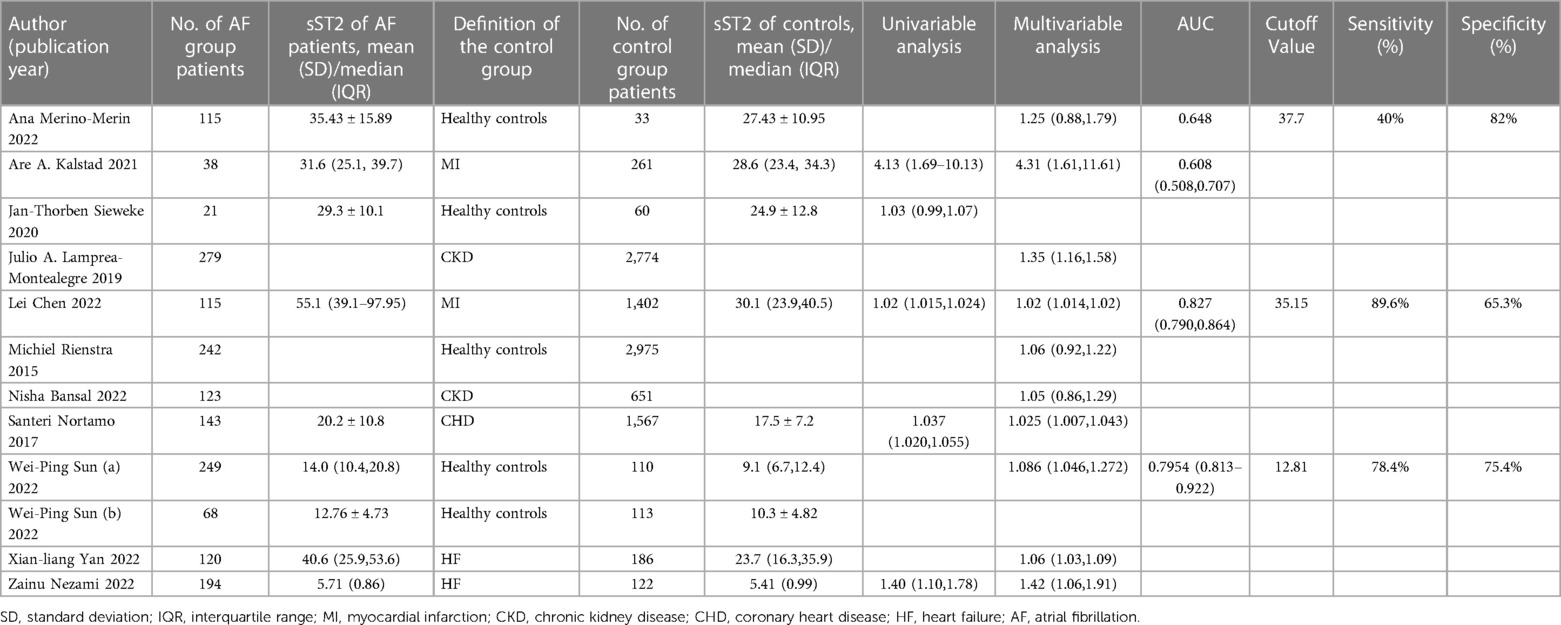
Table 2 diaplayed the characteristics of the included studies for predictive value of sST2 in occurrence of AF. A total of 12 studies were analyzed with 11,961 participants (including 1,707 AF patients and 10,254 controls). The pooled analysis of 10 studies (12, 21, 24, 25, 27, 28, 32, 34, 36, 37) as a continuous variable indicated that sST2 was associated with the risk of AF occurrence (HR:1.04, 95% CI: 1.02–1.07, P < 0.01; I2 = 67.8% Figure 2). Sensitivity analyses showed that the outcomes were stable (Supplementary Figure S2A). The funnel plot and Egger's test revealed publication bias in the results (Egger's test, P = 0.006, Supplementary Figures S2B,C).
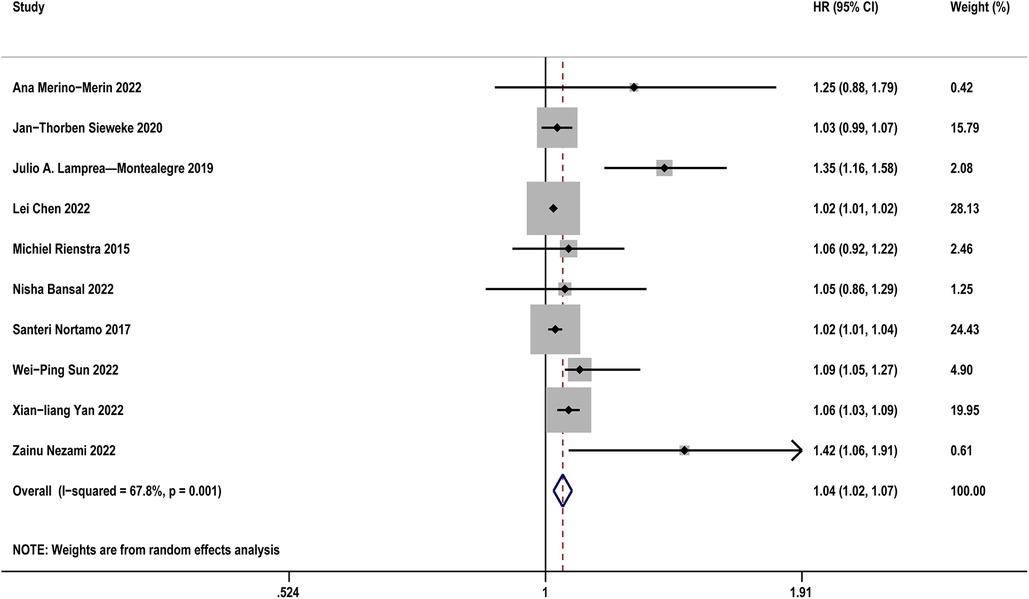
Figure 2. Forest plots show the relationship between sST2 (continuous variables) and the risk of AF occurrence.
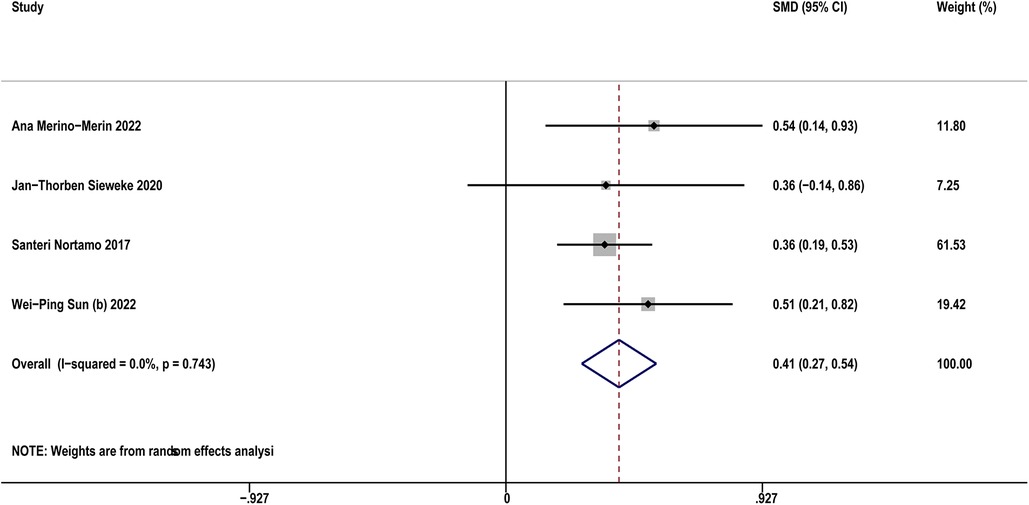
Four studies (12, 21, 32, 35) compared sST2 between AF patients and controls. The result indicated that AF patients showed higher sST2 than controls (SMD: 0.41, 95% CI: 0.27–0.54, P < 0.01; I2 = 0% Figure 3). Sensitivity analyses found that the outcomes were stable (Supplementary Figure S3A). Publication bias was not indicated (Egger's test, P = 0.398, Supplementary Figures S3B,C).
3.4.2 Predictive value of sST2 in AF recurrence after CA
Table 3 diaplayed the characteristics of the included studies for predictive value of sST2 in recurrence of AF. A total of 8 studies were analyzed with 1,021 AF patients (including 255 AF recurrence and 766 not recurrence). The pooled analysis of 7 studies (14, 19, 20, 22, 26, 31, 33) as a continuous variable indicated that sST2 was associated with the risk of AF recurrence (HR:1.09, 95% CI: 1.02–1.16, P < 0.01; I2 = 89.5% Figure 4). Sensitivity analyses showed that the outcomes were stable (Supplementary Figure S4A). Publication bias was not indicated (Egger's test, P = 0.249, Supplementary Figures S4B,C).
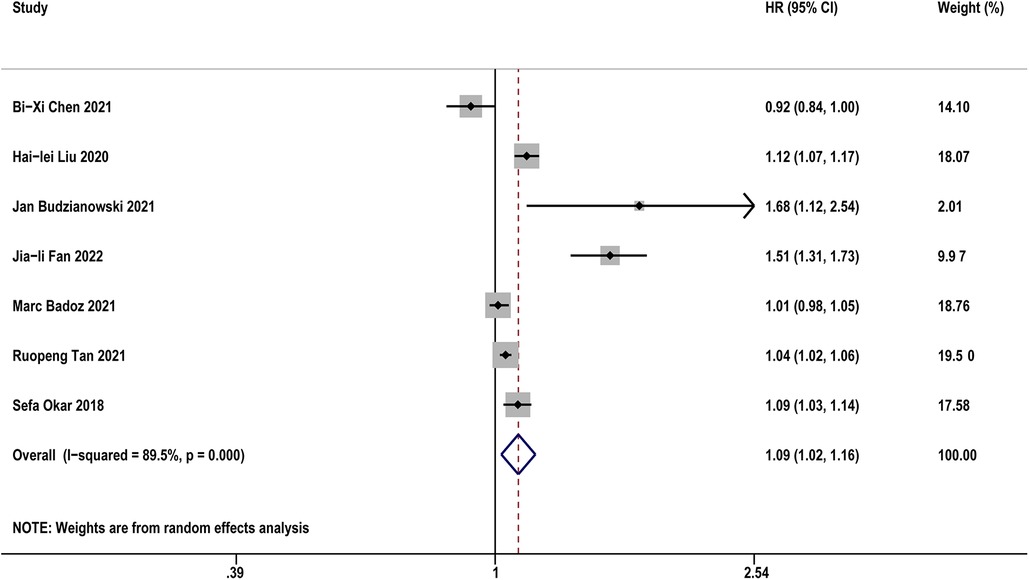
Figure 4. Forest plots show the relationship between sST2 (continuous variables) and the risk of AF recurrence after CA.
Five studies (14, 19, 29, 31, 33) compared sST2 between recurrence and not recurrence in AF patients. The results indicated that AF patients with recurrence showed significantly higher sST2 than those without recurrence (SMD: 0.81, 95% CI: 0.33–1.28, P < 0.01; I2 = 83.9% Figure 5). Sensitivity analyses found that the outcomes were stable (Supplementary Figure S5A). Publication bias was not indicated (Egger's test, P = 0.832, Supplementary Figures S5B,C).
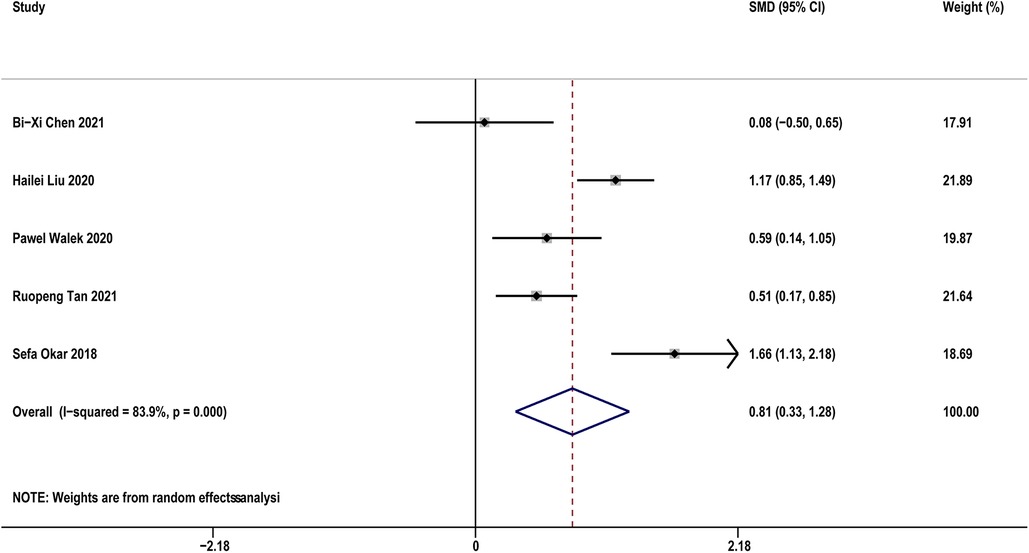
Figure 5. Forest plots show the difference in sST2 values between patients with and without AF recurrence after CA.
3.4.3 Predictive value of sST2 in MACEs following AF
Table 4 diaplayed the characteristics of the included studies for predictive value of sST2 in MACEs following AF. Five studies (17, 18, 23, 30, 37) with 1,614 AF patients examined the relationship between sST2 and the risk of MACEs following AF. The pooled analysis of the estimates as a continuous variable indicated that sST2 was significantly associated with the risk of MACEs (HR:1.60, 95% CI: 1.13–2.27, P < 0.01; I2 = 82.0% Figure 6). Sensitivity analyses showed that the outcomes were stable (Supplementary Figure S6A). The funnel plot and Egger's test revealed publication bias in the results (Egger's test, P = 0.004, Supplementary Figures S6B,C).
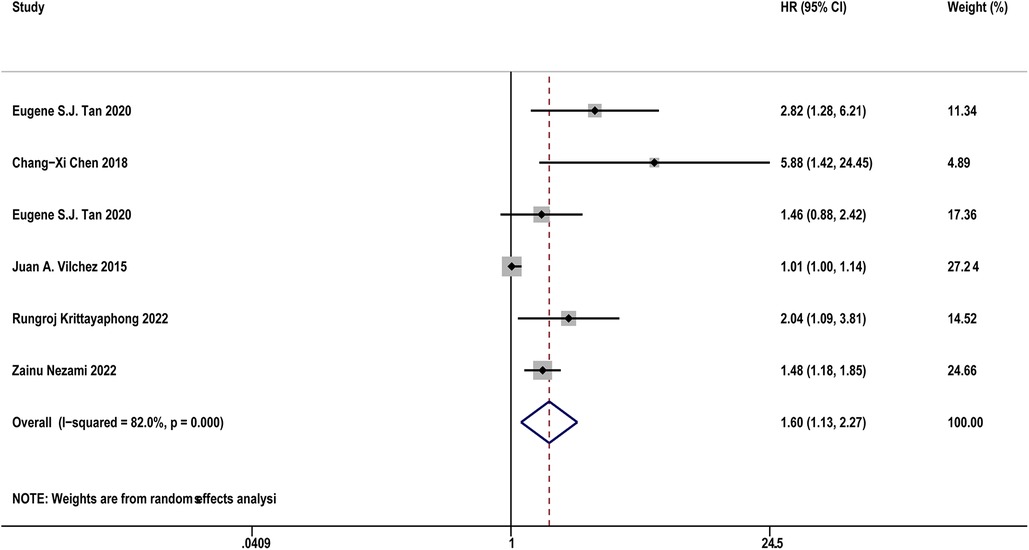
Figure 6. Forest plots show the relationship between sST2 (continuous variables) and the risk of MACEs following AF.
4 Discussion
Our study found that sST2 was associated with the risk of occurrence, recurrence, and MACEs of AF. For every 1 unit increase in sST2, the risk of occurrence, recurrence, and MACEs increased by 4%, 9%, and 60%, respectively. Furthermore, patients with AF showed higher sST2 than controls without AF, and AF patients with recurrence showed significantly higher sST2 than those without recurrence. Sensitivity analyses showed that the outcomes were stable.
AF is partially explained by atrial remodeling determined by myocardial hypertension, dilatation, infiltration, inflammation and fibrosis (38, 39). Atrial remodeling acts in concert with the arrhythmia itself to enhance atrial vulnerability to AF, and might be both cause and consequence of AF (40, 41). Atrial fibrosis is a defining structural feature of atrial remodeling, a process that includes extracellular matrix (ECM) and collagen deposition accumulation and is thought to be initiated and regulated by immune cells (42, 43). Identifying biomarkers associated with atrial fibrosis will increase our understanding of the pathophysiological mechanisms of AF and could be used to develop pharmacological pathways for the prevention of AF, in addition, adding these biomarkers to the risk scale may lead to more accurate predictions of AF risk (44). sST2, as a biomarker closely related to fibrosis and inflammation, plays an important role in the pathogenesis and progression of AF, and also has certain value in predicting the occurrence, progression, recurrence and prognosis of AF.
Studies have found that sST2 reflect fibrosis may be related to the inhibition of IL-33/ST2l pathway (45, 46, 9). IL-33 is the functional ligand of ST2l, and the IL-33/ST2l signaling pathway has been shown plays a role in anti-myocardial fibrosis and cardiomyocyte hypertrophy (47, 48). sST2 is a receptor for IL-1 and competitively binds to IL-33 to inhibit the protective effect of IL-33/ST2l on myocardium (49). When myocardium induced by pressure or volume overload produce a large amount of sST2, high concentrations of sST2 prevent IL-33/ST2l effects and may therefore lead to atrial fibrosis, which is associated with AF via atrial structural remodeling (49, 50). Early atrial dilation in AF patients can lead to physiological stretching of the atrium, causes myofibroblasts to release IL-33, which binds ST2l to myocardial cell membranes and promotes cell integrity and survival. However, during long-term lesions, local and adjacent cells can increase the release of the IL-33 decoy sST2, thereby blocking IL-33/ST2l binding and promoting tissue fibrosis (51, 52). Notably, the novel aspect of IL-33/ST2l signaling mediating cardiac fibrosis represents some novel biomolecular targets for prevention and treatment of AF. In addition, sST2 can also cause myocardial damage by promoting oxidative stress and inflammation. sST2 affects mitochondrial fusion of human cardiac fibroblasts and increases oxidative stress production and inflammatory marker secretion by reducing mitofusins-1 (MFN-1) expression (53).
Fibrosis is associated with impaired cellular coupling, enhanced heterogeneity of intra-atrial conduction, and dispersion of refractory periods, which provide substrates for sustained reentry to drive AF (54, 43). We think that sST2 can reflect fibrosis as well as the degree of fibrosis. Studies have found that the expression of sST2 in patients with persistent AF is higher than that in patients with paroxysmal AF, which may explain that sST2 reflects the degree of fibrosis and the progression of AF (55, 56). Recurrence after AF ablation is directly related to atrial fibrosis and is positively correlated (57). Our study found that sST2 level is an independent predictor of AF recurrence after CA, and every 1 unit increase in sST2, the risk of recurrence increased by 9%. sST2 is also directly associated with MACEs of AF patients. Increased abnormal hemodynamic load leads to atrial dilation, which is a well-known cause of the development of AF, and may also stimulate sST2 and BNP secretion. In addition, sST2 levels are elevated during AF, possibly because the heart rate and atrial pressure in patients with AF are higher than normal, thereby increasing cardiac burden and increasing the risk of MACEs (58, 59). IL-33/ST2l signaling is thought to play an important role in regulating the myocardial response of stretched cardiac fibroblasts and cardiomyocytes to biomechanical overload (60). Loss of IL-33/ST2l signaling leads to hypertrophy of cardiomyocytes, fibrosis, and deterioration of left ventricular function, further aggravating ventricular myocardial remodeling, and increasing the risk of death from HF. Our results show that sST2 has a stronger correlation with the risk of MACEs (HR = 1.60) than the occurrence (HR = 1.04) and recurrence (HR = 1.09) of AF, indicating that sST2 has notable prognostic performance, but low diagnostic performance. We believe that sST2, as a new biomarker of inflammation, fibrosis and cardiac stress, may have a more direct correlation associated with cardiac damage and MACEs. And the prognostic information provided by sST2 is in addition to that provided by other well stablished biomarkers, such as BNP and troponins. However, the mechanism of AF occurrence and recurrence is complex, and sST2, although a marker of fibrosis, is not specific to atrial fibrosis, thus showing a weak clinical correlation.
As a new biomarker, galectin-3 (Gal-3) and sST2 have been found to play an important role in fibrosis. Gal-3 can specifically bind to ECM and fibroblasts, thereby promoting fibroblast proliferation, inflammatory cell infiltration, and remodeling (57). However, the current research is not very clear about the mechanism of Gal-3 and sST2 on atrial fibrosis, and future research should be performed to reveal their role and mechanism, and provide more new basis for the prevention and treatment of atrial fibrosis.
5 Strengths and limitations
Our study performed a detailed meta-analysis and mechanism analysis. First, this is the first meta-analysis to summarize the predictive value of sST2 in AF. Second, this study covers a large sample size from different countries, so the results are relatively stable and reliable. Third, the pooled analysis was based on the most adequately adjusted HRs, so the finding may be independent of potential confounders. Fourth, sensitivity analyses did not significantly affect the results, indicating the results were credible.
There were some limitations in our study. First, the meta-analysis only included observational studies, it carries inherent study design limitations. Second, the heterogeneity in meta-analyses were significant. We used sensitivity analysis and publication bias to explore the source of heterogeneity. Third, some residual factors may affect the results. Fourth, we did not find some suitable case reports to prove our study.
6 Conclusions
Higher sST2 was association with an increased risk of occurrence, recurrence, and MACEs in AF. Assessing sST2 can be used as a potential screening method to predict AF outcomes. Further well-designed cohort studies and randomized clinical trials are warranted to confirm this finding.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
PC: Writing – original draft. JZ: Writing – review & editing. JD: Conceptualization, Writing – original draft. DS: Conceptualization, Investigation, Writing – original draft. HZ: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study was supported by the project of Major New Drug Creation (No. 2018ZX09301-011-001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1308166/full#supplementary-material
References
1. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation. (2019) 139:e56–e528. doi: 10.1161/CIR.0000000000000659
2. Brundel BJJM, Ai X, Hills MT, Kuipers MF, Lip GYH, de Groot NMS. Atrial fibrillation. Nat Rev Dis Primers. (2022) 8(1):21. doi: 10.1038/s41572-022-00347-9
3. Erhard N, Metzner A, Fink T. Late arrhythmia recurrence after atrial fibrillation ablation: incidence, mechanisms and clinical implications. Späte arrhythmierezidive nach ablation von vorhofflimmern—inzidenz, mechanismen und klinische bedeutung. Herzschrittmacherther Elektrophysiol. (2022) 33(1):71–6. doi: 10.1007/s00399-021-00836-6
4. Sagris M, Vardas EP, Theofilis P, Antonopoulos AS, Oikonomou E, Tousoulis D. Atrial fibrillation: pathogenesis, predisposing factors, and genetics. Int J Mol Sci. (2021) 23(1):6. doi: 10.3390/ijms23010006
5. Wijesurendra RS, Casadei B. Mechanisms of atrial fibrillation. Heart. (2019) 105(24):1860–7. doi: 10.1136/heartjnl-2018-314267
6. Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. (2014) 114(9):1453–68. doi: 10.1161/CIRCRESAHA.114.303211
7. O'Neal WT, Venkatesh S, Broughton ST, Griffin WF, Soliman EZ. Biomarkers and the prediction of atrial fibrillation: state of the art. Vasc Health Risk Manag. (2016) 12:297–303. doi: 10.2147/VHRM.S75537
8. Pascual-Figal DA, Januzzi JL. The biology of ST2: the international ST2 consensus panel. Am J Cardiol. (2015) 115(7 Suppl):3B–7B. doi: 10.1016/j.amjcard.2015.01.034
9. Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, et al. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. (2002) 106(23):2961–6. doi: 10.1161/01.CIR.0000038705.69871.D9
10. Kuehn BM. Biomarkers may help stratify patient heart failure risk, guide treatment. Circulation. (2020) 141(5):399–400. doi: 10.1161/CIRCULATIONAHA.119.045477
11. Cui Y, Qi X, Huang A, Li J, Hou W, Liu K. Differential and predictive value of galectin-3 and soluble suppression of tumorigenicity-2 (sST2) in heart failure with preserved ejection fraction. Med Sci Monit. (2018) 24:5139–46. doi: 10.12659/MSM.908840
12. Merino-Merino A, Saez-Maleta R, Salgado-Aranda R, AlKassam-Martinez D, Pascual-Tejerina V, Martin-Gonzalez J, et al. A differential profile of biomarkers between patients with atrial fibrillation and healthy controls. J Pers Med. (2022) 12(9):1406. doi: 10.3390/jpm12091406
13. Kalstad AA, Myhre PL, Laake K, Opstad TB, Tveit A, Solheim S, et al. Biomarkers of ageing and cardiac remodeling are associated with atrial fibrillation. Scand Cardiovasc J. (2021) 55(4):213–9. doi: 10.1080/14017431.2021.1889653
14. Chen BX, Xie B, Zhou Y, Shi L, Wang Y, Zeng L, et al. Association of serum biomarkers and cardiac inflammation in patients with atrial fibrillation: identification by positron emission tomography. Front Cardiovasc Med. (2021) 8:735082. doi: 10.3389/fcvm.2021.735082
15. Margulis AV, Pladevall M, Riera-Guardia N, Varas-Lorenzo C, Hazell L, Berkman ND, et al. Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle-Ottawa scale and the RTI item bank. Clin Epidemiol. (2014) 6:359–68. doi: 10.2147/CLEP.S66677
16. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. (2011) 64(4):383–94. doi: 10.1016/j.jclinepi.2010.04.026
17. Chen C, Qu X, Gao Z, Zheng G, Wang Y, Chen X, et al. Soluble ST2 in patients with nonvalvular atrial fibrillation and prediction of heart failure. Int Heart J. (2018) 59(1):58–63. doi: 10.1536/ihj.16-520
18. Tan ESJ, Chan SP, Liew OW, Chong JPC, Leong GKT, Yeo DPS, et al. Atrial fibrillation and the prognostic performance of biomarkers in heart failure. Clin Chem. (2021) 67(1):216–26. doi: 10.1093/clinchem/hvaa287
19. Liu H, Wang K, Lin Y, Liang X, Zhao S, Li M, et al. Role of sST2 in predicting recurrence of atrial fibrillation after radiofrequency catheter ablation. Pacing Clin Electrophysiol. (2020) 43(11):1235–41. doi: 10.1111/pace.14029
20. Budzianowski J, Hiczkiewicz J, Łojewska K, Kawka E, Rutkowski R, Korybalska K. Biomarkers of early-recurrence atrial fibrillation after catheter ablation in women and men with abnormal body weight. J Clin Med. (2021) 10(12):2694. doi: 10.3390/jcm10122694
21. Sieweke JT, Pfeffer TJ, Biber S, Chatterjee S, Weissenborn K, Grosse GM, et al. miR-21 and NT-proBNP correlate with echocardiographic parameters of atrial dysfunction and predict atrial fibrillation. J Clin Med. (2020) 9(4):1118. doi: 10.3390/jcm9041118
22. Fan J, Li Y, Yan Q, Wu W, Xu P, Liu L, et al. Higher serum sST2 is associated with increased left atrial low-voltage areas and atrial fibrillation recurrence in patients undergoing radiofrequency ablation. J Interv Card Electrophysiol. (2022) 64(3):733–42. doi: 10.1007/s10840-022-01153-9
23. Vílchez JA, Pérez-Cuellar M, Marín F, Gallego P, Manzano-Fernández S, Valdés M, et al. sST2 levels are associated with all-cause mortality in anticoagulated patients with atrial fibrillation. Eur J Clin Invest. (2015) 45(9):899–905. doi: 10.1111/eci.12482
24. Lamprea-Montealegre JA, Zelnick LR, Shlipak MG, Floyd JS, Anderson AH, He J, et al. Cardiac biomarkers and risk of atrial fibrillation in chronic kidney disease: the CRIC study. J Am Heart Assoc. (2019) 8(15):e012200. doi: 10.1161/JAHA.119.012200
25. Chen L, Chen W, Shao Y, Zhang M, Li Z, Wang Z, et al. Association of soluble suppression of tumorigenicity 2 with new-onset atrial fibrillation in acute myocardial infarction. Cardiology. (2022) 147(4):381–8. doi: 10.1159/000524765
26. Badoz M, Serzian G, Favoulet B, Sellal JM, De Chillou C, Hammache N, et al. Impact of midregional N-terminal pro-atrial natriuretic peptide and soluble suppression of tumorigenicity 2 levels on heart rhythm in patients treated with catheter ablation for atrial fibrillation: the biorhythm study. J Am Heart Assoc. (2021) 10(13):e020917. doi: 10.1161/JAHA.121.020917
27. Rienstra M, Yin X, Larson MG, Fontes JD, Magnani JW, McManus DD, et al. Relation between soluble ST2, growth differentiation factor-15, and high-sensitivity troponin I and incident atrial fibrillation. Am Heart J. (2014) 167(1):109–115.e2. doi: 10.1016/j.ahj.2013.10.003
28. Bansal N, Zelnick LR, Soliman EZ, Anderson A, Christenson R, DeFilippi C, et al. Change in cardiac biomarkers and risk of incident heart failure and atrial fibrillation in CKD: the chronic renal insufficiency cohort (CRIC) study. Am J Kidney Dis. (2021) 77(6):907–19. doi: 10.1053/j.ajkd.2020.09.021
29. Wałek P, Gorczyca I, Grabowska U, Spałek M, Wozakowska-Kaplon B. The prognostic value of soluble suppression of tumourigenicity 2 and galectin-3 for sinus rhythm maintenance after cardioversion due to persistent atrial fibrillation in patients with normal left ventricular systolic function. Europace. (2020) 22(10):1470–9. doi: 10.1093/europace/euaa135
30. Krittayaphong R, Pumprueg S, Sairat P. Soluble ST2 in the prediction of heart failure and death in patients with atrial fibrillation. Clin Cardiol. (2022) 45(4):447–56. doi: 10.1002/clc.23799
31. Tan R, Yu H, Han X, Liu Y, Yang X, Xia YL, et al. Circulating soluble suppression of tumorigenicity 2 predicts recurrence after radiofrequency ablation of persistent atrial fibrillation. Front Cardiovasc Med. (2021) 8:653312. doi: 10.3389/fcvm.2021.653312
32. Nortamo S, Ukkola O, Lepojärvi S, Kenttä T, Kiviniemi A, Junttila J, et al. Association of sST2 and hs-CRP levels with new-onset atrial fibrillation in coronary artery disease. Int J Cardiol. (2017) 248:173–8. doi: 10.1016/j.ijcard.2017.07.022
33. Okar S, Kaypakli O, Sahin DY, Koç M. Fibrosis marker soluble ST2 predicts atrial fibrillation recurrence after cryoballoon catheter ablation of nonvalvular paroxysmal atrial fibrillation. Korean Circ J. (2018) 48(10):920–9. doi: 10.4070/kcj.2018.0047
34. Sun WP, Du X, Chen JJ. Biomarkers for predicting the occurrence and progression of atrial fibrillation: soluble suppression of tumorigenicity 2 protein and tissue inhibitor of matrix metalloproteinase-1. Int J Clin Pract. (2022) 2022:6926510. doi: 10.1155/2022/6926510
35. Sun W, Li H, Wang Z, Wu Y, Du J. Clinical and laboratory biomarkers in paroxysmal atrial fibrillation: a single center cross-sectional study. Contrast Media Mol Imaging. (2022) 2022:7012377. doi: 10.1155/2022/7012377
36. Yan X, Guo Y, Li L, Wang Z, Li Z. The sST2 level is an independent influencing factor associated with atrial fibrillation in heart failure patients: a case-control study. J Thorac Dis. (2022) 14(5):1578–87. doi: 10.21037/jtd-22-470
37. Nezami Z, Holm H, Ohlsson M, Molvin J, Korduner J, Bachus E, et al. The impact of myocardial fibrosis biomarkers in a heart failure population with atrial fibrillation-the HARVEST-malmö study. Front Cardiovasc Med. (2022) 9:982871. doi: 10.3389/fcvm.2022.982871
38. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. (2021) 42(5):373–498. doi: 10.1093/eurheartj/ehaa612
39. Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace. (2016) 18(10):1455–90. doi: 10.1093/europace/euw161
40. Sohns C, Marrouche NF. Atrial fibrillation and cardiac fibrosis. Eur Heart J. (2020) 41(10):1123–31. doi: 10.1093/eurheartj/ehz786
41. Quah JX, Dharmaprani D, Tiver K, Lahiri A, Hecker T, Perry R, et al. Atrial fibrosis and substrate based characterization in atrial fibrillation: time to move forwards. J Cardiovasc Electrophysiol. (2021) 32(4):1147–60. doi: 10.1111/jce.14987
42. Lin R, Wu S, Zhu D, Qin M, Liu X. Osteopontin induces atrial fibrosis by activating akt/GSK-3β/β-catenin pathway and suppressing autophagy. Life Sci. (2020) 245:117328. doi: 10.1016/j.lfs.2020.117328
43. Nattel S. Molecular and cellular mechanisms of atrial fibrosis in atrial fibrillation. JACC Clin Electrophysiol. (2017) 3(5):425–35. doi: 10.1016/j.jacep.2017.03.002
44. Staerk L, Preis SR, Lin H, Lubitz SA, Ellinor PT, Levy D, et al. Protein biomarkers and risk of atrial fibrillation: the FHS. Circ Arrhythm Electrophysiol. (2020) 13(2):e007607. doi: 10.1161/CIRCEP.119.007607
45. Bartunek J, Delrue L, Van Durme F, Muller O, Casselman F, De Wiest B, et al. Nonmyocardial production of ST2 protein in human hypertrophy and failure is related to diastolic load. J Am Coll Cardiol. (2008) 52(25):2166–74. doi: 10.1016/j.jacc.2008.09.027
46. Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. (2007) 117(6):1538–49. doi: 10.1172/JCI30634
47. Pascual-Figal DA, Lax A, Perez-Martinez MT, del Carmen, Asensio-Lopez M, Sanchez-Mas J, GREAT Network. Clinical relevance of sST2 in cardiac diseases. Clin Chem Lab Med. (2016) 54(1):29–35. doi: 10.1515/cclm-2015-0074
48. Merino-Merino A, Gonzalez-Bernal J, Fernandez-Zoppino D, Saez-Maleta R, Perez-Rivera JA. The role of galectin-3 and ST2 in cardiology: a short review. Biomolecules. (2021) 11(8):1167. doi: 10.3390/biom11081167
49. Sun Y, Pavey H, Wilkinson I, Fisk M. Role of the IL-33/ST2 axis in cardiovascular disease: a systematic review and meta-analysis. PLoS One. (2021) 16(11):e0259026. doi: 10.1371/journal.pone.0259026
50. Weinberg EO. ST2 protein in heart disease: from discovery to mechanisms and prognostic value. Biomark Med. (2009) 3(5):495–511. doi: 10.2217/bmm.09.56
51. Kotsiou OS, Gourgoulianis KI, Zarogiannis SG. IL-33/ST2 axis in organ fibrosis. Front Immunol. (2018) 9:2432. doi: 10.3389/fimmu.2018.02432
52. Vianello E, Dozio E, Tacchini L, Frati L, Corsi Romanelli MM. ST2/IL-33 signaling in cardiac fibrosis. Int J Biochem Cell Biol. (2019) 116:105619. doi: 10.1016/j.biocel.2019.105619
53. Matilla L, Ibarrola J, Arrieta V, Garcia-Peña A, Martinez-Martinez E, Sádaba R, et al. Soluble ST2 promotes oxidative stress and inflammation in cardiac fibroblasts: an in vitro and in vivo study in aortic stenosis. Clin Sci (Lond). (2019) 133(14):1537–48. doi: 10.1042/CS20190475
54. Benz AP, Aeschbacher S, Krisai P, Moschovitis G, Blum S, Meyre P, et al. Biomarkers of inflammation and risk of hospitalization for heart failure in patients with atrial fibrillation. J Am Heart Assoc. (2021) 10(8):e019168. doi: 10.1161/JAHA.120.019168
55. Liu Y, Niu XH, Yin X, Liu YJ, Han C, Yang J, et al. Elevated circulating fibrocytes is a marker of left atrial fibrosis and recurrence of persistent atrial fibrillation. J Am Heart Assoc. (2018) 7(6):e008083. doi: 10.1161/JAHA.117.008083
56. Lau DH, Linz D, Schotten U, Mahajan R, Sanders P, Kalman JM. Pathophysiology of paroxysmal and persistent atrial fibrillation: rotors, foci and fibrosis. Heart Lung Circ. (2017) 26(9):887–93. doi: 10.1016/j.hlc.2017.05.119
57. Marrouche NF, Wazni O, McGann C, Greene T, Dean JM, Dagher L, et al. Effect of MRI-guided fibrosis ablation vs conventional catheter ablation on atrial arrhythmia recurrence in patients with persistent atrial fibrillation: the DECAAF II randomized clinical trial. JAMA. (2022) 327(23):2296–305. doi: 10.1001/jama.2022.8831
58. Gaggin HK, Januzzi JL Jr. Biomarkers and diagnostics in heart failure. Biochim Biophys Acta. (2013) 1832(12):2442–50. doi: 10.1016/j.bbadis.2012.12.014
59. Yamada T, Murakami Y, Okada T, Yoshida N, Toyama J, Yoshida Y, et al. Plasma brain natriuretic peptide level after radiofrequency catheter ablation of paroxysmal, persistent, and permanent atrial fibrillation. Europace. (2007) 9(9):770–4. doi: 10.1093/europace/eum157
Keywords: soluble suppression of tumorigenicity 2, atrial fibrillation, predictive, occurrence, recurrence, MACEs, meta-analysis, systematic review
Citation: Chen P, Zhang J, Du J, Shi D and Zhang H (2024) Predictive value of soluble suppression of tumorigenicity 2 in atrial fibrillation: a systematic review and meta-analysis. Front. Cardiovasc. Med. 10:1308166. doi: 10.3389/fcvm.2023.1308166
Received: 14 October 2023; Accepted: 22 December 2023;
Published: 11 January 2024.
Edited by:
Emma Louise Robinson, University of Colorado, United StatesReviewed by:
Nikolaos Fragakis, Aristotle University of Thessaloniki, GreeceNavin Suthahar, Erasmus Medical Center, Netherlands
© 2024 Chen, Zhang, Du, Shi and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dazhuo Shi c2hpZGF6aHVvQDEyNi5jb20= He Zhang emhoZTExMTJAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Pengfei Chen
Pengfei Chen Jie Zhang1,†
Jie Zhang1,† Jianpeng Du
Jianpeng Du Dazhuo Shi
Dazhuo Shi He Zhang
He Zhang