- 1Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, Sichuan, China
- 2Traditional Chinese Medicine Regulating Metabolic Diseases Key Laboratory of Sichuan Province, Chengdu, Sichuan, China
Introduction: Reducing multiple cardiovascular risk factors is a key link and a challenging clinical problem to reduce the risk of cardiovascular complications and death in patients with diabetes. Currently, there is a lack of clinical studies on patients with diabetes combined with multiple risk factors. Traditional Chinese medicine is believed to have therapeutic effects that contribute to the comprehensive control of multiple cardiovascular factors. This study aims to provide evidence for the efficacy and safety of Shenqi compound (SQC) for early intervention in diabetic patients at high cardiovascular risk.
Methods and analysis: This study is a multicenter, randomized, double-blind, placebo-controlled trial. A total of 120 diabetic patients with high cardiovascular risk were enrolled in five research centers. After a 2-week run-in period, the intervention group received basic treatment and SQC granules, and the control group received basic treatment and placebo granules for a total of 24 weeks, with a 24-week follow-up. The endpoint outcomes are major adverse cardiovascular events and renal-related and peripheral vascular disease events. The primary efficacy outcome is carotid intima-media thickness, and the secondary efficacy outcomes are carotid shear stress, indicators of glucose and lipid metabolism, pancreatic islets function, hemorheology, traditional Chinese medicine syndrome score, and quality of life scale. Safety indicators and adverse events were used to assess the safety of SQC.
Discussion: This study comprehensively evaluated the efficacy and safety of SQC for early intervention in diabetic patients at high cardiovascular risk from the aspects of overall metabolic level, structure, and function of blood vessels, quality of life, and long-term follow-up of endpoint events, providing evidence-based evidence for the short-term efficacy and long-term benefits of early treatment to reduce the risk of diabetic cardiovascular complications.
Trial Registration: This trial is registered in the Chinese Clinical Trial Registry on March 9, 2023, https://www.chictr.org.cn/showproj.html?proj=192803 (No. ChiCTR2300069219).
1 Introduction
Global diabetes prevention and control strategies have gradually shifted the focus from glycemic control to improving cardiac and renal outcomes (1). Macrovascular disease, mainly atherosclerotic cardiovascular disease (ASCVD), is a major complication and cause of death in patients with type 2 diabetes mellitus (T2DM). The CAPTURE study has shown that the prevalence of cardiovascular disease (CVD) in people with T2DM is 34.8%, with the majority of cases being ASCVD (2). In total, 66.3% of patients with T2DM die from CVD (3). T2DM has been proven to be a CVD risk equivalent (4). The risk of CVD is two to four times higher in diabetic than non-diabetic people (5). The National Cholesterol Education Program shows that patients with T2DM have a more than 20% risk of developing coronary heart disease for the first time within 10 years (6, 7). Therefore, it is a key intervention to delay the deterioration of diabetes complications and reduce mortality to protect the vascular health of diabetic patients as early as possible to maximize cardiovascular benefits.
These risk factors for CVD in patients with T2DM include dyslipidemia, hypertension, obesity, overweight, smoking, and so on. Patients with T2DM often have a combination of these risk factors, which contributes to their high risk of CVD (8). The UKPDS study has shown that the risk factors for coronary artery disease in patients with T2DM are in the order of dyslipidemia, hypertension, hyperglycemia, and smoking (9). Combined control of multiple risk factors can reduce the risk of ASCVD and death in diabetic patients (10–12). However, due to the complexity of chronic disease management, these risk factors are difficult to control effectively in practice. For example, the CCMR-3B study showed that 42% of patients with T2DM had abnormal lipid metabolism, but only 55% received lipid-regulating therapy (8). On the other hand, multiple drug combinations lead to potential side effects, treatment burden, and cost, which can also limit clinical treatment. The trickier question is how to choose the rational clinical treatment options that should maximize benefit. A study has shown that intensive blood glucose control has a limited effect on reducing the risk of CVD and death in patients with T2DM, especially in those with a longer course of disease, older age, and a history of CVD or multiple cardiovascular risk factors (13). The Action to Control Cardiovascular Risk in Diabetes Blood Pressure study has shown that intensive blood pressure control in patients with T2DM did not reduce the total major atherosclerotic cardiovascular events but increased adverse events (14). In conclusion, how to reduce cardiovascular risk factors in this high-risk group is a challenging problem that needs to be addressed in the management of diabetes.
In China, a large number of diabetic patients are at high risk of CVD. Traditional Chinese medicine (TCM) has a wealth of experience in treating diabetes and macrovascular complications, with a variety of treatment methods including herbal compounds, acupuncture, and Tai Chi. We have conducted a meta-analysis of the efficacy and safety of Chinese herbal medicine as an add-on therapy for T2DM patients with carotid atherosclerosis and found that the combination of Chinese herbal medicine may have more advantages than Western medicine alone, which can further reduce carotid intima-media thickness (CIMT) and carotid plaque crouse score, regulate glucose and lipid metabolism, improve insulin resistance, and enhance islet β-cell function (15). However, due to a lack of high-quality clinical trials, the low level of evidence for existing treatment options makes it difficult to promote them widely. Shenqi compound (SQC) is a diabetes medicine used in China for more than 20 years and consists of a number of herbs, as shown in Table 1. A series of studies on the production, extraction methods, and active ingredients of SQC granules have been carried out previously (16–18). Clinical trials have shown that SQC has multiple regulatory benefits in treating diabetic patients by lowering blood glucose, regulating lipids, improving insulin resistance, regulating oxidative stress levels, and reducing carotid intima thickness (19, 20). Further animal studies have shown that SQC can reduce glucose variability, ameliorate gut mucosal barrier damage, regulate gut microbiota and metabolites, protect pancreatic islet function in Goto-Kakizaki rats, and ameliorate vascular damage through mechanisms related to improved apoptosis, oxidative stress, and inflammatory microenvironment in the thoracic aorta (21–24). It also showed significant vasoprotective effects in diabetic mice and was superior to rosiglitazone (25). SQC has shown good cardiovascular protective effects and has been included in the TCM Diagnosis and Treatment Protocol for T2DM by the China National Clinical Research Base of TCM for diabetes. There is an urgent need for a large sample, multicenter randomized controlled trial to obtain high-quality evidence-based proof for patients with diabetes who are at high risk of CVD.
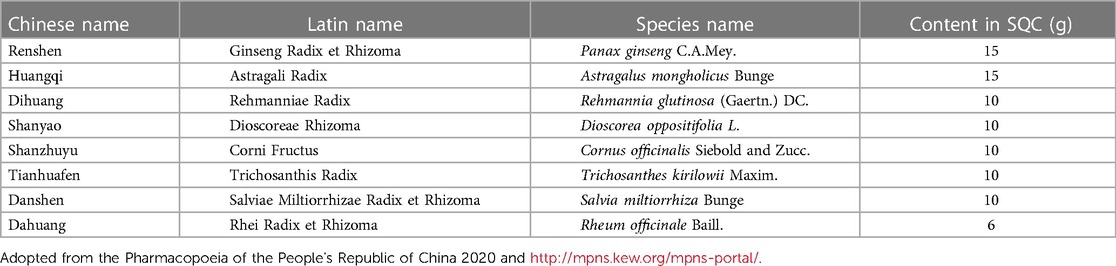
Table 1. Chinese name, Latin name, and species name of each Chinese medicine herb and content in Shenqi compound.
This trial focuses on diabetic patients at high cardiovascular risk and provides evidence-based evidence of the efficacy and safety of SQC in their treatment, provides high-quality evidence-based evidence of the short-term efficacy and long-term benefits of early intervention in TCM in diabetic patients at high cardiovascular risk, and provides solutions to the cardiovascular benefit needs of clinical diabetes management and control.
2 Methods and analyses
2.1 Study design
This study is a multicenter, randomized, double-blind, placebo-parallel controlled clinical trial. Changes in outcomes from baseline, the CIMT, glycometabolism, lipid metabolism, islet function, hemorheology, carotid ultrasound, carotid shear force, TCM syndrome score, and life quality score to provide evidence-based evidence of the efficacy and safety of early intervention of SQC in diabetic patients at high cardiovascular risk.
In this trial, 120 patients with T2DM at high cardiovascular risk were enrolled in five centers. After a 2-week run-in period, participants will be randomized 1:1 to receive SQC or placebo for 24 weeks. The trial flow is shown in Supplementary Figure S1, and the schedule is summarized in Supplementary Table S1 (see Additional File 1). Randomization, allocation concealment, blinding, and data analysis will be entrusted to external experts from the Chinese Evidence-based Medicine Center (the Chinese Cochrane Center), who are not involved in the clinical conduct of this trial. They will act as data monitors and conduct local and email monitoring every 3 months. This study design is in accordance with the Consolidated Standards of Reporting Trials (CONSORT) extension for Chinese herbal medicine formulas and Standard Protocol Items for Clinical Trials with TCM (SPIRIT-TCM Extension 2018) (26, 27).
2.2 Inclusion criteria
1. According to the Guideline for the Prevention and Treatment of T2DM in China published by the Chinese Diabetes Society, patients diagnosed with T2DM were enrolled (5).
2. According to the Guiding Principles for New Drug Clinical Research of TCM, the Guidelines for Prevention and Treatment of Diabetes in TCM, and the diagnostic scale for Qi and Yin deficiency syndrome of diabetes, patients who meet the diagnostic criteria for TCM Qi and Yin deficiency syndromes have the following symptoms: thirst, fatigue and weakness, bulimia, talking laziness, heat in palms, chest, and soles, red tongue with thin white dry fur, and thin and weak pulse (28–30).
3. Patients who were between the ages of 18 and 65 years were included.
4. Patients with CIMT ≥ 1 mm on color Doppler ultrasound were included.
5. Patients who had at least two cardiovascular risk factors (31):
(1) Hypertension: patients who had been diagnosed with hypertension and current blood pressure ≥ 130/80 mmHg when in an unmedicated state.
(2) Dyslipidemia: patients who had low-density lipoprotein cholesterol (LDL-C) > 2.6 mmol/L and at least one of the following conditions: non-HDL-C > 3.4 mmol/L, total cholesterol (TC) > 4.5 mmol/L, triglycerides (TG) > 1.7 mmol/L, or high-density lipoprotein cholesterol (HDL-C) < 1.04 mmol/L.
(3) Obesity/overweight: patients who had body mass index (BMI) ≥ 24 kg/m2 or central obesity (waist circumference ≥90 cm for men and ≥85 cm for women).
(4) Smoking history: patients who have smoked continuously or cumulatively for more than 6 months and are still currently smoking.
(5) Patients who were ≥ 40 years old.
(6) Patients for whom the course of diabetes has been more than 5 years.
6. Patients who participated voluntarily and signed the informed consent form.
2.3 Exclusion criteria
1. Patients who have had an acute metabolic disorder, such as diabetic ketoacidosis, or stringent state in the month before the screening.
2. Patients whose previous coronary angiography confirmed the diagnosis of coronary heart disease, whose electrocardiogram or clinical symptoms suggested coronary heart disease, or whose previous cerebrovascular accident was confirmed, or whose vascular ultrasound showed peripheral arterial plaque formation or arterial stenosis or occlusion.
3. Patients with impaired liver function (serum alanine aminotransferase or aspartate aminotransferase or alkaline phosphatase greater than 1.5 times the upper limit of normal value), impaired renal function [serum creatinine greater than the upper limit of normal value or estimated glomerular filtration rate (eGFR) ≤ 60 mL·min−1·(1.73 m2)−1 at screening].
4. Patients with malignant tumors, severe mental illness, or serious diseases whose life expectancy is less than 2 years.
5. Women who were planning to become pregnant or who were pregnant or breastfeeding.
6. Patients with a known allergy to the herbs in the SQC.
7. Patients who have participated in other clinical trials within the last 3 months.
8. The researcher judges that the patient is unsuitable for this trial due to poor compliance.
2.4 Dropout criteria
1. Participants who voluntarily withdrew.
2. The participant's serum alanine aminotransferase or aspartate aminotransferase is three times greater than the upper limit of normal value, and it is still abnormal after repeat testing within 1 week.
3. The participant's serum creatinine is 1.5 times greater than the upper limit of normal value, and it is still abnormal after repeat testing within 1 week.
4. Participants who do not take the study medication as prescribed; that is, the dose rate is less than 80% or greater than 120%.
5. Participants violate this protocol during the trial by using drugs that are outside the prescribed range and interfere with the efficacy evaluation.
6. Participants who are lost to follow-up without explanation.
7. Participants who are unblinded.
2.5 Recruitment
Participants were recruited from the Hospital of Chengdu University of TCM (Chengdu, China), the Tongji Hospital of Tongji Medical College of Huazhong University of Science and Technology (Wuhan, China), the Affiliated TCM Hospital of Southwest Medical University (Luzhou, China), the Chongqing TCM Hospital (Chongqing, China), and the Affiliated Hospital of Shanxi University of TCM (Xianyang, China). All these institutions are Good Clinical Practice Centers approved and accredited by the China National Medical Products Administration.
2.6 Randomization
This trial was randomized using central stratified block randomization. The PROC PLAN procedure statement of SAS statistical software was used to prespecify the center number, set the seed number, stratified by center, set the number of blocks and block length, and simulate the generation of random sequence numbers for the interventions received by participants. The study drug was packaged in a random sequence. Participants were enrolled sequentially into groups according to the order of random sequence numbers.
2.7 Allocation concealment
Staff blinded the drugs according to the number of centers, grouping sequences, and sequential sequences generated by the random blocks. After blinding, the drugs could not be distinguished by their sequential number. The blind code is a six-column sequential allocation list, where the first column is the center number, the second column is the block group number, the third column is the block length, the fourth column is the participant's serial number, the fifth column is the randomized group identification, and the sixth column is the corresponding allocated number. This list is kept in duplicate, sealed, and locked in a lightproof envelope by the staff who generated the random sequence and by the staff of the pharmaceutical company.
2.8 Blinding
In this study, participants, researchers, outcome assessors, data collection and monitoring staff, and data management and statistical analysis staff were blinded. Drug administrators were not involved in the trial. The whole process was supervised by experts from the Chinese Evidence-based Medicine Center.
SQC and placebo were both produced by the Sichuan Neo-Green Pharmaceutical Technology Development Co. Ltd. (Chengdu, China). The ingredients of the placebo were maltodextrin, bitters, and food colorants, which comply with food safety regulations. The smell, taste, appearance, packaging, dosage form, production batch number, and date of the placebo were identical to those of SQC granules. Based on the previous study experience of the research group, this placebo formulation has the feasibility of a blinding method.
Primary unblinding of the statistical analyst was performed after the data lock, and secondary unblinding was performed after the statistical analysis. Each center received the emergency unblinding letter with study drug information according to the drug allocation. Unblinding may occur in an emergency (serious adverse event, suspected unexpected serious adverse reaction). The participants were treated according to the drug information, and the time, date, and reason for unblinding were recorded. All emergency envelopes must be kept with the trial records at the end of the trial in the clinical research unit.
2.9 Interventions
2.9.1 Run-in period
To standardize basic treatment and ensure compliance, participants entered a 2-week run-in period after screening. They received individualized basic treatment for glucose lowering, blood pressure control, lipid management, and antiplatelet therapy in accordance with the guidelines (5, 31). They received the same lifestyle and behavior education on diabetes self-management, diet, and daily physical activity.
2.9.2 Intervention period
Participants were randomized to intervention and control groups: (1) intervention group: basic treatment and SQC granules; and (2) control group: basic treatment and placebo granules. Both SQC and placebo specifications were 15 g/piece. Dosage indication was three times daily, before meals. For drug administration, granules were dissolved in 100 mL of hot water, stirred, covered, sealed, and taken orally between 30°C and 37°C. The intervention period was 24 weeks.
During this time, participants were not allowed to use acupuncture, massage, or other TCM interventions outside of this study and were also not allowed to use medications with cardiovascular risks.
2.10 Outcomes
Primary and secondary efficacy outcomes were measured once pretreatment and once post-treatment for a total of two times. Endpoint outcomes were measured once pre-treatment, once post-treatment, and once at 24 weeks of follow-up, for a total of three times.
2.10.1 Primary efficacy outcomes
2.10.1.1 CIMT
Equipment: The color Doppler ultrasound machine used uniformly in all study centers was the Philips Diagnostic Ultrasound System and Transducers EPIQ 7 (Philips, Inc.). The specific sites for CIMT measurements were the distal common carotid artery (1.0–1.5 cm below the level of the bifurcation) and the carotid bulb (the relative extension of the beginning of the internal carotid artery).
2.10.2 Secondary efficacy outcomes
1. Glycometabolism outcome: fasting plasma glucose (FPG) and hemoglobin A1c levels.
2. Lipid metabolism outcome: TC, TG, LDL-C, and HDL-C levels.
3. Islet function outcome: fasting insulin (FINS), calculated by HOMA-β = 20 × FINS × (FPG-3.5), HOMA-IR = FPG × FINS/22.5.
4. Hemorheology outcome: plasma viscosity, erythrocyte aggregation index, hematocrit value, and erythrocyte sedimentation rate.
5. Carotid ultrasound: plaque and degree of stenosis, end-diastolic arterial internal diameter (mm), peak systolic velocity (PSV, cm/s), end-diastolic velocity (EDV, cm/s), and mean velocity (Vm, cm/s) in the carotid artery. The following parameters were calculated: pulse index PI = (PSV−EDV)/Vm, resistance index RI = (PSV−EDV)/PSV, and S/D = PSV/EDV.
6. Carotid artery shear stress: τ = 4 × μ × PSV/D (where μ is the plasma viscosity and D is the end diastolic arterial internal diameter).
7. TCM syndrome score (28).
8. Life quality score: (1) Quality of life scale for patients with T2DM (DMQLS) and (2) the Chinese version of the Diabetes Management Self-Efficacy Scale (C-DMSES).
2.10.3 Endpoint outcomes
This trial evaluates two endpoint outcomes: the incidence of endpoint events and the time to progress to endpoint events.
Endpoint events are defined as follows: (1) progression of peripheral vascular disease, defined as carotid plaque (limited IMT ≥ 1.5 mm), stenosis (degree ≥ 50%), or occlusion; (2) major adverse cardiovascular events: cardiovascular death, myocardial infarction, ischemic stroke, and hospitalization due to heart failure; and (3) renal-related events: urinary microalbumin creatinine ratio and eGFR.
2.11 Follow-up
After the end of the treatment period, the study was followed for a further 24 weeks to observe endpoint events.
2.12 Safety evaluation
Safety outcomes included the following: (1) body temperature, respiration, heart rate, and blood pressure, which were monitored at each 4th week of outpatient visit; and (2) blood, urine, and stool routine tests, serum liver function (alanine aminotransferase, aspartate aminotransferase, total bilirubin, alkaline phosphatase, gamma-glutamyl transferase), and renal function (blood urea nitrogen, creatinine) tests, and 12-lead electrocardiography examination, which were performed once before the intervention, once at 4 weeks, once at 12 weeks, and once at 24 weeks after the intervention, for a total of four times. Adverse events (AEs) were monitored throughout the treatment period and graded according to the Common Terminology Criteria for Adverse Events version 5.0.
The trial may be terminated early in the presence of clustered serious safety concerns during the trial, supported by evaluative evidence related to the intervention.
2.13 Data collection and management
Baseline data included the following: (1) demographic data: gender, age, height, weight, waist circumference, hip circumference, calculated BMI, and waist–hip ratio; (2) medical history of diabetes and other diseases; and (3) menstrual history of women of childbearing age; suspected pregnancy should be checked by a urine pregnancy test.
Blood and urine samples were collected on an empty stomach at the hospital in the morning. Test methods and equipment are standardized in each center. The drug package recovery counting method, combined with the medication self-assessment form completed by the participants, was used to monitor the drug-taking rate. A dosing rate of 80%–120% was considered effective dosing.
In this study, paper Case Record Form was used to collect data, and EpiData software V3.1 was used for data management. Two data entry staff entered the data independently using the data verification function for double-checking. After entry, the manual verification plan based on the data verification plan was used to screen for questions. Database locking was performed by a study leader, a statistical analyst, and a data administrator upon signature of the database lock record. REC files were generated, and CHK files were created and exported in Excel for statistical analysis.
2.14 Sample size
This study is a superiority trial using CIMT as the primary outcome for sample size estimation compared to a placebo. Based on published clinical studies, the CIMT mean for the control group on long-term placebo treatment was assumed to be 1.12 mm (32). In this trial, the CIMT mean of the intervention group was estimated to be 1.03 mm, the standard deviation of the two groups was set to 0.10, the superiority value was set to 0.03, and the one-sided test was set to α = 0.025, 1-β = 0.8. The two groups were randomized in a 1:1 ratio. The minimum sample size for each group was calculated using PASS 15.0 software to obtain 45 cases, for a total of 90 cases. Considering the dropout rate and the balance between the two groups and five centers, a total sample size of 120 participants was required, with 60 participants in each group and 24 participants in each center.
2.15 Data analysis
Statistical analysis included baseline data, endpoint, efficacy, and safety outcomes using SPSS 26.0 and SAS 9.1 software. P-value < 0.05 was considered statistically significant. Data files were locked after blinded review and verified as reliable and error-free, and the analysis process was secondary unblinding.
The full analysis set (FAS) was selected for analysis of demographic and other baseline characteristics for comparability analysis. For efficacy and endpoint results, intention-to-treat analysis was mainly used primarily for FAS and also for the per-protocol set (PPS). Differences in results between the two datasets were compared, and if there were inconsistencies, the cause was identified. The safety set (SS) analysis was performed on participants who had received the study drugs at least once after randomization and who had experienced AE.
The Cochran–Mantel–Haenszel test was used for qualitative data, and analysis of variance and analysis of covariance were used for quantitative data. The log-rank test was used to assess the difference in incidence and to plot a curve for the endpoint outcome. The Cox proportional hazards regression model was used for multifactorial analysis. Subgroup analysis was conducted to explore differences in intervention efficacy among different populations based on age (>50 or ≤50 years), gender, smoking history, hypertension, hyperlipidemia, and drug combination (statin use or not, ACEI/ARB drugs ≥50% of the target dose).
If participants withdrew from the study, they were asked to return for a final visit, if possible, and their data were used using the last observation carried forward (LOCF) method. Participants who took the study drug and had a baseline follow-up visit were included in the FAS for analysis, and those who had an AE were included in the SS for analysis. If the rate of missing data was high (≥20%) and the variable containing the missing value was important for the question under study, the missing data were analyzed using the multiple imputation method.
3 Discussion
The carotid artery is a common site of atherosclerosis, with lesions often appearing earlier than in the coronary and cerebral arteries. Clinical studies have shown that a reduction in CIMT reduces the risk of cardiovascular events; therefore, CIMT progression is considered an effective surrogate marker for cardiovascular events and can predict cardiovascular risk in relatively low-risk populations (33, 34). On the other hand, CIMT screening allows cardiovascular risk stratification in patients with diabetes (35). As a non-invasive test, ultrasound has qualitative and quantitative value in the repeated detection of IMT and plaque changes. Currently, many clinical guidelines recommend using CIMT and plaque characteristics to help predict the risk of macrovascular complications in patients with T2DM (36). Therefore, this study focused on CIMT changes in diabetic patients to assess ASCVD risk reduction.
This study focused on changes in hemorheology and hemodynamics, which are indicators of early changes in vascular structure and function. Hematocrit value and plasma viscosity have been shown to correlate with heart rate variability in patients with T2DM (37). The erythrocyte sedimentation rate has been shown to be a predictor of coronary heart disease and heart failure (38, 39). Increased erythrocyte aggregation leads to an inability to pass through the capillaries, which is an important factor in the development of macroangiopathy in diabetic patients (40). On the other hand, the vessel wall is exposed to prolonged blood flow and the direction of fluid flow in normal vessels is laminar, which has certain anti-inflammatory, antioxidant stress, and antiatherosclerotic effects, while hemodynamic changes have an important effect on the elasticity and contractile function of the vessels, with wall shear stress in the parallel direction being closely relevant to atherosclerosis (41). Vascular endothelial cells, smooth muscle cells, and fibroblasts can sense shear stimulation and convert them into intracellular signals (42–44). Shear stress changes are present in patients with T2DM and atherosclerosis, and shear stress changes on the carotid artery wall in patients with risk factors for atherosclerosis are significantly associated with the number of risk factors such as hyperglycemia, hypertension, and dyslipidemia (45, 46). Metformin has been shown to improve aortic wall shear stress abnormalities in youngsters with type 1 diabetes (47). Pioglitazone has been shown to improve diastolic shear stress of the brachial artery in patients with impaired glucose tolerance (48). However, there is a temporary lack of clinical evidence on the effect of TCM on arterial shear force in diabetic patients. Therefore, carotid artery shear stress is selected in this study to identify early changes in the vascular microenvironment in patients with diabetes.
In efficacy trials for early intervention in chronic diseases, it is difficult to observe sufficient endpoint events during the trial, but there is undeniable value in focusing on the early stages of the disease. The highlight of this study is that patients with T2DM at high cardiovascular risk were selected as the research population, and in response to this contradiction, multiple outcomes such as islet function, vascular structure, and vascular function were selected to evaluate the early efficacy of SQC from multiple perspectives. The shortcoming of this study is that only 24 weeks of intervention and 24 weeks of follow-up were carried out, which is relatively short for the duration of a chronic disease.
Ethics statement
The studies involving humans were approved by the Ethical Review Committee of the Hospital of Chengdu University of Traditional Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YL: Conceptualization, Methodology, Writing – original draft, Writing – review and editing. ZZ: Investigation, Methodology, Writing – review and editing. NY: Investigation, Methodology, Writing – review and editing. XF: Data curation, Methodology, Writing – review and editing. HX: Data curation, Methodology, Writing – review and editing. HG: Conceptualization, Methodology, Supervision, Writing – review and editing. CX: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing – review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study is supported by the innovation team and talents cultivation program of the National Administration of Traditional Chinese Medicine (No. ZYYCXTD-C-202209) and a major research and development project from the Sichuan Science and Technology Program (No. 2022ZDZX0022).
Acknowledgments
All the authors thank all the researchers, staff, and participants involved in the conduct of the study, as well as the support of the hospitals and institutions for the five study centers and the external experts from the Chinese Evidence-based Medicine Center for guidance and supervision.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1290240/full#supplementary-material
References
1. Das SR, Everett BM, Birtcher KK, Brown JM, Januzzi JL Jr., Kalyani RR, et al. 2020 expert consensus decision pathway on novel therapies for cardiovascular risk reduction in patients with type 2 diabetes: a report of the American College of Cardiology solution set oversight committee. J Am Coll Cardiol. (2020) 76(9):1117–45. doi: 10.1016/j.jacc.2020.05.037
2. Mosenzon O, Alguwaihes A, Leon JLA, Bayram F, Darmon P, Davis TME, et al. CAPTURE: a multinational, cross-sectional study of cardiovascular disease prevalence in adults with type 2 diabetes across 13 countries. Cardiovasc Diabetol. (2021) 20(1):154. doi: 10.1186/s12933-021-01344-0
3. Cavallari I, Bhatt DL, Steg PG, Leiter LA, McGuire DK, Mosenzon O, et al. Causes and risk factors for death in diabetes: a competing-risk analysis from the SAVOR-TIMI 53 trial. J Am Coll Cardiol. (2021) 77(14):1837–40. doi: 10.1016/j.jacc.2021.02.030
4. Juutilainen A, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Type 2 diabetes as a “coronary heart disease equivalent”: an 18-year prospective population-based study in Finnish subjects. Diabetes Care. (2005) 28(12):2901–7. doi: 10.2337/diacare.28.12.2901
5. Chinese Diabetes Society. Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition). Chin J Diabetes Mellitus. (2021) 13(4):305–409. doi: 10.3760/cma.j.cn115791-20210221-00095
6. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. (2001) 285(19):2486–97. doi: 10.1001/jama.285.19.2486
7. Hunt KJ, Resendez RG, Williams K, Haffner SM, Stern MP and San Antonio Heart Study. National Cholesterol Education Program versus World Health Organization metabolic syndrome in relation to all-cause and cardiovascular mortality in the San Antonio Heart Study. Circulation. (2004) 110(10):1251–7. doi: 10.1161/01.CIR.0000140762.04598.F9
8. Ji L, Hu D, Pan C, Weng J, Huo Y, Ma C, et al. Primacy of the 3B approach to control risk factors for cardiovascular disease in type 2 diabetes patients. Am J Med. (2013) 126(10):925.e11–e22. doi: 10.1016/j.amjmed.2013.02.035
9. Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). Br Med J. (1998) 316(7134):823–8. doi: 10.1136/bmj.316.7134.823
10. Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. (2008) 358(6):580–91. doi: 10.1056/NEJMoa0706245
11. Brunström M, Carlberg B. Effect of antihypertensive treatment at different blood pressure levels in patients with diabetes mellitus: systematic review and meta-analyses. Br Med J. (2016) 352:i717. doi: 10.1136/bmj.i717
12. Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. (2015) 313(6):603–15. doi: 10.1001/jama.2014.18574
13. Ray KK, Seshasai SR, Wijesuriya S, Sivakumaran R, Nethercott S, Preiss D, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. Lancet. (2009) 373(9677):1765–72. doi: 10.1016/S0140-6736(09)60697-8
14. ACCORD Study Group, Cushman WC, Evans GW, Byington RP, Goff DC, Grimm RH, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. (2010) 362(17):1575–85. doi: 10.1056/NEJMoa1001286
15. Zhang Z, Leng Y, Chen Z, Fu X, Liang Q, Peng X, et al. The efficacy and safety of Chinese herbal medicine as an add-on therapy for type 2 diabetes mellitus patients with carotid atherosclerosis: an updated meta-analysis of 27 randomized controlled trials. Front Pharmacol. (2023) 14:1091718. doi: 10.3389/fphar.2023.1091718
16. Shan L, Yang H, Cao L, Yuan C, Zhang L, Gao T, et al. Optimization of extraction process of Shenqi compound granules with multi-index comprehensive evaluation method and investigation of its thermal stabilit. Chin J Exp Tradit Med Formulae. (2016) 22(7):24–7. doi: 10.13422/j.cnki.syfjx.2016070024
17. Shan L, Cao L, Yang H, Yuan C, Song Y, Tan J, et al. Optimization of water extraction process of saponins from Shenqi Fufang granular by response surface methodology. Nat Product Res Dev. (2016) 28(4):568–74. doi: 10.16333/j.1001-6880.2016.4.018
18. Shan L, Yuan C, Yang H, Cao L, Tan J, Gao T, et al. Simultaneous determination of saponins in Shenqi compound granules by HPLC-ELSD. Chin J Exp Tradit Med Formulae. (2016) 22(14):102–5. doi: 10.13422/j.cnki.syfjx.2016140102
19. Zhang X, Liu Y, Xiong D, Xie C. Insulin combined with Chinese medicine improves glycemic outcome through multiple pathways in patients with type 2 diabetes mellitus. J Diabetes Investig. (2015) 6(6):708–15. doi: 10.1111/jdi.12352
20. Xie H, Xie C, Gao H. Effect of traditional Chinese medicine compound on Hs-CRP and IL6 of patients with vascular lesions in type 2 diabetes. Chin J Exp Tradit Med Formulae. (2014) 20(10):209–12. doi: 10.13422/j.cnki.syfjx.2014100209
21. Zhang X, Wang H, Xie C, Hu Z, Zhang Y, Peng S, et al. Shenqi compound ameliorates type-2 diabetes mellitus by modulating the gut microbiota and metabolites. J Chromatogr B Analyt Technol Biomed Life Sci. (2022) 1194:123189. doi: 10.1016/j.jchromb.2022.123189
22. Fu X, Zhou X, Liu Y, Lei Y, Xie H, Leng Y, et al. Exploration of SQC formula effect on type 2 diabetes mellitus by whole transcriptome profile in rats. Endocr Metab Immune Disord Drug Targets. (2021) 21(7):1261–9. doi: 10.2174/1871530321666210225125141
23. Liu Y, Kang J, Gao H, Zhang X, Chao J, Gong G, et al. Exploration of the effect and mechanism of ShenQi compound in a spontaneous diabetic rat model. Endocr Metab Immune Disord Drug Targets. (2019) 19(5):622–31. doi: 10.2174/1871530319666190225113859
24. Tian Y, Xu G, Gao H, Xie HY, Leng YL, Fu XX, et al. The mitigatory effect of Shen-Qi compound on the diabetic thoracic aortic complications through inhibiting the inflammatory microenvironment by miR-223-3p/RBP-J/IRF8 axis. Evid Based Complement Alternat Med. (2022) 2022:6686931. doi: 10.1155/2022/6686931
25. Gao H, Duan Y, Fu X, Xie H, Liu Y, Yuan H, et al. Comparison of efficacy of SHENQI compound and rosiglitazone in the treatment of diabetic vasculopathy analyzing multi-factor mediated disease-causing modules. PLoS One. (2018) 13(12):e0207683. doi: 10.1371/journal.pone.0207683
26. Cheng CW, Wu TX, Shang HC, Li YP, Altman DG, Moher D, et al. CONSORT extension for Chinese herbal medicine formulas 2017: recommendations, explanation, and elaboration (simplified Chinese version). Ann Intern Med. (2017) 167(2):W21–34. doi: 10.7326/IsTranslatedFrom_M17-2977_2
27. Dai L, Cheng CW, Tian R, Zhong LL, Li YP, Lyu AP, et al. Standard protocol items for clinical trials with traditional Chinese medicine 2018: recommendations, explanation and elaboration (SPIRIT-TCM extension 2018). Chin J Integr Med. (2019) 25(1):71–9. doi: 10.1007/s11655-018-2999-x
28. Zheng X. Guiding principles for new drug clinical research of traditional Chinese medicine (trial). Beijing: China Medical Science Press (2002). 233–7.
29. China Association of Chinese Medicine. Guidelines for prevention and treatment of diabetes in TCM. Chin Med Mod Distance Educ China. (2011) 9(4):148–51. doi: 10.3969/j.issn.1672-2779.2011.04.111
30. Xu G. Diagnostic scale for qi and yin deficiency syndrome of type 2 diabetes mellitus [Master’s thesis]. Chengdu (Sichuan): Chengdu University of TCM (2020).
31. American Diabetes Association. 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2021. Diabetes Care. (2021) 44(Suppl 1):S125–50. doi: 10.2337/dc21-S010
32. Mita T, Katakami N, Shiraiwa T, Yoshii H, Onuma T, Kuribayashi N, et al. Sitagliptin attenuates the progression of carotid intima-media thickening in insulin-treated patients with type 2 diabetes: the sitagliptin preventive study of intima-media thickness evaluation (SPIKE): a randomized controlled trial. Diabetes Care. (2016) 39(3):455–64. doi: 10.2337/dc15-2145
33. Willeit P, Tschiderer L, Allara E, Reuber K, Seekircher L, Gao L, et al. Carotid intima-media thickness progression as surrogate marker for cardiovascular risk: meta-analysis of 119 clinical trials involving 100 667 patients. Circulation. (2020) 142(7):621–42. doi: 10.1161/CIRCULATIONAHA.120.046361
34. Geisel MH, Bauer M, Hennig F, Hoffmann B, Lehmann N, Möhlenkamp S, et al. Comparison of coronary artery calcification, carotid intima-media thickness and ankle-brachial index for predicting 10-year incident cardiovascular events in the general population. Eur Heart J. (2017) 38(23):1815–22. doi: 10.1093/eurheartj/ehx120
35. Malik S, Budoff MJ, Katz R, Blumenthal RS, Bertoni AG, Nasir K, et al. Impact of subclinical atherosclerosis on cardiovascular disease events in individuals with metabolic syndrome and diabetes: the multi-ethnic study of atherosclerosis. Diabetes Care. (2011) 34(10):2285–90. doi: 10.2337/dc11-0816
36. Jellinger PS, Handelsman Y, Rosenblit PD, Bloomgarden ZT, Fonseca VA, Garber AJ, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. (2017) 23(Suppl 2):1–87. doi: 10.4158/EP171764.APPGL
37. Velcheva I, Damianov P, Mantarova S, Antonova N. Hemorheology and heart rate variability in patients with diabetes mellitus type 2. Clin Hemorheol Microcirc. (2011) 49(1–4):513–8. doi: 10.3233/CH-2011-1500
38. Andresdottir MB, Sigfusson N, Sigvaldason H, Gudnason V. Erythrocyte sedimentation rate, an independent predictor of coronary heart disease in men and women: the Reykjavik study. Am J Epidemiol. (2003) 158(9):844–51. doi: 10.1093/aje/kwg222
39. Ingelsson E, Arnlöv J, Sundström J, Lind L. Inflammation, as measured by the erythrocyte sedimentation rate, is an independent predictor for the development of heart failure. J Am Coll Cardiol. (2005) 45(11):1802–6. doi: 10.1016/j.jacc.2005.02.066
40. Vague P, Juhan I. Red cell deformability, platelet aggregation, and insulin action. Diabetes. (1983) 32(Suppl 2):88–91. doi: 10.2337/diab.32.2.s88
41. Osman MW, Nath M, Khalil A, Webb DR, Robinson TG, Mousa HA. Haemodynamic differences amongst women who were screened for gestational diabetes in comparison to healthy controls. Pregnancy Hypertens. (2018) 14:23–8. doi: 10.1016/j.preghy.2018.07.007
42. Lee DY, Chiu JJ. Atherosclerosis and flow: roles of epigenetic modulation in vascular endothelium. J Biomed Sci. (2019) 26(1):56. doi: 10.1186/s12929-019-0551-8
43. Shi ZD, Tarbell JM. Fluid flow mechanotransduction in vascular smooth muscle cells and fibroblasts. Ann Biomed Eng. (2011) 39(6):1608–19. doi: 10.1007/s10439-011-0309-2
44. Mack CP. Signaling mechanisms that regulate smooth muscle cell differentiation. Arterioscler Thromb Vasc Biol. (2011) 31(7):1495–505. doi: 10.1161/ATVBAHA.110.221135
45. Kearney-Schwartz A, Virion JM, Stoltz JF, Drouin P, Zannad F. Haemorheological disturbances in hypertensive type 2 diabetic patients—influence of antihypertensive therapy. Fundam Clin Pharmacol. (2007) 21(4):387–96. doi: 10.1111/j.1472-8206.2007.00496.x
46. Jiang Y, Kohara K, Hiwada K. Association between risk factors for atherosclerosis and mechanical forces in carotid artery. Stroke. (2000) 31(10):2319–24. doi: 10.1161/01.str.31.10.2319
47. Bjornstad P, Schäfer M, Truong U, Cree-Green M, Pyle L, Baumgartner A, et al. Metformin improves insulin sensitivity and vascular health in youth with type 1 diabetes mellitus. Circulation. (2018) 138(25):2895–907. doi: 10.1161/CIRCULATIONAHA.118.035525
Keywords: type 2 diabetes mellitus, cardiovascular risk, randomized controlled trial, traditional Chinese medicine, Shenqi compound
Citation: Leng Y, Zhang Z, Yao N, Fu X, Xie H, Gao H and Xie C (2024) Chinese herbal medicine Shenqi compound for early intervention in patients at high cardiovascular risk of type 2 diabetes mellitus: the protocol of a multicenter, randomized, double-blind, placebo-controlled trial. Front. Cardiovasc. Med. 10:1290240. doi: 10.3389/fcvm.2023.1290240
Received: 7 September 2023; Accepted: 13 December 2023;
Published: 8 January 2024.
Edited by:
Juncheng Wei, Temple University, United States© 2024 Leng, Zhang, Yao, Fu, Xie, Gao and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Gao Y2RnaDc2QDE2My5jb20= Chunguang Xie eGllY2dAY2R1dGNtLmVkdS5jbg==
Abbreviations AEs, adverse events; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CIMT, carotid intima-media thickness; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; FINS, fasting insulin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SQC, Shenqi compound; T2DM, type 2 diabetes mellitus; TC, total cholesterol; TCM, traditional Chinese medicine; TGs, triglycerides.
 Yulin Leng
Yulin Leng Zehua Zhang
Zehua Zhang Nairong Yao1
Nairong Yao1 Hongyan Xie
Hongyan Xie Chunguang Xie
Chunguang Xie