
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 15 December 2023
Sec. Coronary Artery Disease
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1284491
 Aihong Peng1,†
Aihong Peng1,† Bing Zhang1,†
Bing Zhang1,† Siyin Wang1,†
Siyin Wang1,† Yujia Feng1
Yujia Feng1 Shengnan Liu1
Shengnan Liu1 Cuiyi Liu1
Cuiyi Liu1 Shu Li2
Shu Li2 Fei Li1
Fei Li1 Yuanyuan Peng1*
Yuanyuan Peng1* Jing Wan1*
Jing Wan1*
Background: Inflammation and lipid infiltration play crucial roles in the development of atherosclerosis. This study aimed to investigate the association between various complex indexes of blood cell types and lipid levels with the severity of coronary artery stenosis and their predictive value in coronary heart disease (CHD).
Methods: The retrospective study was conducted on 3,201 patients who underwent coronary angiography at the Department of Zhongnan Hospital of Wuhan University. The patients were divided into two groups: CHD group and non-CHD group. The CHD group was further classified into three subgroups (mild, moderate, severe) based on the tertiles of their Gensini score or SYNTAX score I. Various complex indexes of blood cell types and lipid levels were compared between the groups.
Results: It revealed a positive correlation between all complex indexes and the severity of coronary artery stenosis. The systemic inflammation-response index/high-density lipoprotein cholesterol count (SIRI/HDL) exhibited the strongest correlation with both severity scores (Gensini score: r = 0.257, P < 0.001; SYNTAX score I: r = 0.171, P < 0.001). The monocyte to high-density lipoprotein cholesterol ratio (MHR) was identified as a stronger independent risk factor for CHD. However, SIRI/HDL had higher diagnostic efficacy for CHD (sensitivity 66.7%, specificity 60.4%, area under curve 0.680, 95% CI: 0.658–0.701). Notably, the pan-immune-inflammation value multiplied by low-density lipoprotein cholesterol count (PIV × LDL) exhibited the highest sensitivity of 85.2%.
Conclusion: All complex indexes which we investigated exhibited positive correlations with the severity of coronary artery stenosis. SIRI/HDL demonstrated higher diagnostic efficiency for CHD and a significant correlation with the severity of coronary artery stenosis.
The number of deaths attributed to non-communicable diseases (NCDs) worldwide in 2019 was 42.03 million, accounting for 74.36% of all deaths. Among NCDs, cardiovascular diseases accounted for the highest number of deaths, with coronary heart disease (CHD) being a major contributor (1). CHD accounted for 32.7% of the global burden of cardiovascular disease (2). Atherosclerosis, a sustained and dynamic inflammatory process in the vascular system, is a major underlying cause of CHD. It involves the interaction between immune responses and metabolic disturbances, leading to the formation and activation of coronary artery lesions (3, 4). Inflammatory cells (such as monocytes, neutrophils, and lymphocytes), lipid infiltration, platelet activation, coagulation cascade reactions, and platelet-fibrin clot formation play crucial roles in the development of atherosclerosis (5). In recent years, emerging indicators of inflammation and oxidative stress, such as the monocyte to high-density lipoprotein cholesterol ratio (MHR), neutrophil to lymphocyte ratio (NLR), systemic immune-inflammation index (SII), and systemic inflammation-response index (SIRI), have gained attention in assessing the severity and prognosis of CHD. Another parameter, the pan-immune-inflammation value (PIV) (6), has been defined as a comprehensive and cost-effective indicator of chronic low-grade inflammation. Bektas et al. found that PIV outperformed NLR and SII in predicting primary and secondary outcomes in ST-segment elevation myocardial infarction (STEMI) (7), with one-year all-cause mortality as the primary outcome. However, the correlation between PIV and the severity of coronary artery stenosis has not been explored in previous studies. In this study, we aimed to investigate the relationship between PIV and CHD while also examining complex indexes that combine leukocyte subtypes, platelet count, and lipid levels. We compared the diagnostic value of SIRI/HDL, SIRI × LDL, PIV/HDL, PIV × LDL, PIV, SIRI, SII, MHR, and NLR in predicting CHD and the severity of coronary artery stenosis.
This retrospective study included a total of 3,201 patients who underwent coronary angiography at the Department of Cardiology, Zhongnan Hospital of Wuhan University, between January 2013 and December 2021. The diagnosis of coronary heart disease (CHD) was made based on the latest diagnostic criteria for coronary heart disease. Based on the results of coronary angiography, the patients were categorized into two groups: non-CHD group and CHD group. The non-CHD group consisted of 727 patients, including 401 males and 326 females. The CHD group comprised 2,474 patients, including 1,725 males and 749 females. Within the CHD group, further classification was performed based on the tertiles of either the Gensini score or the SYNTAX score I, resulting in three subgroups: mild, moderate, and severe.
(1) With chest tightness, chest pain and other suspected coronary artery disease clinical manifestations; (2) Coronary angiography was performed and recorded; (3) With complete admission general data and relevant clinical examination data.
(1) Various acute and chronic infectious diseases; (2) Hematologic diseases; (3) Surgical procedures and severe trauma within 3 months; (4) Autoimmune diseases or being treated with immunosuppressive drugs; (5) Malignant tumors; (6) Previous history of myocardial infarction; (7) Previous history of percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG); (8) Cardiomyopathy or decompensated heart failure; (9) Severe hepatic and renal insufficiency; (10) Steroids use and incomplete clinical data.
Two experienced interventional cardiologists conducted coronary angiography and assessed the findings. The procedure involved multiple projections using the radial or femoral route, following the Judkins method. The cardiologists evaluated the degree of stenosis in the left main artery, left anterior descending artery, left circumflex artery, and right coronary artery. A stenosis of 50% or more in any of these coronary arteries can be diagnosed as coronary heart disease.
Based on the results of the coronary angiography, the Gensini score and the SYNTAX score I were computed for each patient. In the coronary artery disease group, the patients were categorized into three groups based on the tertiles of the Gensini score. The groups were as follows: mild group (Gensini score < 20), moderate group (20 ≤ Gensini score ≤ 45), and severe group (Gensini score > 45). The SYNTAX score I was determined using the SYNTAX scoring website (https://syntaxscore.org/). For the patients with coronary heart disease, they were divided into three groups according to the tertiles of the SYNTAX score: mild group (SYNTAX score < 8), moderate group (8 ≤ SYNTAX score ≤ 16), and severe group (SYNTAX score > 16).
General information of the patients was collected, which included their hospitalization number, age, gender, smoking status, hypertension, and diabetes mellitus. All patients underwent venous blood collection from the elbow in the early morning after admission, on an empty stomach (fasting for more than 10 h). The blood samples were tested by the Department of Clinical Laboratory at Zhongnan Hospital of Wuhan University. Various biochemical parameters were measured, including the following: neutrophil count (NEUT), lymphocyte count (LYMP), monocyte count (MONO), platelet count (PLT), white blood cell count (WBC), erythrocyte distribution width coefficient of variation (RDW-CV), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), alkaline phosphatase (ALP), creatinine (Cr), Uric acid (UA), fasting glucose (FBG). These parameters were measured to assess the patients' biochemical profile and provide important information regarding their health status.
PIV = [neutrophil count (×109/L) × platelet count (×109/L) × monocyte count (×109/L)/lymphocyte count (×109/L)]; SIRI = [neutrophil count (×109/L) × monocyte count (×109/L)/lymphocyte count (×109/L)]; SII = [neutrophil count (×109/L) × platelet count (×109/L)/lymphocyte count (×109/L)]; MHR = monocyte to high-density lipoprotein cholesterol ratio; NLR = neutrophil to lymphocyte ratio; SIRI/HDL = [SIRI/high-density lipoprotein cholesterol count(mmol/L)]; SIRI × LDL = [SIRI × low-density lipoprotein cholesterol count(mmol/L)]; PIV/HDL = [PIV/high-density lipoprotein cholesterol count(mmol/L)]; PIV × LDL = [PIV × low-density lipoprotein cholesterol count(mmol/L)].
The measurement data were presented as mean ± standard deviation () when they followed a normal distribution. For non-normally distributed measurement data, the median and interquartile range [M(Q3–Q1)] were used to describe the distribution. Count data were expressed as percentages (%). To compare two groups with normally distributed measures, the t-test was employed. When comparing multiple groups, the Analysis of Variance (ANOVA) was utilized. For conducting post-hoc comparisons between groups, the Least Significant Difference (LSD) analysis was employed. In cases where the measurement data did not conform to a normal distribution, the Mann-Whitney nonparametric test was employed for comparing two groups. For comparing multiple groups with non-normally distributed data, the Kruskal-Walli test was used. When dealing with count data, the Χ² test was employed for analysis. In the case of continuous variables following a normal distribution, Pearson correlation analysis was used. For variables that did not conform to a normal distribution, Spearman's rank correlation was employed. In the risk factor analysis of coronary heart disease, all variables underwent univariate Logistic analysis initially. Subsequently, composite variables showing statistical significance in the univariate analysis and other variables with statistical significance were subjected to multivariate Logistic regression analysis. The diagnostic efficacy was determined by employing Receiver Operating Characteristic (ROC) curve analysis. All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 25 (IBM SPSS Inc., Chicago, USA). A significance level of 5% (P < 0.05) was considered statistically significant.
It revealed that the majority of patients in the CHD group were male, accounting for 69.7% of the total. This suggests a higher prevalence of CHD in males compared to females. The prevalence of smoking, diabetes mellitus, and hypertension was significantly higher in the CHD group compared to the non-CHD group. Approximately 42.5% of patients with CHD were smokers, 27.8% had diabetes mellitus, and 63.8% had hypertension. These findings suggest that these factors contribute to the development of CHD and are more common among individuals with the disease. In terms of biomarkers and indices, the values of SIRI/HDL, SIRI × LDL, PIV/HDL, PIV × LDL, PIV, SIRI, SII, MHR, and NLR were higher in the CHD group compared to the non-CHD group. These biomarkers and indices represent different aspects of inflammatory immune responses and metabolic disturbances in the body. The observed differences in their values between the two groups indicate a potential association between these biomarkers and indices and the presence of CHD. These findings highlight the importance of considering these factors and biomarkers in the diagnosis and management of CHD (Table 1).
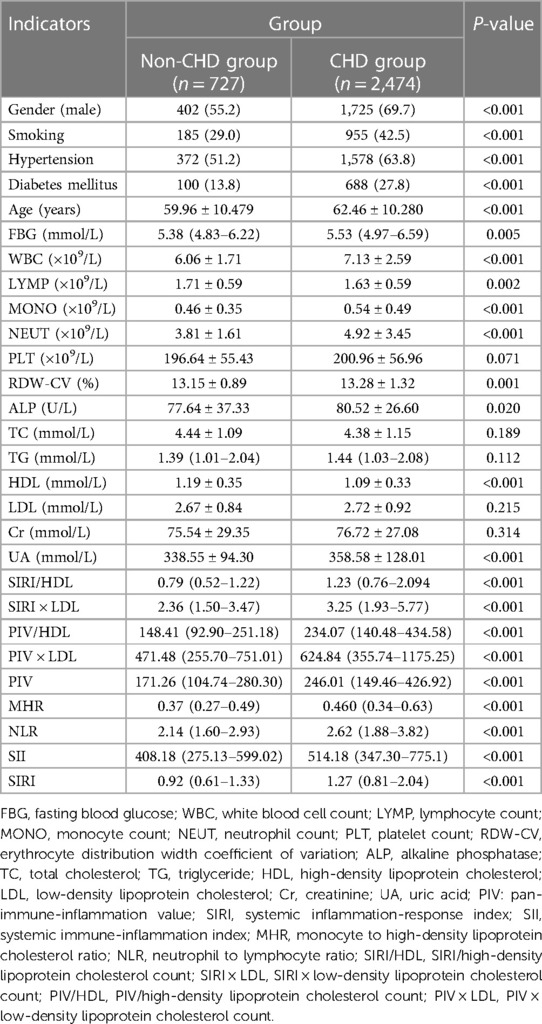
Table 1. Comparison of general information and complex indexes between the non-CHD group and the CHD group [(), M (Q3–Q1), n (%)].
The CHD group was further divided into mild, moderate, and severe categories based on the Gensini score tertiles. Comparing the complex indexes, it was observed that the severe group had higher values of SIRI/HDL, SIRI × LDL, PIV/HDL, PIV × LDL, PIV, SIRI, SII, MHR, and NLR compared to the other two groups. Additionally, the moderate and severe groups had higher values of SIRI/HDL, SIRI × LDL, PIV/HDL, PIV × LDL, PIV, SIRI, and NLR compared to the mild group. These differences were statistically significant, with a P-value of less than 0.05 (Table 2). Similarly, the CHD group was also categorized into mild, moderate, and severe groups based on the SYNTAX score I tertiles. In this analysis, the severe group exhibited higher values of SIRI/HDL, SIRI × LDL, PIV/HDL, PIV, SIRI, SII, MHR, and NLR compared to the other two groups. Moreover, the moderate and severe groups had higher values of SIRI/HDL, SIRI × LDL, PIV/HDL, PIV × LDL, PIV, SIRI, SII, and NLR compared to the mild group. These differences were statistically significant, with a P-value of less than 0.05 (Table 3). In summary, when categorizing the CHD group based on the severity of coronary artery lesions determined by the Gensini score and SYNTAX score I tertiles, the severe groups consistently exhibited higher values of various composite indexes compared to the mild and moderate groups. These findings suggest that these composite indexes are associated with the severity of coronary artery lesions and can potentially serve as indicators for assessing the extent and severity of CHD.
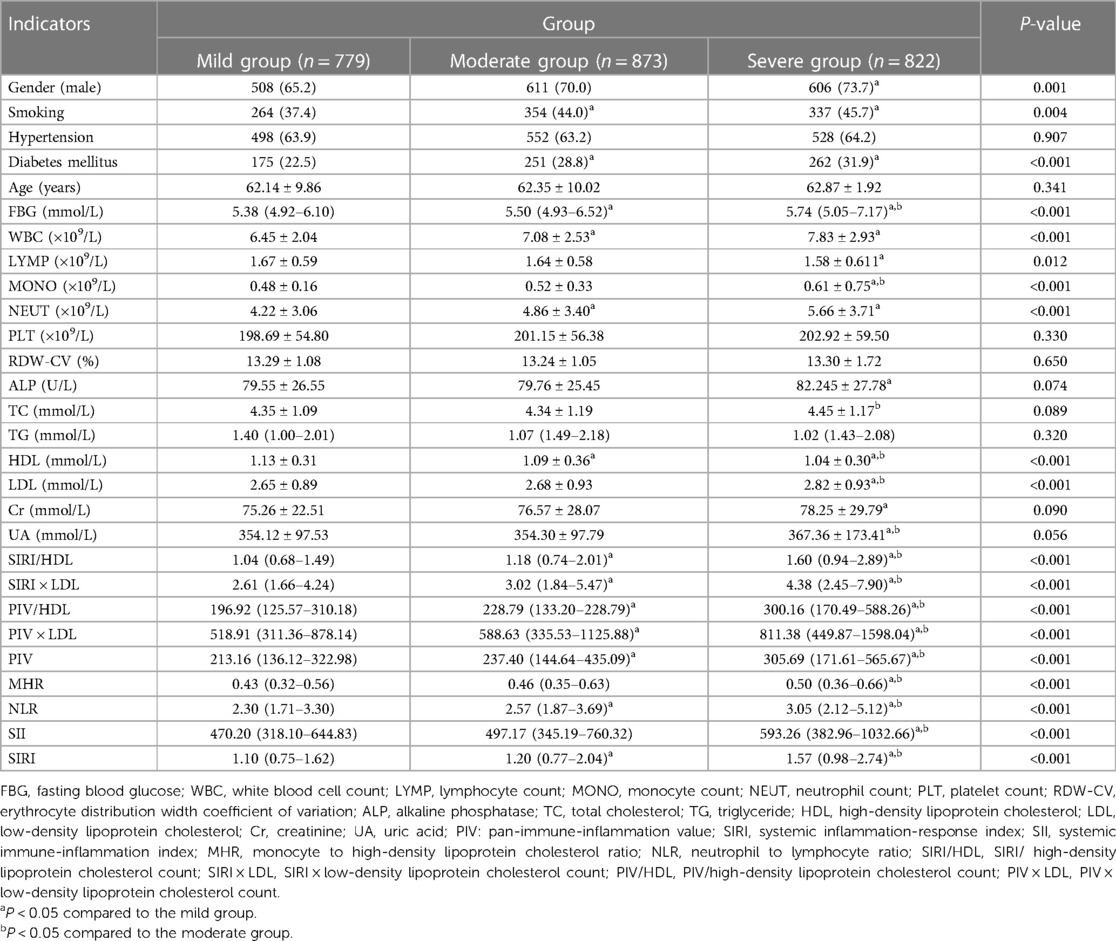
Table 2. Comparison of general information and complex indexes in the gensini score triple quartile group for CHD [(), M (Q3–Q1), n (%)].
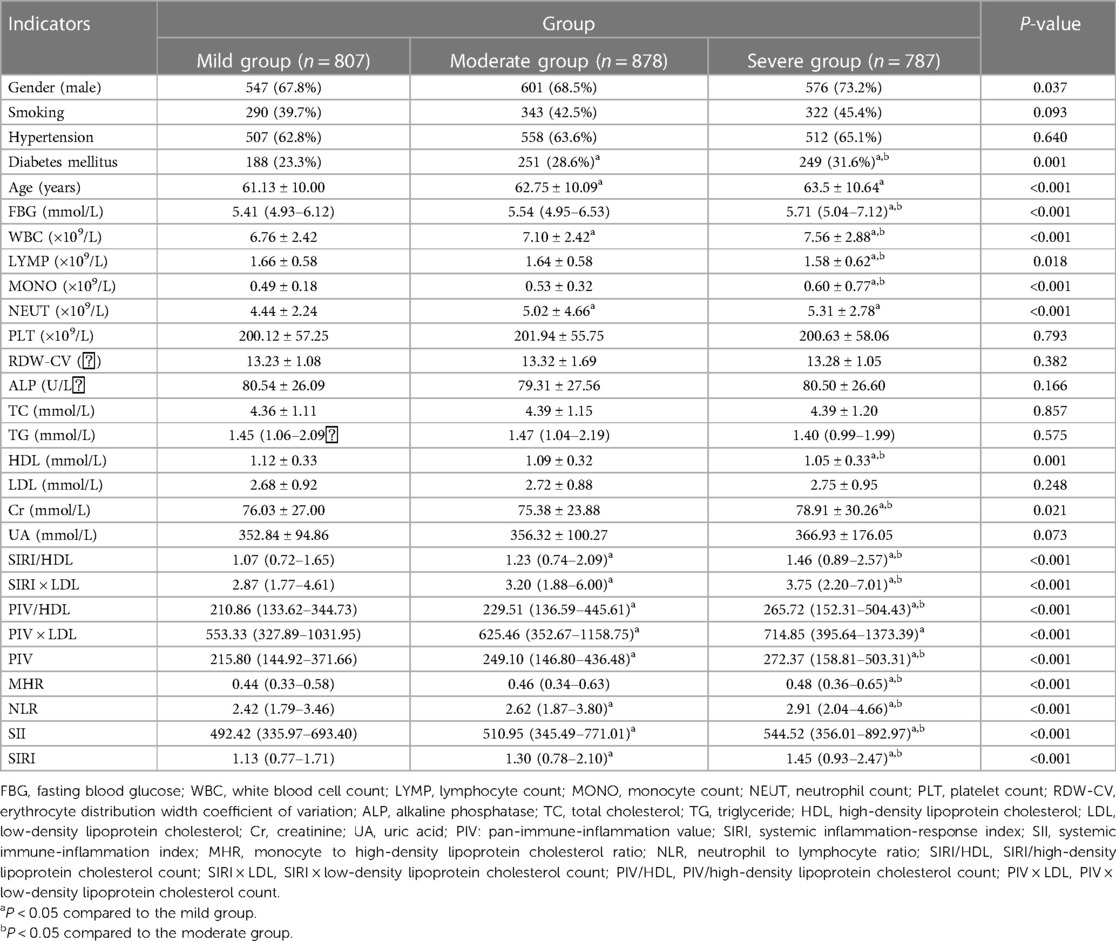
Table 3. Comparison of general information and complex indexes in the SYNTAX score triple quartile group for CHD [(), M (Q3–Q1), n (%)].
Spearman correlation analysis was conducted to examine the relationship between various factors, including SIRI/HDL, SIRI × LDL, PIV/HDL, PIV × LDL, PIV, SIRI, SII, MHR, NLR, and Gensini score or SYNTAX score I. The findings revealed that SIRI/HDL, SIRI × LDL, PIV/HDL, PIV × LDL, PIV, SIRI, SII, MHR, and NLR exhibited positive correlations with the Gensini score. Among these factors, SIRI/HDL demonstrated the strongest correlation with a coefficient of 0.257 (P < 0.001) (Figure 1). Additionally, SIRI/HDL, SIRI × LDL, PIV/HDL, PIV × LDL, PIV, SIRI, SII, MHR, and NLR were also positively correlated with SYNTAX scores I, although the correlation was relatively weak. SIRI/HDL again displayed the strongest correlation with a coefficient of 0.171 (P < 0.001) (Table 4). This analysis suggests that SIRI/HDL, SIRI × LDL, PIV/HDL, PIV × LDL, PIV, SIRI, SII, MHR, and NLR are associated with both Gensini score and SYNTAX score I, indicating their potential relevance to the severity of coronary artery disease. Notably, SIRI/HDL appears to have the strongest correlation with both scoring systems.
The presence of CHD was used as the dependent variable to assess its association with various factors. Further analysis was performed using multivariate logistic regression to identify independent influencing factors for the occurrence of CHD. The complex indexes with statistically significant associations in the single-factor analysis, along with other variables that showed statistical significance in the single-factor analysis, were included in the multivariate logistic regression analysis. The results demonstrated that SIRI/HDL, SIRI × LDL, PIV/HDL, PIV, SIRI, SII, MHR, and NLR could be considered as independent influencing factors for the occurrence of CHD (Table 5). Among these factors, MHR was found to be a stronger independent risk factor for CHD compared to other complex indexes. Overall, it suggests that SIRI/HDL, SIRI × LDL, PIV/HDL, PIV, SIRI, SII, MHR, and NLR have significant associations with the development of CHD. These factors may serve as valuable indicators for assessing the risk of CHD, with MHR showing the strongest independent association.
The results demonstrated that SIRI/HDL exhibited superior effectiveness in diagnosing coronary heart disease compared to SIRI × LDL, PIV/HDL, PIV × LDL, PIV, SIRI, SII, MHR, NLR, with the highest efficacy observed when the cut-off value for SIRI/HDL was set at 1.015. The sensitivity of SIRI/HDL was found to be 0.667%, specificity was 0.604%, and the area under the curve was 0.680 (95% CI: 0.658–0.701), indicating good diagnostic performance. On the other hand, PIV × LDL demonstrated the highest sensitivity of 85.2%, while PIV/HDL exhibited the highest specificity of 0.623% (Table 6 and Figure 1).
To the best of our knowledge, the correlation PIV and the severity of coronary artery stenosis has not been explored in previous studies. Furthermore, to gain a deeper understanding, we combined leukocyte subtypes, platelet count, and lipid profile to create complex indexes. This allowed us to analyze the relationship between the severity of coronary artery stenosis and these complex indexes, including SIRI/HDL, SIRI × LDL, PIV/HDL, PIV × LDL, PIV, SIRI, SII, MHR, and NLR, and their predictive value for CHD. There are some key findings. First, we demonstrated that PIV were positively correlated with the severity of coronary artery lesions through a large sample analysis. Second, although PIV is a new inflammatory indicator that can diagnose CHD, its diagnostic ability is not as good as that of the previous inflammatory indicator SIRI. Third, by combining the inflammatory indicator SIRI with HDL, we derived the SIRI/HDL index, which may enhance the diagnostic accuracy of CHD. Forth, compared with single inflammatory indicators, the complex index obtained by combining them with HDL can improve the diagnostic ability of CHD. In contrast, the diagnostic ability of the complex index obtained by combining them with LDL becomes weaker.
The incidence of CHD has been increasing due to changes in lifestyle and societal and economic development. Additionally, there is a concerning trend of CHD occurring at younger ages (8). AS we know, CHD is considered a chronic inflammatory disease and is a result of the narrowing or obstruction of coronary arteries due to atherosclerosis. It is characterized by the accumulation of lipids, local inflammation, proliferation of smooth muscle cells (SMCs), apoptosis, necrosis, and fibrosis (9). It involves a complex interplay of various inflammatory cells, including monocytes, neutrophils, and lymphocytes, as well as platelets and lipid metabolism (10–15). In addition, arterial stiffness is a recognized predictor of cardiovascular (CV) morbidity and death, and is an early indicator of arteriosclerosis. Inflammatory biomarkers and biomarkers of lipid metabolism were associated with arterial stiffness, a surrogate marker of cardiovascular events, indirectly emphasizing the prophylactic importance of those biomarkers (16, 17). Peripheral blood inflammatory cell counts and their derivatives have gained popularity in clinical practice as easily accessible and cost-effective indicators of chronic low-grade inflammation. These indicators have proven useful in assessing the severity and prognosis of coronary artery disease. Many studies have highlighted the importance of parameters such as MHR, NLR, SII, SIRI in evaluating CHD severity and prognosis (18–26). Moreover, the novel parameter called pan-immune inflammatory value (PIV) has been proposed as a more reliable predictor of clinical outcomes in patients with advanced colorectal cancer (6). Bektas et al. discovered that PIV was able to predict both early and late prognosis in patients with ST-segment elevation myocardial infarction (STEMI) (7). However, relying on a single inflammatory indicator may not provide a complete understanding of inflammation severity. Therefore, early and accurate screening should involve a combination of multiple inflammatory factors and biomarkers of lipid metabolism to predict the severity of the disease more accurately. Based on the above studies, our study focused on the relationship between PIV and the severity of coronary lesions. In addition, while exploring the relationship between PIV and the severity of coronary artery lesions, we boldly combined leukocyte subtypes, platelets and lipids to form complex indexes. To further explore and compare the relationship between SIRI/HDL, SIRI*LDL, PIV/HDL, PIV*LDL, PIV, SIRI, SII, MHR, NLR and the degree of coronary artery stenosis and their predictive value to coronary heart disease.
The Gensini score reflects the plaque load, fully considers the number, location, and degree of stenosis of coronary artery lesions, and can better evaluate the degree of coronary artery lesions. It has been widely used in clinical practice. The SYNTAX score I reflects the type of plaque and the complexity of PCI, and is a guideline for decision-making of revascularization in patients with coronary artery disease, and its predictive value for adverse events has been confirmed in many studies (27). In this study, we assessed the severity of coronary artery lesions using both the Gensini score and the SYNTAX score I in a cohort of 3,201 patients who underwent coronary angiography. Our findings demonstrated a positive correlation between each complex index and the degree of coronary artery stenosis. Notably, SIRI/HDL exhibited the strongest correlation with both the Gensini score and SYNTAX score I. As the level of SIRI/HDL increased, indicating a higher value, the Gensini score also increased, suggesting a more severe coronary artery lesion. Moreover, SIRI/HDL showed superior diagnostic efficacy compared to other composite indices. Using a threshold value of 1.015 for SIRI/HDL, the sensitivity and specificity were determined to be 0.667% and 0.604%, respectively. The area under the curve for SIRI/HDL was 0.680.
In our study, we demonstrated the link between PIV and the severity of coronary artery lesions through a large sample analysis. By combining the inflammatory marker SIRI with HDL, we derived the SIRI/HDL index, which provided a more comprehensive understanding of lipid infiltration, systemic inflammation, and immune system activation in patients with CHD. The blood cell counts and lipid profile, required for calculating SIRI/HDL, can be easily and quickly obtained. Utilizing SIRI/HDL may enhance the diagnostic accuracy of CHD. Additionally, it is worth noting that the complex index PIV × LDL exhibited the highest sensitivity in diagnosing CHD. In clinical practice, it is crucial to recognize the significance of using complex indicators in diagnosing CHD. By employing these complex indexes, we can develop effective strategies for early detection and treatment, benefiting a larger number of patients and improving their prognosis.
In this study, we acknowledged several limitations. One of the most significant limitations was the retrospective design, which was based on the study's retrospective nature. Additionally, our participants were from a single center rather than multiple centers, and the study design was cross-sectional. Furthermore, we only assessed atherosclerosis through coronary angiography, while intravascular ultrasonography and coronary computed tomography might provide more precise information about the extent of coronary atherosclerosis. And due to the existence of multicollinearity among the composite indicators, we conducted multifactorial logistic regression analysis on these indicators independently from the other variables in the study of coronary heart disease. This approach allowed for a more accurate assessment of the impact of the composite indicators on the development of the disease. It was worth noting that some patients may be taking statins, which can affect their TC and LDL levels. However, even with lower LDL levels resulting from medication use, cardiovascular events associated with AS can still occur. This might be due to other lipid compositions contributing to residual risk and ongoing inflammatory reactions during the active AS process (28). While we did discover correlations, it was imperative that additional research could be conducted to verify the clinical relevance of the composite indices of different leukocyte subtypes, lipid, and platelet in relation to AS.
All complex indexes which we investigated, including PIV, exhibited positive correlations with the severity of coronary artery stenosis. SIRI/HDL, a complex index combing blood cell type and lipid measurements, demonstrated higher diagnostic efficiency for CHD and a significant correlation with the severity of coronary artery stenosis. Incorporating SIRI/HDL into diagnostic protocols may improve the accuracy of CHD diagnosis.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
JW: Writing – review & editing, Writing – original draft. AP: Writing – original draft, Data curation, Methodology. SW: Methodology, Writing – review & editing. BZ: Methodology, Writing – review & editing. YF: Data curation, Writing – review & editing. SL: Data curation, Writing – review & editing. CL: Data curation, Writing – review & editing. LS: Data curation, Writing – review & editing. YP: Writing - review & editing, Writing – original draft. FL: Data curation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We would like to gratefully acknowledge the support of Zhongnan Hospital of Wuhan University for investigators participating in this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bai J, Cui J, Shi F, Yu C. Global epidemiological patterns in the burden of main non-communicable diseases, 1990–2019: relationships with socio-demographic Index. Int J Public Health. (2023) 68:1605502. doi: 10.3389/ijph.2023.1605502
2. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet. (2017) 390(10100):1211–59. doi: 10.1016/S0140-6736(17)32154-2
3. Natorska J, Wypasek E, Grudzień G, Sobczyk D, Marek G, Filip G, et al. Does diabetes accelerate the progression of aortic stenosis through enhanced inflammatory response within aortic valves? Inflammation. (2012) 35(3):834–40. doi: 10.1007/s10753-011-9384-7
4. Bäck M, Yurdagul A, Tabas I, Öörni K, Kovanen PT. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat rev Cardiol. (2019) 16(7):389–406. doi: 10.1038/s41569-019-0169-2
5. Mustafic S, Ibralic AM, Loncar D. Association of inflammatory and hemostatic parameters with values of high sensitive troponin in patients with acute coronary syndrome. Med Arch. (2022) 76(2):84–9. doi: 10.5455/medarh.2022.76.84-89
6. Fucà G, Guarini V, Antoniotti C, Morano F, Moretto R, Corallo S, et al. The pan-immune-inflammation value is a new prognostic biomarker in metastatic colorectal cancer: results from a pooled-analysis of the valentino and TRIBE first-line trials. Brit J Cancer. (2020) 123(3):403–9. doi: 10.1038/s41416-020-0894-7
7. Murat B, Murat S, Ozgeyik M, Bilgin M. Comparison of pan-immune-inflammation value with other inflammation markers of long-term survival after ST-segment elevation myocardial infarction. Eur J Clin Invest. (2023) 53(1):e13872. doi: 10.1111/eci.13872
8. Koopman C, Bots ML, van Dis I, Vaartjes I. Shifts in the age distribution and from acute to chronic coronary heart disease hospitalizations. Eur J Prev Cardiol. (2016) 23(2):170–7. doi: 10.1177/2047487314544975
9. Wu MY, Li CJ, Hou MF, Chu PY. New insights into the role of inflammation in the pathogenesis of atherosclerosis. Int J Mol Sci. (2017) 18(10):2034. doi: 10.3390/ijms18102034
10. Schaftenaar F, Frodermann V, Kuiper J, Lutgens E. Atherosclerosis: the interplay between lipids and immune cells. Curr Opin Lipidol. (2016) 27(3):209–15. doi: 10.1097/MOL.0000000000000302
11. Kologrivova I, Shtatolkina M, Suslova T, Ryabov V. Cells of the immune system in cardiac remodeling: main players in resolution of inflammation and repair after myocardial infarction. Front Immunol. (2021) 12:664457. doi: 10.3389/fimmu.2021.664457
12. Massberg S, Brand K, Grüner S, Page S, Müller E, Müller I, et al. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med. (2002) 196(7):887–96. doi: 10.1084/jem.20012044
13. Nording HM, Seizer P, Langer HF. Platelets in inflammation and atherogenesis. Front Immunol. (2015) 6:98. doi: 10.1084/jem.20012044
14. Pasalic L, Wang SS, Chen VM. Platelets as biomarkers of coronary artery disease. Semin Thromb Hemost. (2016) 42(3):223–33. doi: 10.1055/s-0036-1572328
15. Choi HY, Hafiane A, Schwertani A, Genest J. High-density lipoproteins: biology, epidemiology, and clinical management. Can J Cardiol. (2017) 33(3):325–33. doi: 10.1016/j.cjca.2016.09.012
16. Baba M, Maris M, Jianu D, Luca CT, Stoian D, Mozos I. The impact of the blood lipids levels on arterial stiffness. J Cardiovasc Dev Dis. (2023) 10(3):127. doi: 10.3390/jcdd10030127
17. Mozos I, Malainer C, Horbańczuk J, Gug C, Stoian D, Luca CT, et al. Inflammatory markers for arterial stiffness in cardiovascular diseases. Front Immunol. (2017) 8:1058. doi: 10.3389/fimmu.2017.01058
18. Ganjali S, Gotto AM, Ruscica M Jr, Atkin SL, Butler AE, Banach M, et al. Monocyte-to-HDL-cholesterol ratio as a prognostic marker in cardiovascular diseases. J Cell Physiol. (2018) 233(12):9237–46. doi: 10.1002/jcp.27028
19. Kaya H, Ertaş F, Soydinç MS. Association between neutrophil to lymphocyte ratio and severity of coronary artery disease. Clin Appl Thromb Hem. (2014) 20(2):221. doi: 10.1177/1076029613499821
20. Sahin DY, Elbasan Z, Gür M, Yildiz A, Akpinar O, Icen YK, et al. Neutrophil to lymphocyte ratio is associated with the severity of coronary artery disease in patients with ST-segment elevation myocardial infarction. Angiology. (2013) 64(6):423–9. doi: 10.1177/0003319712453305
21. Han K, Shi D, Yang L, Wang Z, Li Y, Gao F, et al. Prognostic value of systemic inflammatory response index in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Ann Med. (2022) 54(1):1667–77. doi: 10.1080/07853890.2022.2083671
22. Duffy BK, Gurm HS, Rajagopal V, Gupta R, Ellis SG, Bhatt DL. Usefulness of an elevated neutrophil to lymphocyte ratio in predicting long-term mortality after percutaneous coronary intervention. JACC. (2006) 97(7):993–6. doi: 10.1016/j.amjcard.2005.10.034
23. Dziedzic EA, Gąsior JS, Tuzime KA, Paleczny J, Junka A, Dąbrowski M, et al. Investigation of the associations of novel inflammatory biomarkers-systemic inflammatory index (SII) and systemic inflammatory response index (SIRI)-with the severity of coronary artery disease and acute coronary syndrome occurrence. Int J Mol Sci. (2022) 23(17):9553. doi: 10.3390/ijms23179553
24. Sun M, Zhao D, Zhang Y, Zhai Y, Ye M, Wang X, et al. Prognostic utility of monocyte to high-density lipoprotein ratio in patients with acute coronary syndrome: a meta-analysis. Am J Med Sci. (2020) 359(5):281–6. doi: 10.1016/j.amjms.2020.01.018
25. Akboga MK, Inanc IH, Sabanoglu C, Akdi A, Yakut I, Yuksekkaya B, et al. Systemic immune-inflammation index and C-reactive protein/albumin ratio could predict acute stent thrombosis and high SYNTAX score in acute coronary syndrome. Angiology. (2023) 74(7):693–701. doi: 10.1177/00033197221125779
26. Çelik Aİ, Bezgin T, Karaaslan MB, Coşkun R, Çağdaş M. The relationship between coronary artery calcium score and monocyte to high-density lipoprotein cholesterol ratio in patients with stable angina pectoris. Turk Kardiyol Dern A. (2022) 50(8):583–9. doi: 10.5543/tkda.2022.22412
27. Palmerini T, Genereux P, Caixeta A, Cristea E, Lansky A, Mehran R, et al. Prognostic value of the SYNTAX score in patients with acute coronary syndromes undergoing percutaneous coronary intervention: analysis from the ACUITY (acute catheterization and urgent intervention triage StrategY) trial. Jam Coll Cardiol. (2011) 57(24):2389–97. doi: 10.1016/j.jacc.2011.02.032
Keywords: coronary heart disease, Gensini score, SYNTAX score I, systemic inflammation-response index/high-density lipoprotein cholesterol count (SIRI/HDL), complex indexes
Citation: Peng A, Zhang B, Wang S, Feng Y, Liu S, Liu C, Li S, Li F, Peng Y and Wan J (2023) Comparison of the value of various complex indexes of blood cell types and lipid levels in coronary heart disease. Front. Cardiovasc. Med. 10:1284491. doi: 10.3389/fcvm.2023.1284491
Received: 28 August 2023; Accepted: 29 November 2023;
Published: 15 December 2023.
Edited by:
Tommaso Gori, University Medical Centre, Johannes Gutenberg University Mainz, GermanyReviewed by:
Ioana Mozos, Victor Babes University of Medicine and Pharmacy, Romania© 2023 Peng, Zhang, Wang, Feng, Liu, Liu, Li, Li, Peng and Wan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanyuan Peng cGVuZ3l5QHdodS5lZHUuY24= Jing Wan d2FuamluZ196bkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.