
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 29 November 2023
Sec. Pediatric Cardiology
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1290482
This article is part of the Research Topic Rising Stars in Pediatric Cardiology 2023 View all 12 articles
Background: Cardioneuroablation (CNA) is recognized as a promising therapeutic option for adults with severe symptomatic bradycardia caused by excessive vagal tone. However, no pediatric cases have been reported to date. Therefore, the aim of this study is to evaluate the feasibility and efficacy of CNA in children.
Methods: A 12-year-old male patient was hospitalized with symptoms of fatigue, palpitations, and syncope for more than 2 months, and was definitively diagnosed with functional sinoatrial node dysfunction by using a 12-lead electrocardiogram, 24-h Holter monitoring, loading dose of atropine test (0.04 mg/kg), and treadmill exercise test. Simultaneously, whole-exome sequencing was performed on the child and his core family members. After completing the preoperative examination and signing the informed consent form, the child underwent CNA therapy.
Results: First, the electroanatomic structures of both atria were mapped out by using the Carto 3 system, according to the protocol of purely anatomy-guided and local fractionated intracardiac electrogram–guided CNA methods. Then, the local fractionated intracardiac electrograms of each cardiac ganglionated plexus (GP), including the GP between the aortic root and the medial wall of the superior vena cava, the GP between the posterior wall of the coronary sinus ostium and the left atrium, the GP between the anterior antrum of the right superior pulmonary vein and the superior vena cava, the GP in the superolateral area around the root of the left superior pulmonary vein, the GP around the root of the right inferior pulmonary vein, and the GP around the root of the left inferior pulmonary vein, were used as targets for ablation at a power of 30 W with an ablation index of 350–400. At a 6-month follow-up, the child's heart rhythm saw a complete restoration to sinus rhythm and clinical symptoms disappeared.
Conclusion: The first application of CNA in a child with symptomatic sinus bradycardia was achieved with better clinical outcomes. CNA can be carried out cautiously in children under suitable indications.
Severe bradycardia with different etiologies can lead to serious clinical manifestations such as fatigue, palpitations, syncope, and even sudden death. The traditional therapy for patients with severe bradycardia is cardiac pacemaker implantation. However, when these patients are adolescents, or much younger, the children or their guardians refuse pacemaker implantations because of concerns about long-term complications (1–3). In recent years, studies have reported that cardioneuroablation (CNA) has become a promising therapeutic strategy for bradycardia caused by excessive vagal tone, and has achieved good clinical outcomes in adults (4–6). However, so far no case of a pediatric patient receiving CNA therapy has been reported. This study aims to fill this gap by presenting the case of a child with sinus arrest, sinus bradycardia, and junctional escape rhythms and showing improvement in clinical outcomes with the relieving of symptoms and rhythm disorders using CNA therapy.
A 12-year-old male patient was hospitalized with symptoms of fatigue, palpitations, and syncope for more than 2 months. The 12-lead electrocardiogram (ECG) showed sinus bradycardia and junctional escape rhythm (Figure 1A). A 24 h Holter monitoring displayed a total of 62,108 heartbeats in 24 h, with 5,094 sinus arrests greater than 2 s and 971 sinus arrests greater than 3 s, with the longest cardiac arrest time being 7.424 s (Figure 1B). The child had no history of myocarditis prior to the onset of the disease, no structural cardiac abnormalities, no similar family history, and no positive findings on laboratory examinations. Normal sinus rhythm appeared after a loading dose of atropine test (0.04 mg/kg) and treadmill exercise test (Figure 1C). The child was diagnosed as having functional sinoatrial node dysfunction possibly due to excessive vagal tone based on the above results. Meanwhile, to exclude inherited heart disease, blood samples from the child, his elder sister, and his parents were collected, and whole-exome sequencing (WES) was performed by MyGenostics (Beijing, China) using the Illumina HiSeq X ten system. After strongly rejecting pacemaker implantation, the child's parents agreed to CNA therapy, for which they signed an informed consent form.
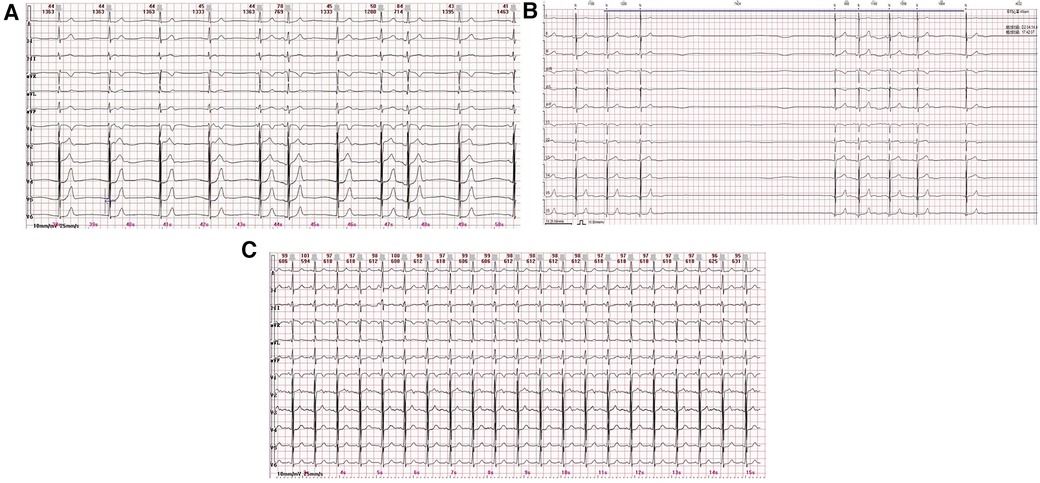
Figure 1. (A) 12-lead ECG displays a sinus bradycardia and junctional escape rhythm. (B) The 24 h Holter monitoring displays a sinus arrest of 7.424 s. (C) Normal sinus rhythm appears by a loading dose of atropine test (only the ECG graph after 1 min of atropine administration is displayed).
After a satisfactory administration of anesthesia, the bilateral femoral veins were punctured successfully and a quadripolar electrode catheter was inserted into the right ventricle for an intracardiac electrophysiological study and right ventricle pacing. When severe bradycardia occurred in the patient during the procedure, a decapolar steerable electrode catheter was inserted into the coronary sinus for the intracardiac electrophysiological study. Surface ECG and intracardiac electrograms were continually monitored by using a multichannel recording system during the whole procedure (LEAD-7000; JJET, Chengdu, China). The three-dimensional (3D) electroanatomic structures of both atria were mapped out by using the Carto 3 system (Biosense Webster, Diamond Bar, CA, USA). The sinus atrial node, phrenic nerve, coronary sinus, and His bundle were labeled. A Thermocool® ST (Biosense Webster, Diamond Bar, CA, USA) irrigated catheter was inserted in the right/left atrium for the ablation therapy. According to the protocol reported by Pachon et al. and Aksu et al. (3, 7), we administered the CNA therapy by sequentially targeting the ganglionated plexus (GP) between the aortic root and the medial wall of the superior vena cava (Ao-SVC GP), the GP between the posterior wall of the coronary sinus ostium and the left atrium (PMLGP), the GP between the anterior antrum of the right superior pulmonary vein and the superior vena cava (RAGP), the GP in the superolateral area around the root of the left superior pulmonary vein (LSGP), the GP around the root of the right inferior pulmonary vein (RIGP), and the GP around the root of the left inferior pulmonary vein (LIGP) (Figure 2A). The local fractionated intracardiac electrograms (Figure 2B) of each cardiac GP, including Ao-SVC GP, PMLGP, RAGP, LSGP, RIGP, and LIGP, were used as ablation targets, and the radio frequency of the GPs was performed in the power-controlled mode in a point-by-point fashion through the irrigated ST catheter. Energy was delivered at a power of 30 W for 30 s with an ablation index of 350–400. The ablation endpoint was the complete elimination of local fractionated intracardiac electrograms in each GP. Low-molecular heparin was administrated intravenously at 100 IU/kg during the CNA procedure with an additional half-dose of over 2 h of operating time. The duration of radiofrequency ablation in the child was 3 h with a fluoroscopy time of 11 m and dose of 5.4 mGy. Aspirin antiplatelet aggregation was routinely administered for 6 months after the CNA procedure.
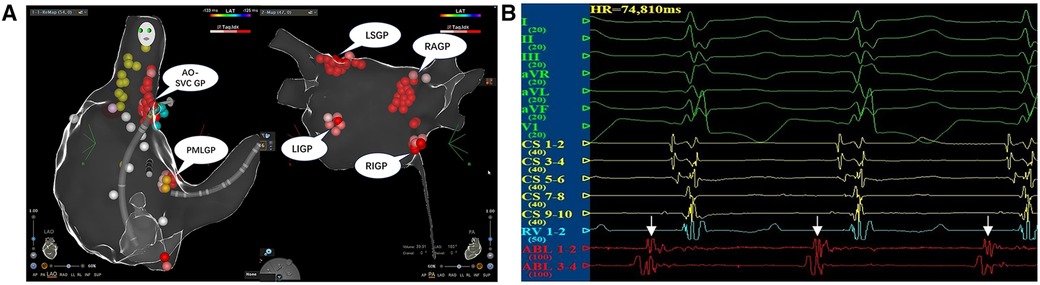
Figure 2. (A) The CNA procedure. The local fractionated intracardiac electrograms of each cardiac ganglionated plexus, including Ao-SVC GP, PMLGP, RAGP, LSGP, RIGP, and LIGP, were used as targets for ablation using a power of 30 W for 30 s with an ablation index of 350–400. (B) The local fractionated intracardiac electrograms of the ablation catheter from AO-SVC GP were used as the target potential during the CNA procedure.
After the completion of the purely anatomy-guided and local fractionated intracardiac electrogram–guided CNA procedure in the locations of the GPs, the child's surface ECG and intracardiac electrograms instantly shifted to sinus rhythm (Figure 3A). One week after the CNA procedure, the 12-lead ECG still presented sinus bradycardia and junctional escape rhythm, but the 24-h Holter monitoring displayed a total of 78,434 heartbeats in 24 h, with 143 sinus arrests greater than 2 s and one sinus arrest greater than 3 s, with the longest cardiac arrest time being 3.780 s (Figure 3B). Meanwhile, the child's palpitations were relieved significantly. At the 3-month follow-up, the child's 12-lead ECG completely restored to normal sinus rhythm, and 24 h Holter monitoring displayed a total of 120,457 heartbeats in 24 h without sinus arrest. At the 6-month follow-up, the child's 12-lead ECG still remained in normal sinus rhythm, and the 24 h Holter monitoring displayed a total of 102,912 heartbeats in 24 h without sinus arrest. Interestingly, the child's palpitations and syncope disappeared completely. A comparison of Holter features before and after the CNA procedure revealed a gradual increase in the total number of heartbeats, a marked improvement in cardiac rhythm, and a gradual disappearance of sinus arrests (Table 1). The deceleration capacity (DC) of the heart rate, a new technique for assessing autonomic tone, is used to quantitatively assess vagal tone by analyzing the overall trend of sinus rhythm over a 24 h period. Normally, DC is positively correlated with vagal tone, but in our patient, the DC was 14.4 before CNA treatment and only 8.3 after CNA treatment, which confirmed that the child's symptoms and rhythm disturbances were closely related to high vagal tone.
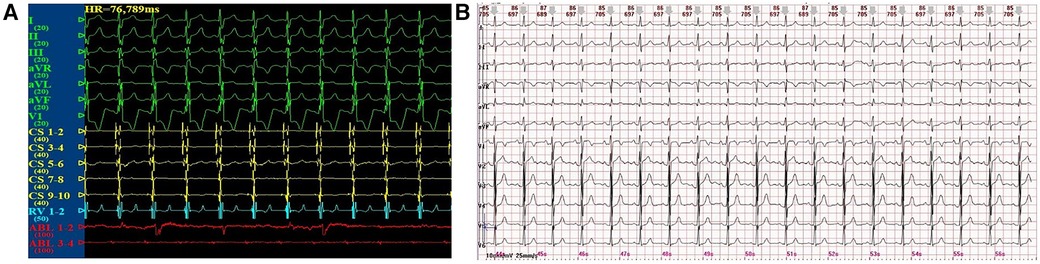
Figure 3. (A) Sinus rhythm after the CNA procedure. (B) 12-lead ECG at the 6-month follow-up after the CNA procedure.
The child’s parents and sister had no previous history of similar illnesses. WES was performed on the child and his core family members. It showed the child had a novel c.611 + 1G>T heterozygous mutation in the SCN5A gene (Figure 4), which resulted in a splicing mutation of amino acids. No abnormalities were found in the other family members.
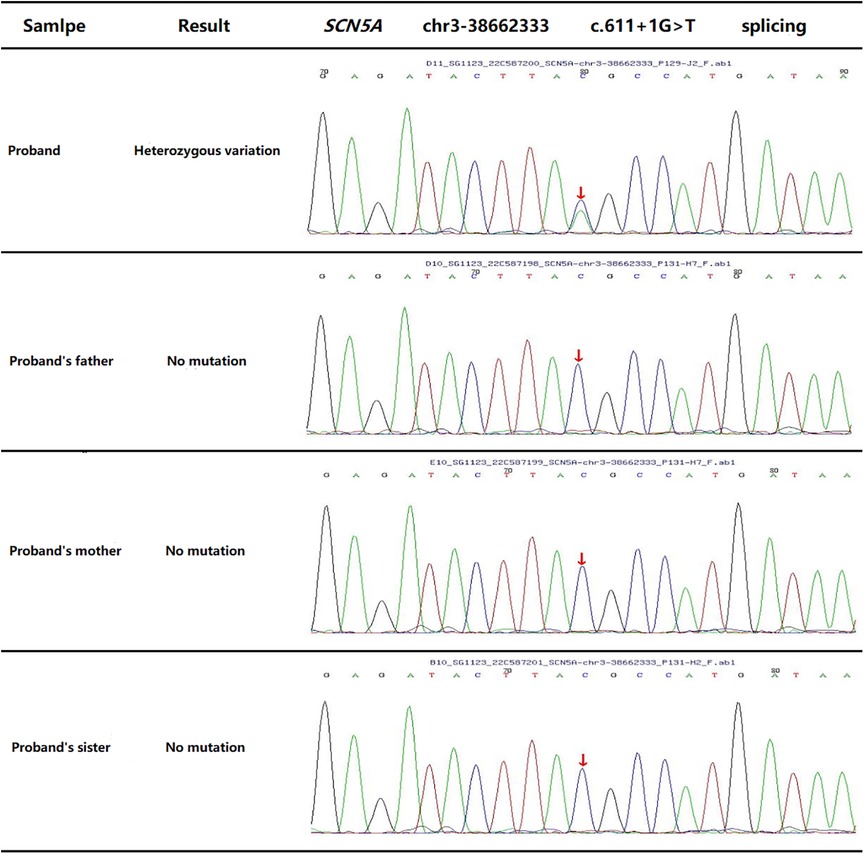
Figure 4. WES outcome of proband (the child) and his family members displays a novel c.611 + 1G>T heterozygous mutation in the SCN5A gene.
No intraoperative or postoperative puncture/ablation-related complications occurred in the child, such as vascular injury, arrhythmia, laryngeal reentrant nerve injury, and pericardial tamponade. Heart structure and function remained normal during follow-up.
This study displayed the first application of CNA in a child with symptomatic bradycardia. The child's heart rhythm was completely restored to sinus rhythm and clinical symptoms disappeared without any perioperative and long-term complications during the 6-month follow-up. Overall, this preliminary result was inspiring, showing that CNA may be safe and feasible for children with symptomatic bradycardia.
It is well-known that the autonomic nervous system plays an important role in cardiac functions. An imbalance in autonomic tone, mainly excessive vagal tone, is the common cause of functional sinoatrial node dysfunction (SND), functional atrioventricular block (AVB), and vasovagal syncope (VVS), except organic cardiac lesions (7–9). Traditionally, bradycardic patients who are at a high risk for clinically serious cardiovascular events if they developed hemodynamic disturbances may require pacemaker implantation to prevent sudden fatal events (1, 2). The cell bodies of cardiac efferent vagal postganglionic neurons have been shown to be contained in the intrinsic cardiac GP, which is embedded in the atrial wall and in epicardial fat pads (10, 11). Common GPs include Ao-SVC GP, PMLGP, RAGP, RIGP, LSGP, and LIGP with different physiological functions, and they interact with one another (6, 9, 10). Therefore, a disruption of nerve fibers by radiofrequency ablation can inhibit excessive activation of the vagal nerve, thereby eliminating or improving clinical symptoms. That is why CNA has proven to be a reliable therapeutic target for patients with excessive vagal tone.
In our study, our child patient presented with abnormal rhythm (sinus bradycardia, sinus arrest, and junctional escape rhythm) and symptoms of fatigue, palpitations, and syncope, while organic cardiac disorders and family history were excluded. Moreover, the resting heart rate of the child was approximately 58 bpm with sinus bradycardia and junctional escape rhythm, which rose to 97 bpm with sinus rhythm after atropine intravenous administration, which proved that the result of the atropine test was negative. Therefore, symptomatic bradycardia was considered to have been caused by excessive vagal tone. All these show that CNA is able to achieve better clinical outcomes.
Currently, there is a lack of guidelines for the selection of suitable candidates for CNA. Some authors have reported that indications for CNA in adults include the following: (1) patients with severe symptomatic bradycardia of functional SND or AVB [bradycardia was documented by performing ECG and 24 h Holter monitoring and was associated with excessive vagal tone with evidence of correcting the bradycardia by a loading dose of atropine], or patients with a symptomatic vasovagal syncope (cardioinhibitory type or mixed type), which was documented by performing a head-up tilt table test (HUT); (2) patients aged between 14 and 65 years; (3) those with no structural heart diseases; and (4) those who rejected the option of implanting a cardiac pacemaker on them (6). However, no indications were noted for CNA in children.
The child patient in our study had no structural heart diseases, except for a novel c.611 + 1G>T heterozygous mutation in the SCN5A gene. Many studies have reported that SCN5A, encoding the pore-forming ion-conducting α-subunit of the cardiac sodium channel (Nav1.5), was the causative gene for ion channel disease, including sick sinus syndrome, long Q-T syndrome, and Brugada syndrome (12–14). It is worth considering that the child had a heterozygous mutation in the SCN5A gene but a negative atropine test, along with a satisfactory clinical outcome and DC value reduction after CNA. However, further basic research is needed to clarify the relationship between the novel c.611 + 1G>T heterozygous mutation and disease development. Meanwhile, this study provides probable clinical evidence for the development of CNA indications in pediatric patients in the future.
There is no guideline for the therapeutic strategy, including labeling methods, ablation strategies, and ablation endpoints, during the CNA procedure. Purely anatomy-guided CNA (15, 16) and intracardiac high-frequency stimulation (HFS)-guided CNA (17) were clinically performed in patients with symptomatic bradycardia caused by excessive vagal tone. The former procedure tends to enlarge the ablation area first and the latter procedure perhaps causes myocardial scarring, leading to other types of arrhythmias. The latter easily causes severe discomfort to the patient during HFS. With regard to the target GPs and chamber during the performance of the CNA procedure, the team of Dr. Pachon and Dr. Aksu tended to ablate all of the atrial GP groups in both atria (4, 8, 16, 17), while Dr. Yao’s team (5) mainly focused on the GP groups in the left atrium, and Dr. Debruyne (18, 19) ablated the GP groups only in the right atrium. The determination of ablation endpoints has not been standardized to date. The techniques of inhibition of the vagal response, elimination of the intracardiac fractionated electrograms, and effecting improvements in electrophysiological parameters, such as increasing the heart rate and shortening the PR interval, have all been used by clinical investigators as ablation endpoints during the performance of the CNA procedure.
In our study, purely anatomy-guided CNA rather than intracardiac HFS–guided CNA was applied because most of the child's rhythms displayed junctional escape rhythms at the onset of the CNA procedure, all of the atrial GP locations in both atria were ablated, and the intracardiac fractionated electrograms in each GP location were used as ideal target intracardiac electrograms. Each ablation target employed 30 W of energy for 30 s with an ablation index of 350–400. Partial return to sinus rhythm and increased heart rate were used as ablation endpoints. The above ablation strategy helped achieve satisfactory clinical outcomes with no perioperative complications.
Despite our obtaining satisfactory clinical results at a 6-month follow-up in our study, the CNA technique is not without its problems and limitations. GPs located in the atrial epicardium, and the medium- and long-term effects (20, 21) of ablation at the endocardial surface, need to be observed before conclusions can be drawn. Meanwhile, a partial reinnervation of the cardiac ganglion after GP ablation in dogs has been reported in the literature (22). It has also been found that the regenerative capacity of nerve fibers is higher in children than in adults, which may be a high-risk factor for producing poor long-term results or recurrence. Hence, multi-center, large-sample clinical studies are needed for the application of CNA in pediatrics.
In our study, CNA was successfully performed in a child whose rhythm returned to sinus rhythm and clinical symptoms disappeared after 6 months of follow-up. CNA may be an ideal therapeutic strategy for severely symptomatic bradycardic children with appropriate indications. However, the long-term safety and efficacy of CNA in children need to be further evaluated.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
XX: Writing – original draft. SH: Writing – original draft. QL: Writing – original draft. RL: Writing – original draft. LZ: Writing – original draft. WC: Writing – review & editing. YY: Writing – review & editing. TL: Writing – original draft, Writing – review & editing.
The authors declare that financial support was received for the research, authorship, and/or publication of this article.
This study was supported by the National Natural Science Foundation of China (81570218), the Program for Youth Innovation in Future Medicine, Chongqing Medical University (w0176), and the National Clinical Key Specialty Construction Project (010140).
The authors would like to thank all those who participated in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer PF declared a past co-authorship with the author YY to the handling editor.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Varosy PD, et al. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society [published correction appears in Circulation 2019 Aug 20;140(8):e506-e508]. Circulation. (2019) 140(8):e382–482. doi: 10.1161/CIR.0000000000000628
2. Brignole M, Moya A, de Lange FJ, Deharo JC, Elliott PM, van Dijk JG, et al. 2018 ESC guidelines for the diagnosis and management of syncope. Eur Heart J. (2018) 39(21):1883–948. doi: 10.1093/eurheartj/ehy037
3. Sandhu RK, Raj SR, Thiruganasambandamoorthy V, Kaul P, Morillo CA, Sivilotti M, et al. Canadian cardiovascular society clinical practice update on the assessment and management of syncope. Can J Cardiol. (2020) 36(8):1167–77. doi: 10.1016/j.cjca.2019.12.023
4. Pachon JC, Pachon EI, Pachon JC, Lobo TJ, Pachon MZ, Jatene AD, et al. “Cardioneuroablation”–new treatment for neurocardiogenic syncope, functional AV block and sinus dysfunction using catheter RF-ablation. Europace. (2005) 7(1):1–13. doi: 10.1016/j.eupc.2004.10.003
5. Yao Y, Shi R, Wong T, Zheng L, Chen W, Zhang S, et al. Endocardial autonomic denervation of the left atrium to treat vasovagal syncope: an early experience in humans. Circ Arrhythm Electrophysiol. (2012) 5(2):279–86. doi: 10.1161/CIRCEP.111.966465
6. Chen W, Liu Z, Xiao P, Du H, Yin Y, Ling Z, et al. Extracardiac vagal stimulation-assisted cardioneuroablation: dynamically evaluating the impact of sequential ganglionated plexus ablation on vagal control of SAN and AVN in patients with sinoatrial node dysfunction. J Cardiovasc Dev Dis. (2022) 9(6):188. doi: 10.3390/jcdd9060188
7. Aksu T, Golcuk E, Yalin K, Guler TE, Erden I. Simplified cardioneuroablation in the treatment of reflex syncope, functional AV block, and sinus node dysfunction. Pacing Clin Electrophysiol. (2016) 39(1):42–53. doi: 10.1111/pace.12756
8. Aksu T, Guler TE, Yalin K, Mutluer FO, Ozcan KS, Calò L. Catheter ablation of bradyarrhythmia: from the beginning to the future. Am J Med Sci. (2018) 355(3):252–65. doi: 10.1016/j.amjms.2017.11.016
9. Alboni P, Holz A, Brignole M. Vagally mediated atrioventricular block: pathophysiology and diagnosis. Heart. (2013) 99(13):904–8. doi: 10.1136/heartjnl-2012-303220
10. Hanna P, Dacey MJ, Brennan J, Robbins S, Ardell JL, Shivkumar K, et al. Innervation and neuronal control of the mammalian sinoatrial node a comprehensive atlas. Circ Res. (2021) 128(9):1279–96. doi: 10.1161/CIRCRESAHA.120.318458
11. Pauza DH, Skripka V, Pauziene N, Stropus R. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat Rec. (2000) 259(4):353–82. doi: 10.1002/1097-0185(20000801)259:4%3C353::AID-AR10%3E3.0.CO;2-R
12. Wilde AAM, Amin AS. Clinical spectrum of SCN5A mutations: long QT syndrome, Brugada syndrome, and cardiomyopathy. JACC Clin Electrophysiol. (2018) 4(5):569–79. doi: 10.1016/j.jacep.2018.03.006
13. Benson DW, Wang DW, Dyment M, Knilans TK, Rhodes TH, George AL Jr, et al. Congenital sick sinus syndrome caused by recessive mutations in the cardiac sodium channel gene (SCN5A). J Clin Invest. (2003) 112(7):1019–28. doi: 10.1172/JCI18062
14. Wallace MJ, El Refaey M, Mesirca P, Hund TJ, Mangoni ME, Mohler PJ. Genetic complexity of sinoatrial node dysfunction. Front Genet. (2021) 12:654925. doi: 10.3389/fgene.2021.654925
15. Aksu T, De Potter T, John L, Osorio J, Gupta D, Davila A, et al. Procedural and short-term results of electroanatomic-mapping-guided ganglionated plexus ablation by first-time operators: a multicenter study. J Cardiovasc Electrophysiol. (2022) 33(1):117–22. doi: 10.1111/jce.15278
16. Aksu T, Gopinathannair R, Bozyel S, Yalin K, Gupta D. Cardioneuroablation for treatment of atrioventricular block. Circ Arrhythm Electrophysiol. (2021) 14(9):e010018. doi: 10.1161/CIRCEP.121.010018
17. Aksu T, Padmanabhan D, Shenthar J, Yalin K, Olshansky RB, Gopinathannair R, et al. The benefit of cardioneuroablation to reduce syncope recurrence in vasovagal syncope patients: a case-control study. J Interv Card Electrophysiol. (2022) 63(1):77–86. doi: 10.1007/s10840-020-00938-0
18. Debruyne P, Wijns W. Cardio-neuromodulation: the right-sided approach. JACC Clin Electrophysiol. (2017) 3(9):1056–7. doi: 10.1016/j.jacep.2016.12.027
19. Debruyne P, Rossenbacker T, Collienne C, Roosen J, Dewilde W, Wijns W, et al. Unifocal right-sided ablation treatment for neurally mediated syncope and functional sinus node dysfunction under computed tomographic guidance. Circ Arrhythm Electrophysiol. (2018) 11(9):e006604. doi: 10.1161/CIRCEP.118.006604
20. Aksu T, Guler TE, Bozyel S, Golcuk SE, Yalin K, Lakkireddy D, et al. Medium-term results of cardioneuroablation for clinical bradyarrhythmias and vasovagal syncope: effects on QT interval and heart rate. J Interv Card Electrophysiol. (2021) 60(1):57–68. doi: 10.1007/s10840-020-00704-2
21. Pachon-M JC, Pachon-M EI, Pachon CTC, Santillana-P TG, Lobo TJ, Pachon-M JC, et al. Long-term evaluation of the vagal denervation by cardioneuroablation using Holter and heart rate variability. Circ Arrhythm Electrophysiol. (2020) 13(12):e008703. doi: 10.1161/CIRCEP.120.008703
Keywords: cardioneuroablation (CNA), sinus bradycardia, sinus arrest, ganglionated plexus, vagal tone, children
Citation: Xu X, He S, Liu Q, Liu R, Zhang L, Chen W, Yin Y and Lu T (2023) Cardioneuroablation for successful treatment of symptomatic bradycardia in a 12-year-old child after a 6-month follow-up. Front. Cardiovasc. Med. 10:1290482. doi: 10.3389/fcvm.2023.1290482
Received: 7 September 2023; Accepted: 7 November 2023;
Published: 29 November 2023.
Edited by:
Liqun Sun, University of Toronto, CanadaReviewed by:
Dan Wichterle, Institute for Clinical and Experimental Medicine (IKEM), Czechia© 2023 Xu, He, Liu, Liu, Zhang, Chen, Yin and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tiewei Lu bHR3MjAwMTQ1QDE2My5jb20=
AbbreviationsAo-SVC GP, the GP between the aortic root and the medial wall of the superior vena cava; PMLGP, the GP between the posterior wall of the coronary sinus ostium and the left atrium; RAGP, the GP between the anterior antrum of the right superior pulmonary vein and the superior vena cava; LSGP, the GP in the superolateral area around the root of the left superior pulmonary vein; RIGP, the GP around the root of the right inferior pulmonary vein; LIGP, the GP around the root of the left inferior pulmonary vein.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.