- Department of Neurology, Huzhou Central Hospital, Affiliated Central Hospital of HuZhou University, Huzhou, Zhejiang, China
Background: This review aims to examine the association of Parkinson's disease (PD) with the increased risk of cardiovascular events.
Methods: PubMed, Embase, CENTRAL, and Scopus databases were electronically searched for papers published up to 5 May 2023. Studies reporting the association between PD and the subsequent risks of stroke, myocardial infarction (MI), and cardiovascular mortality were included.
Results: Sixteen studies were included in this review. The clinical data of 101,712 PD patients were compared with that of the control group of 204,901 patients without PD in the included studies. Meta-analysis showed that PD patients had an increased risk of stroke compared with patients without PD (odds ratio (OR): 1.49; 95% confidence interval (CI): 1.30, 1.72; I2 = 76%). The pooled analysis demonstrated no significant increase in the risk of MI (OR: 1.16; 95% CI: 0.85, 1.59; I2 = 82%) and cardiovascular mortality (OR: 1.20; 95% CI: 0.93, 1.54; I2 = 65%) in PD patients. However, data from cohort studies indicated a possibility of higher risk of MI (OR: 1.36; 95% CI: 1.01, 1.84; I2 = 80%) and cardiovascular mortality (OR: 1.22; 95% CI: 1.00, 1.60; I2 = 62%) in patients with PD.
Conclusion: Patients with PD may have an increased risk of stroke as compared with the age- and gender-matched general population. While our results show that PD does not increase the overall risk of MI and cardiovascular mortality, analysis of cohort studies alone demonstrated that these risks may be higher in patients with PD. The current evidence is of very low quality. Further prospective cohort studies from different countries that would account for important cardiovascular risk factors are needed to improve the current evidence.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, PROSPERO (CRD42023421924).
Introduction
Parkinson's disease (PD) is a progressive neurodegenerative movement disorder that commonly affects old people. The European data show that the prevalence rate of PD is about 108–257 per 100,000 individuals with an annual incidence of around 11–19 per 100,000 people (1). Data from the Chinese population show that around 1.37% of the aging population is affected by PD, that is, a total of 3.62 million patients (2). PD leads to movement abnormalities and other non-motor symptoms like memory loss, depression, visual disturbances, and autonomic dysfunction. The significant disability and poor quality of life of patients with PD are associated with a high economic burden on the healthcare system, and with increased physical and emotional dependence of patients on caregivers (3). These patients usually require full-time nursing care for their day-to-day activities and daily monitoring of the illness.
The aging population is also at an increased risk of cardiovascular diseases, a common cause of mortality in this age group. According to the American Heart Association on Heart Disease and Stroke Statistics (4), the risk of cardiovascular diseases rises from 35% to 40% in the 40–60 years age group to 75%–78% in the 60–80 years age group and exceeds 85% in people over 80 years of age. The accumulation of cardiovascular risk factors such as diabetes and obesity over a period of time also has a deleterious effect on the overall health of the aging population (5).
Currently, the association of PD with a higher risk of important cardiovascular events like stroke and myocardial infarction (MI) is not clear. Recently, a systematic review by Alves et al. (6) attempted to answer this clinical question, but it could only include 11 studies. The present updated meta-analysis aims to assess if PD increases the risk of cardiovascular events as compared with the age- and gender-matched control group.
Material and methods
Search source and strategy
The protocol of the review was prepared and published on PROSPERO (CRD42023421924) before beginning the study. A systematic search of PubMed, Embase, CENTRAL, and Scopus databases was carried out independently by two reviewers. The last date of the search was 5 May 2023. In addition, gray literature was searched by means of Google Scholar.
We aimed to include the following studies: (1) Cohort or case-control in design. (2) Studies comparing patient’s with PD with the general population matched at least for age and sex. (3) Studies examining the risk of stroke, MI, or cardiovascular mortality. (4) Studies reporting the effect size of the association or providing sufficient data to calculate the same.
The exclusion criteria were as follows: (1) Studies not on any cardiovascular outcome. (2) Cross-sectional studies. (3) Abstracts, editorials, thesis, and non-peer-reviewed studies. (4) In cases of duplication of data source, only a study with a higher sample size or better matching of cases and controls was included.
To conduct a wide but targeted search, we employed the following keywords: Parkinson's disease; cardiac; cardiovascular; heart failure; myocardial infraction; coronary artery disease; cerebrovascular; stroke; mortality; and cardiovascular mortality. Further details are mentioned in Supplementary Material Table S1.
Study selection
Two reviewers independently examined all the search results. First, the retrieved data were congregated and deduplicated electronically. The titles and abstracts of all articles were screened to identify relevant studies. Non-relevant articles were excluded, and the remaining studies underwent full-text analysis based on the eligibility criteria. Any disagreements were solved by consensus. We also examined the reference lists of the included studies for any other missed articles.
Extracted data and outcomes
Two reviewers were independently involved in the data extraction. A preformatted table was generated before beginning the review. The extracted data included the name(s) of the author(s), year of publication, location and database used, study type, sample size (PD and control), age and gender of the sample, diagnosis of PD, disease duration, matched variables, outcome reported, measurement of outcome, confounders adjusted, and follow-up period. Study details were then cross-matched, and any discrepancies were resolved by consensus.
The outcome of the review was selected based on the data reported by the studies. Quantitative analysis was done if at least four studies reported the same variable. Based on the initial analysis, stroke, MI, and cardiovascular mortality were selected as the outcomes of this review.
Risk of bias analysis
Two reviewers assessed the quality of the included studies using the ROBINS-1 tool (7). The tool has seven domains of bias. Each one of the domains is examined using a series of signaling questions that extract information about the study and the analysis being done. Once the signaling questions are completed, the results are classified as serious risk of bias, moderate risk of bias, or low risk of bias. The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was also utilized using the GRADEpro GDT software for examining the certainty of the evidence.
Statistical analysis
The review was conducted as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (8). The quantitative analysis was carried out using the software ReviewManager [RevMan, version 5.3; Nordic Cochrane Centre (Cochrane Collaboration), Copenhagen, Denmark; 2014]. The effect size of the association between PD and cardiovascular outcomes was extracted and combined using the generic inverse variation function of RevMan to generate an odds ratio (OR) with 95% confidence intervals (CI). Results were presented in the form of a forest plot. The meta-analysis was conducted in a random-effects model. And the subgroup analysis was done based on study type.
Funnel plots were generated using RevMan to judge publication bias. Interstudy heterogeneity was assessed by I2 statistics. A value of I2 < 50% indicated low heterogeneity and that >50% indicated substantial heterogeneity. A sensitivity analysis was performed to check for outliers in the analysis. One study at a time was removed from the meta-analysis and the results were regenerated.
Results
Search results
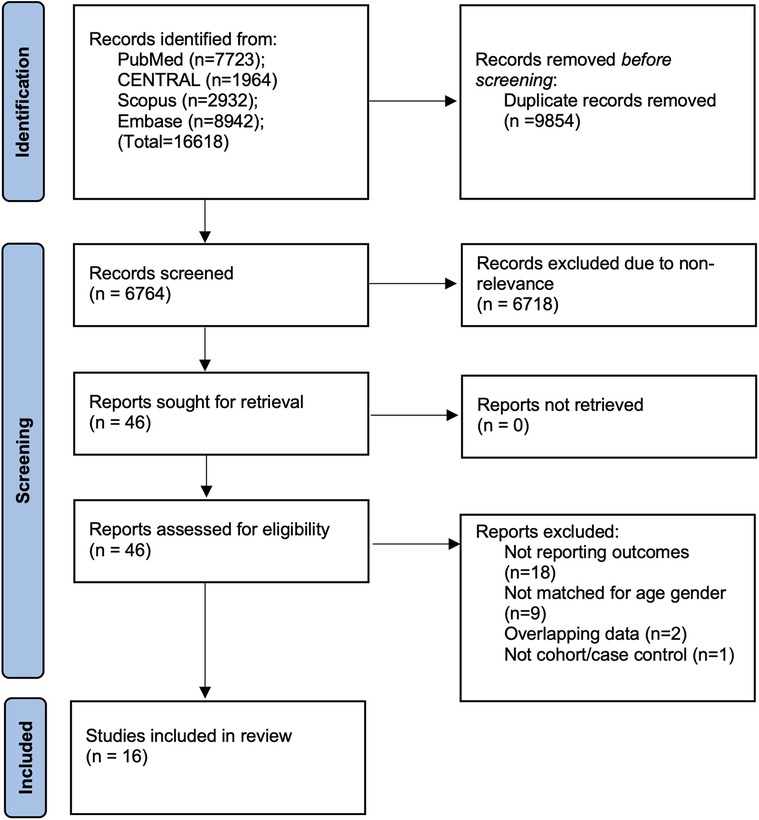
The results generated during the search strategy are shown in Figure 1. A total of 16,618 articles were initially retrieved. Of them, 6,764 records remained after deduplication, and 46 were subsequently found suitable for full-text analysis. Eventually, 16 studies were included in the final review (9–24). The inter-reviewer agreement for the selection of studies was high (kappa = 0.95). No additional studies were found on Google Scholar.
Study details
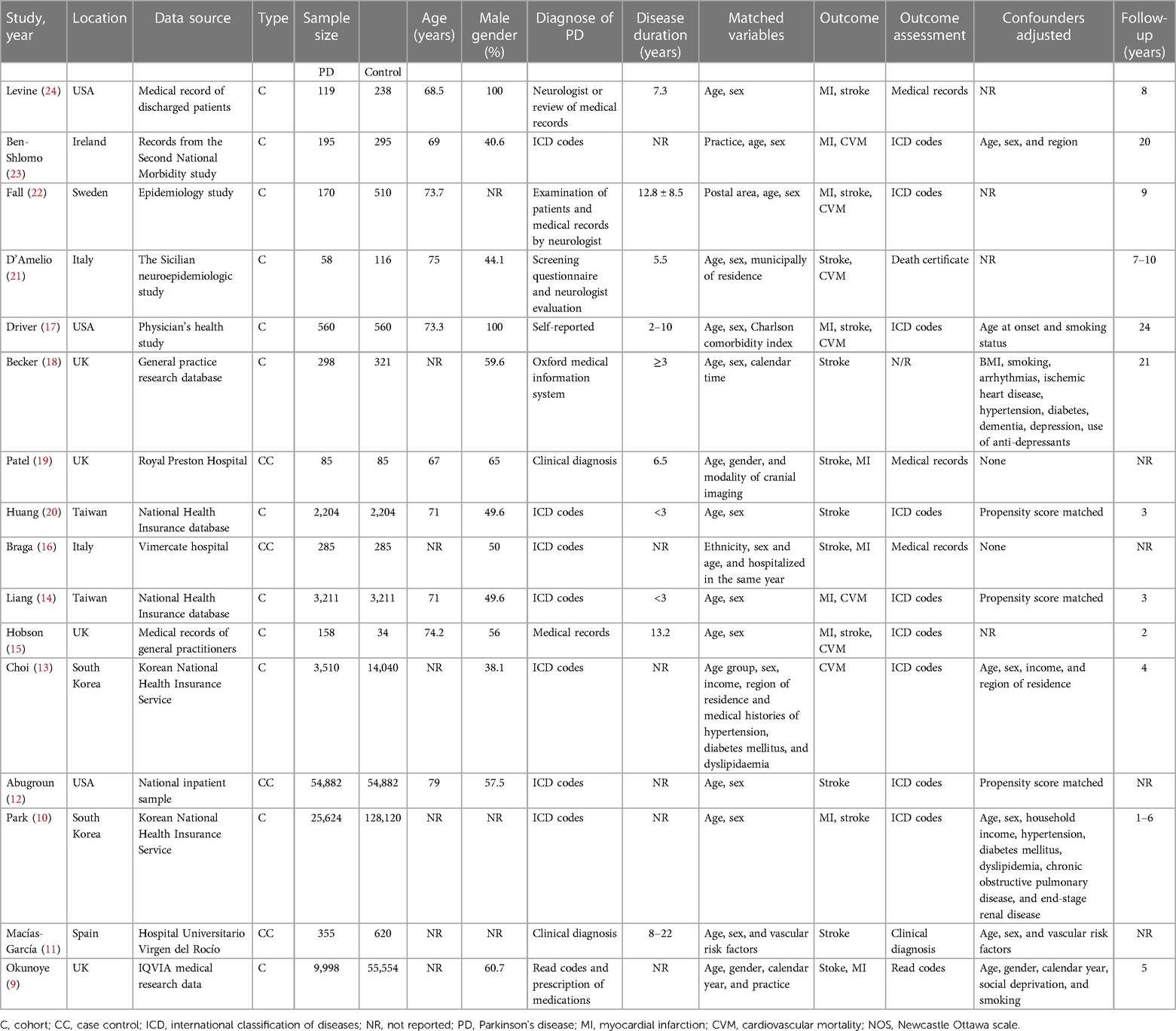
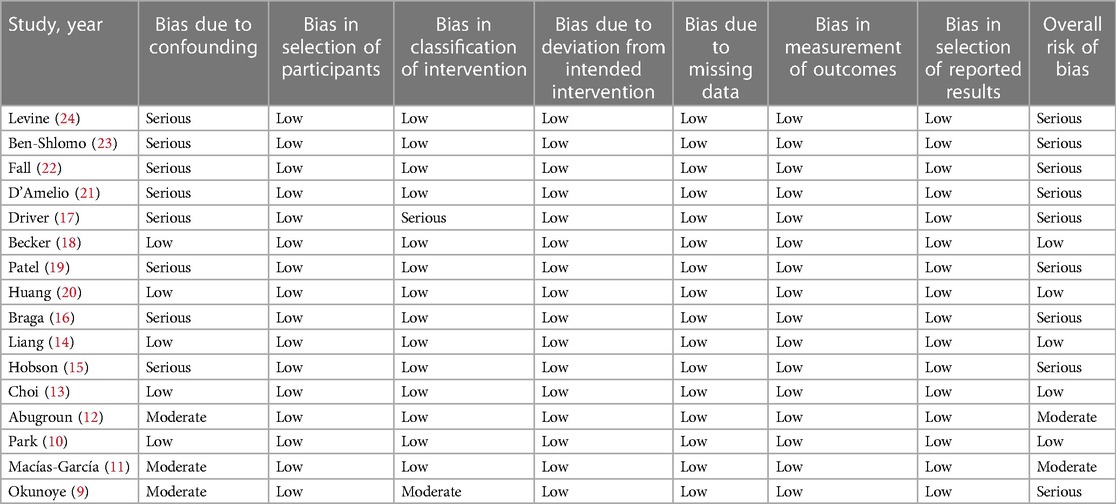
The data sourced from the included studies are provided in Table 1. These studies were published over a wide timeline, ranging from 1992 to 2022. The study locations were the USA, the UK, Sweden, Ireland, Italy, Spain, South Korea, and Taiwan. All studies were retrospective. Four were case-control studies, while the remaining 12 were cohort studies. A total of 101,712 patients with PD were compared with a control group of 204,901 individuals without PD in the included studies. PD was diagnosed clinically in only four studies, while the rest used medical records to identify patients with PD. The study and control groups in the included studies were matched for age, gender, and other different variables. Most studies used medical records to identify the outcome of interest. There was a high variability in the follow-up duration in the included studies. The risk of bias assessment is presented in Table 2. Five studies had a low risk of bias, two had a moderate risk of bias, while the remaining had a high risk of bias.
Outcomes
A total of 13 studies reported data on stroke. The meta-analysis showed that patients with PD had an increased risk of stroke as compared with the non-PD group (OR: 1.49; 95% CI: 1.30, 1.72; I2 = 76%) (Figure 2). The results were significant for the cohort studies (OR: 1.60; 95% CI: 1.30, 1.98; I2 = 71%) but not for the case-control studies (OR: 1.29; 95% CI: 0.98, 1.70; I2 = 25%). Visual inspection of the funnel plot did not show any publication bias (Supplementary Material Figure S1). Furthermore, sensitivity analysis did not detect any effect of the singular exclusion of individual studies.
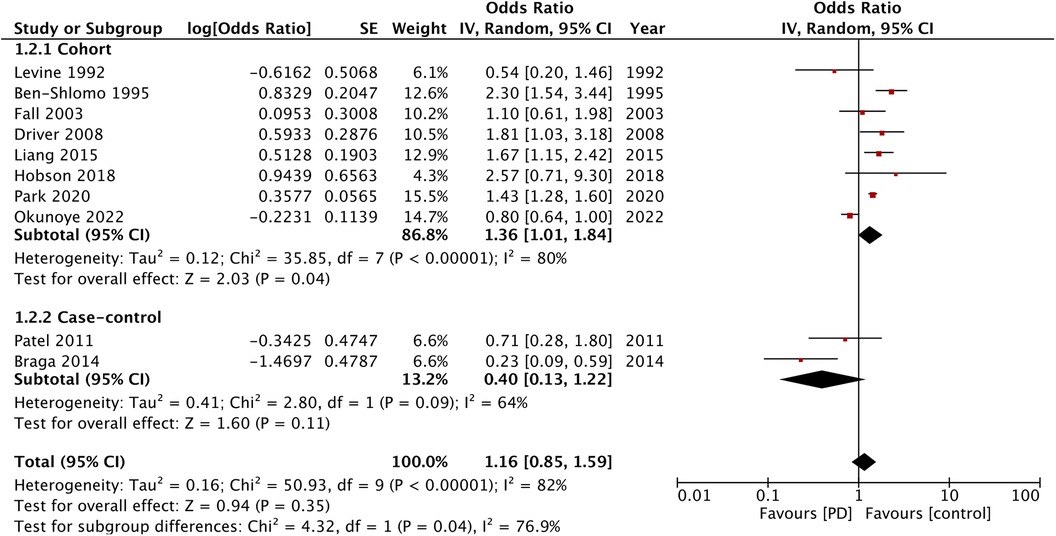
For the outcome of MI, 10 studies were available. A pooled analysis demonstrated no significant difference in the risk of MI in patients with PD as compared with the non-PD group (OR: 1.16; 95% CI: 0.85, 1.59; I2 = 82%) (Figure 3). However, the results of the cohort studies (OR: 1.36; 95% CI: 1.01, 1.84; I2 = 80%) showed a significant increase in the risk of MI in patients with PD, while no such association was noted for the case-control studies (OR: 0.40; 95% CI: 0.13, 1.22; I2 = 64%). Visual inspection of the funnel plot did not show any publication bias (Supplementary Material Figure S2), and sensitivity analysis did not detect any change in the results.
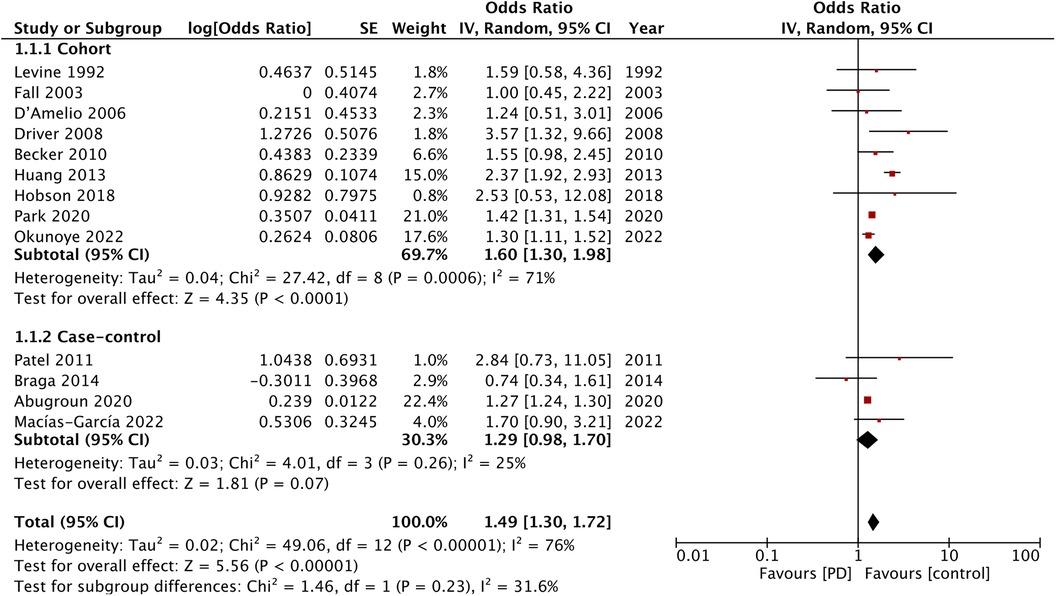
Eight studies reported data on cardiovascular mortality. The meta-analysis found no difference in cardiovascular mortality between the PD and non-PD groups (OR: 1.20; 95% CI: 0.93, 1.54; I2 = 65%). Sensitivity analysis showed that the exclusion of the study of Fall et al. (14) resulted in a significant increase in the risk of cardiovascular mortality (OR: 1.36; 95% CI: 1.11, 1.65; I2 = 40%). There was a tendency for increased cardiovascular mortality in the cohort studies (OR: 1.22; 95% CI: 1.00, 1.60; I2 = 62%) but not for the case-control studies (OR: 0.44; 95% CI: 0.15, 1.29) (Figure 4).
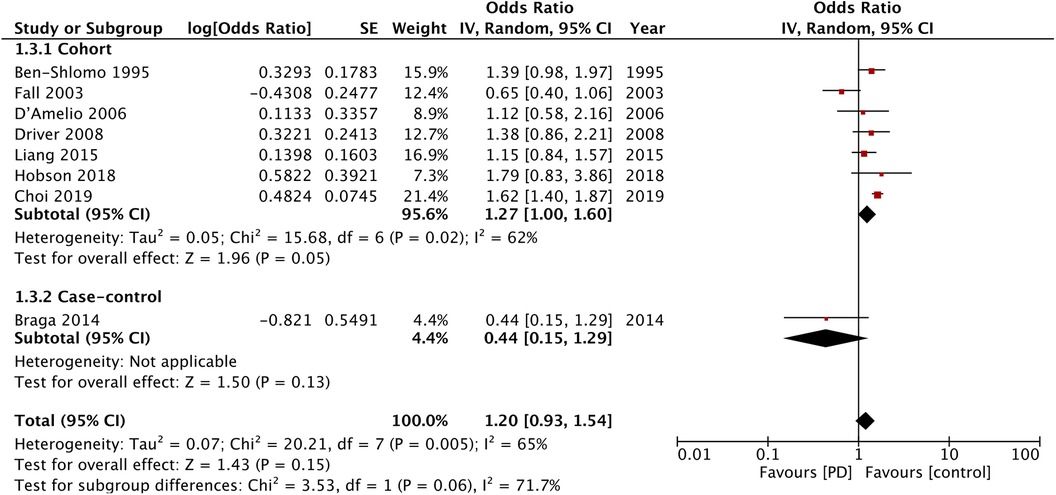
Figure 4. Meta-analysis of the risk of cardiovascular mortality among patients with PD and non-PD controls.
Certainty of evidence
GRADE assessment of evidence is provided in the Supplementary Material Table S2. Since the majority of the studies had serious bias in the adjustment of confounders, the certainty of evidence was downgraded. Overall, all outcomes had very low certainty of evidence.
Discussion
This updated systematic review and meta-analysis included 16 studies examining the association between PD and adverse cardiovascular outcomes. Our results showed that PD was associated with a 49% higher risk of stroke as compared with the age- and gender-matched general population. Nevertheless, there was no statistically significant association between PD and the risk of MI or cardiovascular mortality. Our results concur with the previous review (6) on this topic, but with a higher number of studies.
With a gradual aging of the global population, the prevalence of age-related neurological and cardiovascular disorders is also on the rise (25). PD constitutes one of the major neurological disorders that causes significant disability and results in a poor quality of life in the aging population (1). Importantly, this group is also at a higher risk of cardiovascular disorders. Comorbidities like stroke and MI could worsen the prognosis of PD. Therefore, understanding the potential impact of PD on cardiovascular outcomes in aging patients is crucial. This review examined the association between PD and three important cardiovascular outcomes, namely, stroke, MI, and cardiovascular mortality. It is important to note that age and gender could be important confounders while assessing such associations. If the control group is younger, the risk could be overestimated leading to false positive results. Therefore, we included only studies with age- and gender-matched controls to generate better-quality evidence.
The pooled analysis of 13 studies found a significantly higher risk of stroke in patients with PD. When stratified based on the study type, the association was significant for the cohort but not for the case-control studies. The cohort studies are a better source of scientific evidence as they have a temporal framework to assess causality (26). Also, the number of cohort studies was higher compared with the case-control studies in our analysis. The lack of change in the significance of the results, demonstrated by the sensitivity analysis, also adds to the credibility of this outcome.
There are several mechanisms by which PD could increase the risk of stroke (27). Motor dysfunction leading to reduced physical activity is the hallmark of PD. Several studies have shown that physical activity is important for stroke prevention (28, 29). Second, autonomic dysfunction, which affects >40% of patients with PD (30), is an important regulator of the heart–brain axis and could be a key factor in the heightened risk of stroke. According to one study (30), about a third of the patients with PD have orthostatic hypotension owing to the autonomic dysfunction. Another study with almost 25 years of follow-up has demonstrated a twofold higher risk of stroke in patients with orthostatic hypotension (31). It is plausible that the episodes of hypotension could lead to brain hypoperfusion, thereby increasing the risk of ischemic stroke.
Another possible mechanism for the association between PD and stroke may be related to homocysteine metabolism. Homocysteine is an amino acid that has a proinflammatory effect on arteries and is a recognized risk factor for cardiovascular and neurodegenerative disease (32). Studies show that patients with PD may have increased levels of homocysteine compared with controls, and the difference is more pronounced in patients who were treated with L-dopa (33, 34). Homocysteine with its proinflammatory function has been shown to increase oxidative stress and reduce nitric oxide synthesis, thereby causing endothelial dysfunction (35). Clinically, it has been associated with atherosclerosis and an increased risk of stroke (36).
Stroke and coronary artery disease have several common risk factors. However, while PD in our study was associated with the increased risk of stroke, this meta-analysis did not find such a correlation between the risk of MI and cardiovascular mortality. The analysis of only the cohort studies did demonstrate a 36% increased risk of MI and a 22% increased risk of cardiovascular mortality. In addition, we identified one outlier study (14) in the analysis of cardiovascular mortality. Its exclusion resulted in a 36% increased risk of cardiovascular mortality, associated with PD. The methodological differences in the study designs could have been important factors leading to such variability in the results. More importantly, the number of cohort studies in the meta-analysis of MI and cardiovascular mortality was not high, and the high interstudy heterogeneity prohibits wider interpretation of the results.
A recent review of cohort, case-control, and cross-sectional studies has also demonstrated no association between PD and MI (37). Another review by Chua et al. (38), published in 2022, combined data from three cohort studies and two case-control studies examining the relationship between PD and coronary artery disease. Similarly to our study, this review showed a significant association between PD and coronary artery disease in cohort studies but not in case-control studies. Hence, it seems that the design of the study is an important component in assessing the relationship between PD and cardiovascular events.
Mechanisms that increase the risk of stroke have also been linked with the pathogenesis of MI in patients with PD. A meta-analysis of 13 studies has shown that orthostatic hypotension is associated with an increased risk of coronary artery disease, heart failure, and all-cause mortality (39). Patients with PD suffer from baroreflex dysfunction, which increases sympathetic activity and reduces parasympathetic control, leading to reduced diastolic perfusion pressure and reduced myocardial blood flow (40). Orthostatic hypotension also activates compensatory neuroendocrine mechanisms, which in turn could trigger platelets and coagulation factors and lead to adverse cardiovascular events (39). One such example includes changes in the endothelin system that are seen in cases of orthostatic hypotension. Physiological vasoconstrictors like endothelin-1 and vasopressin that have an adaptive role in hypotensive episodes could increase the risk of atherothrombosis in the long term (41).
Second, the higher levels of homocysteine in patients with PD can lead to a hypercoagulable state that increases the risk of coronary thrombosis and adverse cardiovascular events (34–36). In addition, inflammation could be a shared risk factor between PD and MI. Chronic neuroinflammation causing activation of microglia and elevated levels of proinflammatory mediators has been an important contributor to the pathophysiology of PD (42). Correspondingly, oxidative stress and systemic inflammation are also known risk factors for atherosclerosis and coronary artery disease (43). Therefore, it is plausible that the baseline systemic inflammation is higher in patients with PD and contributes to the increased risk of MI.
There are several limitations to this review. The number of studies in each analysis was not very high, especially in terms of cardiac outcomes. The high heterogeneity is a significant limitation and could be due to variations in study populations, methodology, and region of the study. Furthermore, there were differences in the methods used to diagnose patients with PD. Only four studies performed a clinical diagnosis, while the rest relied on medical records, which could introduce an important source of bias. Also, there was no uniformity in the adjusted confounders among the included studies. Important cardiovascular risk factors, such as diabetes, hypertension, obesity, and smoking, were not adjusted in multiple studies, which can potentially skew the results. In addition, we observed variability in the duration of follow-ups. Currently, it is unclear how long it takes for PD to increase the risk of adverse cardiovascular events. Therefore, studies with shorter follow-ups could potentially miss such outcomes. Finally, owing to the unavailability of data, other important cardiovascular outcomes like heart failure could not be examined by this review.
The strength of this review lies in its updated and comprehensive literature search involving several databases. Importantly, only age- and gender-matched studies were included to optimize the outcomes. Also, we avoided cross-sectional studies due to their inherent bias and inability to demonstrate causality (26). Separate analysis for the case-control and the cohort studies was conducted with sensitivity analysis to provide a detailed evaluation of the results.
The results have clinical implications. Patients with PD should be considered at high risk for adverse cardiovascular events. They should be closely monitored, especially by nursing personnel involved in their care, for acute symptoms of stroke, MI, and other cardiovascular diseases. However, it must also be considered that the quality of evidence in our study was low. The certainty of evidence was downgraded due to the risk of bias in the included studies as a result of the lack of adjustment of confounders. Therefore, our results should be interpreted with caution, and there is a need to strengthen the evidence by conducting more robust studies. Studies should also assess the pathophysiological relationship between PD and cardiovascular events and the possible interventional strategies to reduce such risk.
Conclusions
Patients with PD may have an increased risk of stroke as compared with the age- and gender-matched general population. Overall, our results show that PD does not increase the risk of MI and cardiovascular mortality. However, analysis of only the cohort studies demonstrated that patients with PD may have an increased risk of MI and cardiovascular mortality. Current evidence is of very low quality. Further prospective cohort studies from different countries and taking into account important cardiovascular risk factors are needed to improve the current evidence.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Author contributions
YH: Conceptualization, Investigation, Methodology, Software, Supervision, Validation, Writing – original draft. SX: Visualization, Writing – review and editing, Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Project administration, Software, Validation.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
Huzhou City Science and Technology Plan Project (2019GYB49).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1284826/full#supplementary-material
References
1. Balestrino R, Schapira AHV. Parkinson disease. Eur J Neurol. (2020) 27:27–42. doi: 10.1111/ENE.14108
2. Qi S, Yin P, Wang L, Qu M, Kan GL, Zhang H, et al. Prevalence of Parkinson’s disease: a community-based study in China. Mov Disord. (2021) 36:2940–4. doi: 10.1002/MDS.28762
3. Muangpaisan W, Hori H, Brayne C. Systematic review of the prevalence and incidence of Parkinson’s disease in Asia. J Epidemiol. (2009) 19:281–93. doi: 10.2188/JEA.JE20081034
4. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. (2019) 139:e56–28. doi: 10.1161/CIR.0000000000000659
5. Ciumărnean L, Milaciu MV, Negrean V, Orășan OH, Vesa SC, Sălăgean O, et al. Cardiovascular risk factors and physical activity for the prevention of cardiovascular diseases in the elderly. Int J Environ Res Public Health. (2021) 19:207. doi: 10.3390/IJERPH19010207
6. Alves M, Caldeira D, Ferro JM, Ferreira JJ. Does Parkinson’s disease increase the risk of cardiovascular events? A systematic review and meta-analysis. Eur J Neurol. (2020) 27:288–96. doi: 10.1111/ENE.14076
7. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Br Med J. (2016) 355:i4919. doi: 10.1136/BMJ.I4919
8. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
9. Okunoye O, Horsfall L, Marston L, Walters K, Schrag A. Rate of hospitalizations and underlying reasons among people with Parkinson’s disease: population-based cohort study in UK primary care. J Parkinsons Dis. (2022) 12:411–20. doi: 10.3233/JPD-212874
10. Park JH, Kim DH, Park YG, Kwon DY, Choi M, Jung JH, et al. Association of Parkinson disease with risk of cardiovascular disease and all-cause mortality: a nationwide, population-based cohort study. Circulation. (2020) 141:1205–7. doi: 10.1161/CIRCULATIONAHA.119.044948
11. Patel M, Coutinho C, Emsley HCA. Prevalence of radiological and clinical cerebrovascular disease in idiopathic Parkinson’s disease. Clin Neurol Neurosurg. (2011) 113:830–4. doi: 10.1016/J.CLINEURO.2011.05.014
12. Huang Y-P, Chen L-S, Yen M-F, Fann C-Y, Chiu Y-H, Chen H-H, et al. Parkinson’s disease is related to an increased risk of ischemic stroke-a population-based propensity score-matched follow-up study. PLoS One. (2013) 8:e68314. doi: 10.1371/JOURNAL.PONE.0068314
13. D’Amelio M, Ragonese P, Morgante L, Reggio A, Callari G, Salemi G, et al. Long-term survival of Parkinson’s disease: a population-based study. J Neurol. (2006) 253:33–7. doi: 10.1007/S00415-005-0916-7
14. Fall PA, Saleh A, Fredrickson M, Olsson JE, Granérus AK. Survival time, mortality, and cause of death in elderly patients with Parkinson’s disease: a 9-year follow-up. Mov Disord. (2003) 18:1312–6. doi: 10.1002/MDS.10537
15. Ben-Shlomo Y, Marmot MG. Survival and cause of death in a cohort of patients with Parkinsonism: possible clues to aetiology? J Neurol Neurosurg Psychiatry. (1995) 58:293–9. doi: 10.1136/JNNP.58.3.293
16. Levine RL, Jones JC, Bee N. Stroke and Parkinson’s disease. Stroke. (1992) 23:839–42. doi: 10.1161/01.STR.23.6.839
17. Macías-García D, Periñán MT, Muñoz-Delgado L, Jesús S, Jimenez-Jaraba MV, Buiza-Rueda D, et al. Increased stroke risk in patients with Parkinson’s disease with LRRK2 mutations. Mov Disord. (2022) 37:225–7. doi: 10.1002/MDS.28863
18. Abugroun A, Taha A, Abdel-Rahman M, Patel P, Ali I, Klein LW. Cardiovascular risk among patients ≥65 years of age with Parkinson’s disease (from the national inpatient sample). Am J Cardiol. (2020) 136:56–61. doi: 10.1016/J.AMJCARD.2020.09.021
19. Choi HG, Lim JS, Lee YK, Sim S, Kim M. Mortality and cause of death in South Korean patients with Parkinson’s disease: a longitudinal follow-up study using a national sample cohort. BMJ Open. (2019) 9:e029776. doi: 10.1136/BMJOPEN-2019-029776
20. Liang HW, Huang YP, Pan SL. Parkinson disease and risk of acute myocardial infarction: a population-based, propensity score-matched, longitudinal follow-up study. Am Heart J. (2015) 169:508–14. doi: 10.1016/J.AHJ.2014.11.018
21. Hobson P, Meara J. Mortality and quality of death certification in a cohort of patients with Parkinson’s disease and matched controls in North Wales, UK at 18 years: a community-based cohort study. BMJ Open. (2018) 8:e018969. doi: 10.1136/BMJOPEN-2017-018969
22. Braga M, Pederzoli M, Antonini A, Beretta F, Crespi V. Reasons for hospitalization in Parkinson’s disease: a case-control study. Parkinsonism Relat Disord. (2014) 20:488–92. doi: 10.1016/J.PARKRELDIS.2014.01.022
23. Driver JA, Kurth T, Buring JE, Gaziano JM, Logroscino G. Parkinson disease and risk of mortality: a prospective comorbidity-matched cohort study. Neurology. (2008) 70:1423–30. doi: 10.1212/01.WNL.0000310414.85144.EE
24. Becker C, Jick SS, Meier CR. Risk of stroke in patients with idiopathic Parkinson disease. Parkinsonism Relat Disord. (2010) 16:31–5. doi: 10.1016/J.PARKRELDIS.2009.06.005
25. Hahad O, Frenis K, Kuntic M, Daiber A, Münzel T. Accelerated aging and age-related diseases (CVD and neurological) due to air pollution and traffic noise exposure. Int J Mol Sci. (2021) 22:1–23. doi: 10.3390/IJMS22052419
26. Song JW, Chung KC. Observational studies: cohort and case-control studies. Plast Reconstr Surg. (2010) 126:2234. doi: 10.1097/PRS.0B013E3181F44ABC
27. Elfil M, Bayoumi A, Sayed A, Aladawi M, Aboutaleb PE, Grieb L, et al. Stroke in Parkinson’s disease: a review of epidemiological studies and potential pathophysiological mechanisms. Acta Neurol Belg. (2023) 123:773–83. doi: 10.1007/S13760-023-02202-4
28. Howard VJ, McDonnell MN. Physical activity in primary stroke prevention: just do it! Stroke (2015) 46:1735–9. doi: 10.1161/STROKEAHA.115.006317
29. Kokubo Y. Traditional risk factor management for stroke: a never-ending challenge for health behaviors of diet and physical activity. Curr Opin Neurol. (2012) 25:11–7. doi: 10.1097/WCO.0B013E32834EB58E
30. Merola A, Romagnolo A, Rosso M, Suri R, Berndt Z, Maule S, et al. Autonomic dysfunction in Parkinson’s disease: a prospective cohort study. Mov Disord. (2018) 33:391–7. doi: 10.1002/MDS.27268
31. Rawlings AM, Juraschek SP, Heiss G, Hughes T, Meyer ML, Selvin E, et al. Association of orthostatic hypotension with incident dementia, stroke, and cognitive decline. Neurology. (2018) 91:e759–68. doi: 10.1212/WNL.0000000000006027
32. Mattson M, Haberman F. Folate and homocysteine metabolism: therapeutic targets in cardiovascular and neurodegenerative disorders. Curr Med Chem. (2003) 10:1923–9. doi: 10.2174/0929867033456864
33. Todorović Z, Džoljić E, Novaković I, Mirković D, Stojanović R, Nešić Z, et al. Homocysteine serum levels and MTHFR C677T genotype in patients with Parkinson’s disease, with and without levodopa therapy. J Neurol Sci. (2006) 248:56–61. doi: 10.1016/J.JNS.2006.05.040
34. Rogers JD, Sanchez-Saffon A, Frol AB, Diaz-Arrastia R. Elevated plasma homocysteine levels in patients treated with levodopa: association with vascular disease. Arch Neurol. (2003) 60:59–64. doi: 10.1001/ARCHNEUR.60.1.59
35. Stamler JS, Osborne JA, Jaraki O, Rabbani LE, Mullins M, Singel D, et al. Adverse vascular effects of homocysteine are modulated by endothelium-derived relaxing factor and related oxides of nitrogen. J Clin Invest. (1993) 91:308–18. doi: 10.1172/JCI116187
36. Chrysant SG, Chrysant GS. The current status of homocysteine as a risk factor for cardiovascular disease: a mini review. Expert Rev Cardiovasc Ther. (2018) 16:559–65. doi: 10.1080/14779072.2018.1497974
37. Nabizadeh F, Valizadeh P, Sharifi P, Zafari R, Mirmosayyeb O. Risk of myocardial infarction in Parkinson’s disease: a systematic review and meta-analysis. Eur J Neurol. (2023) 30:2557–69. doi: 10.1111/ENE.15838
38. Chua SKK, Saffari SE, Lee SJY, Tan EK. Association between Parkinson’s disease and coronary artery disease: a systematic review and meta-analysis. J Parkinsons Dis (2022) 12:1737–48. doi: 10.3233/JPD-223291
39. Ricci F, Fedorowski A, Radico F, Romanello M, Tatasciore A, Di Nicola M, et al. Cardiovascular morbidity and mortality related to orthostatic hypotension: a meta-analysis of prospective observational studies. Eur Heart J. (2015) 36:1609–17. doi: 10.1093/EURHEARTJ/EHV093
40. Verwoert GC, Mattace-Raso FUS, Hofman A, Heeringa J, Stricker BHC, Breteler MMB, et al. Orthostatic hypotension and risk of cardiovascular disease in elderly people: the Rotterdam study. J Am Geriatr Soc. (2008) 56:1816–20. doi: 10.1111/J.1532-5415.2008.01946.X
41. Fedorowski A, Burri P, Struck J, Juul-Möller S, Melander O. Novel cardiovascular biomarkers in unexplained syncopal attacks: the SYSTEMA cohort. J Intern Med. (2013) 273:359–67. doi: 10.1111/JOIM.12043
42. Collins LM, Toulouse A, Connor TJ, Nolan YM. Contributions of central and systemic inflammation to the pathophysiology of Parkinson’s disease. Neuropharmacology. (2012) 62:2154–68. doi: 10.1016/J.NEUROPHARM.2012.01.028
Keywords: neurodegenerative disease, cardiac disease, mortality, risk, stroke
Citation: Hu Y and Xu S (2023) Association between Parkinson's disease and the risk of adverse cardiovascular events: a systematic review and meta-analysis. Front. Cardiovasc. Med. 10:1284826. doi: 10.3389/fcvm.2023.1284826
Received: 31 August 2023; Accepted: 21 November 2023;
Published: 7 December 2023.
Edited by:
Amit K. Dey, National Institutes of Health (NIH), United StatesReviewed by:
Nicholas Streicher, MedStar Georgetown University Hospital, United States Kumar Ashish, CarolinaEast Medical Center, United States© 2023 Hu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shanxia Xu eHVzaGFueGlheGlhQDE2My5jb20=
 Yan Hu
Yan Hu Shanxia Xu
Shanxia Xu