- 1Laboratório de Imunologia, Instituto do Coração (InCor), LIM19, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, (HCFMUSP), São Paulo, Brazil
- 2Department of Internal Medicine, School of Medical Sciences, State University of Campinas (UNICAMP), São Paulo, Brazil
- 3Department of Basic Pharmaceutical Sciences, Fred Wilson School of Pharmacy, High Point University, High Point, NC, United States
Editorial on the Research Topic
COVID-19 Mechanisms on Cardio-Vascular Dysfunction: From membrane receptors to immune response, Volume II
Introduction
This second edition of the Research Topic “COVID-19 Mechanisms on Cardio-Vascular Dysfunction: From membrane receptors to immune response” reiterates our commitment to fill some aspects of this research lacuna by searching for original advanced and contemporary knowledge on this theme/issue. In this edition we present three relevant manuscripts: an original research article, a case report, and a review, which are summarized in this Editorial.
Since 2020, when the World Health Organization (WHO) declared a worldwide pandemic in relation to COVID-19, it has been known that several comorbidities influence patients' outcomes, with varying degrees of impact on the severity of the disease. Nevertheless, the cause–effect relationships between molecular/cellular mechanisms and clinical manifestations remain poorly understood. In this context, cardiovascular manifestations independent of or dependent on pre-existing diseases are associated with greater severity and poor prognosis in COVID-19 patients (Figure 1).
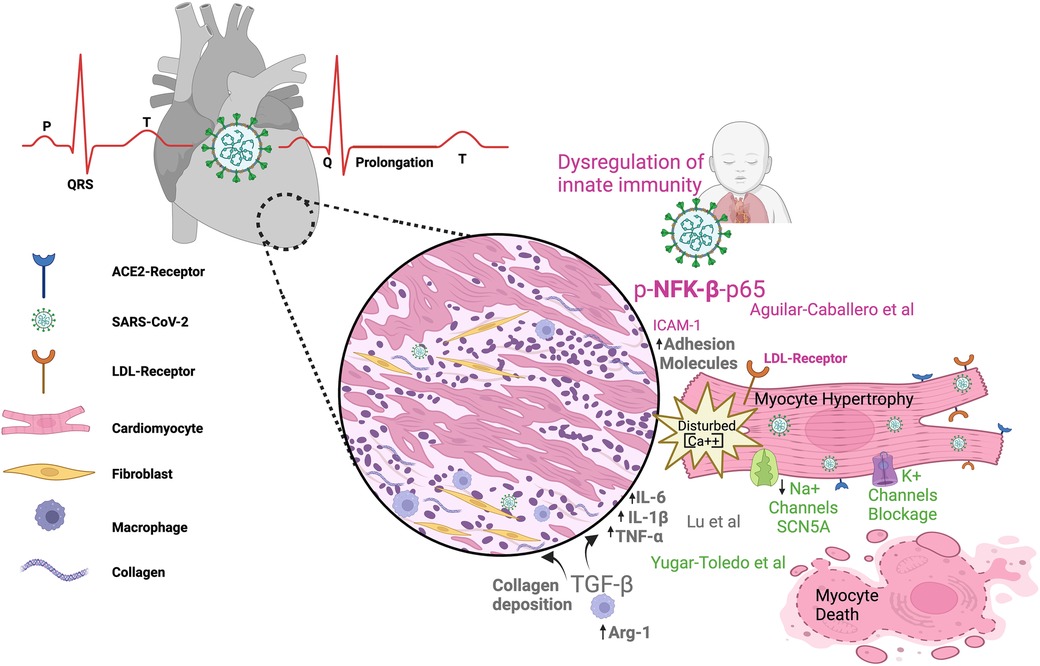
Figure 1. SARS-CoV-2 infection can impact the cardiovascular system in both children and adults. The figure illustrates electrocardiogram P, Q, R, S, T waves and their alterations in SARS-CoV-2 infection; dysregulated metabolism; inflammation; and immunity as components of the link between COVID-19 infection and heart damage. ACE2, angiotensin-converting enzyme 2; Arg-1, arginase 1; LDL, low-density lipoprotein receptor; TNFα, tumor necrosis factor alpha; (IL)-6, (IL)-1β, IL, interleukin; TGF-β, transforming growth factor–beta; NFK-β, nuclear factor kappa B.
Growing evidence indicates changes in gene signature in association with phenotype manifestations of pathophysiological conditions. Recent progress regarding the ways in which COVID-19 contributes to cardiovascular complications is assessed by Lu et al. Using bioinformatic analysis, the authors demonstrate how COVID-19 and atrial fibrillation (AF) are potentially associated with the gene signature. Transcriptome analyses unveil 54 shared differentially expressed genes (DEGs) between COVID-19 and AF, of which 34 are upregulated and 20 are downregulated genes. Among these shared DEGs, 10 key genes may become potential biomarkers and/or therapeutic targets (RPS8, BMP4, SFN, TYMS, NOG, AK5, WNT11, RLN, ARG1, and ACSL). The relationship between COVID-19 and AF includes regulatory mechanisms such as transcriptional factors, miRNAs, and drugs. Additionally, three genes (ARG1, GIMAP7, and RFX2) emerge as severity/prognosis markers linking COVID-19 to AF development. Similar studies (1, 2) have shown that, during COVID-19 disease, concomitant complications such as hypertension, heart failure, renal diseases, and diabetes mellitus type 2 share some genes and/or regulatory molecules with AF/COVID-19, suggesting that some mechanisms resulting from SARS-CoV-2 infection are common between different preexisting comorbidities. Apparently, some genes involved in negative complications that link comorbidities to COVID-19 symptoms and prognosis are not related to whether the disease onset occurred before or post SARS-CoV-2 infection.
Lu et al. also describe how the mechanisms by which COVID-19 triggers AF are associated with predominant DEGs that cause disturbances in signaling pathways related to cell metabolism, inflammation, and immunity. The emerging principles that link cellular energy disturbances and deregulated immune/inflammatory systems to disease progression are not new. Immunometabolism relationships contribute to the progression and severity of both COVID-19 (3, 4) and cardiovascular diseases (5) (Raghavan et al.), independently of their association. The cytokine release and immune cell differentiation that occurs during COVID-19 is under metabolic control (3, 4), and an understanding of how the immune response depends on intricate metabolic cell pathways is crucial in order to improve patient outcomes.
The lethality of pulmonary hyperinflammation in a preterm newborn (28 weeks) after intrauterine transmission of SARS-CoV-2 is the focus of the case report by Aguilar-Caballero et al. The authors report progressive and irreversible worsening lung hyperinflammation mediated by an imbalance of the immune response and inflammatory mediators, leading to severe and systemic clinical complications culminating in death. Elevated expression levels were observed in lung and heart detection of biomarkers related to hyperinflammation (LDLr, phospho-NF-κβ-p65, OPN), vascular dysfunction (ICAM-1), and NETs (citH3). The OPN molecule is expressed not only by immune cells but also in the vascular cells (EC and SMC) and cardiomyocytes, and circulating OPN levels predict outcomes and mechanical ventilation exigency in both adults (6) and children (7). Extreme prematurity (less than 28 weeks) is not associated with a severe course and/or poor prognosis of COVID-19 (8). Importantly, this premature infant suffered from long COVID-19 symptoms, with persistent infection consistent with the disease severity. Chronic and persistent immune dysregulation, together with an active viral infection, prevented all efforts to treat the patient and avoid progression of disease severity (9).
The review by by Yugar-Toledo et al. summarizes the main aspects of cardiovascular infection, including the clinical symptoms, during COVID-19 and sheds light on previously established comorbidities and their association with disease progression. The authors review SARS-CoV-2 damage in the myocardium, demonstrating the dependence of the inflammatory response and the three main pathways responsible for cardiac disease. These three mechanisms can affect the myocardium through the direct impact of viral entrance, through consequential damage (through down- and up-regulation of ACE2 and AT1 receptor expression, respectively), and finally, through indirect action in the form of recruitment and activation of immune cells mediating the systemic inflammatory response. The relevant consequences for the cardiovascular system include Kawasaki-like disease in children and acute coronary artery disease, arrhythmias, Takotsubo syndrome, and other cardiovascular and pulmonary complications in adults. The molecular mechanisms of cardiac arrythmias, ECG alterations, and associated findings are also reviewed, as shown by imaging assessments of cardiovascular comorbidities.
The collection of articles presented in this second special issue represents the landscape of the state of the art in cardiovascular diseases and their relationship to SARS-CoV-2 infection and COVID-19 development. In conclusion, this Research Topic highlights a two-way road connecting cardiovascular diseases and COVID-19: cardiovascular complications may be preexisting factors or consequences that aggravate COVID symptoms and prognosis. DEGs, transcriptional factors, drugs, and molecular routes emerge as a promising therapeutic targets, biomarkers for assessment of severity, and drivers of drug administration. Independently of the causal relationships, the improvement of our understanding of cardiovascular dysfunction and its underlying mechanisms will contribute to our understanding of COVID-19 pathology, progression, and prediction of clinical outcomes.
Author contributions
AW: Writing – review & editing. RS: Writing – review & editing. AM: Writing – original draft, Writing – review & editing.
Acknowledgments
We thank Dr. Kent Stewart for the editing work on the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nashiry A, Sarmin Sumi S, Islam S, Quinn JMW, Moni MA. Bioinformatics and system biology approach to identify the influences of COVID-19 on cardiovascular and hypertensive comorbidities. Brief Bioinformatics. (2021) 22(2):1387–401. doi: 10.1093/bib/bbaa426
2. Dolan ME, Hill DP, Mukherjee G, McAndrews MS, Chesler EJ, Blake JA. Investigation of COVID-19 comorbidities reveals genes and pathways coincident with the SARS-CoV-2 viral disease. Sci Rep. (2020) 10(1):20848. doi: 10.1038/s41598-020-77632-8
3. Xiao N, Nie M, Pang H, Wang B, Hu J, Meng X, et al. Integrated cytokine and metabolite analysis reveals immunometabolic reprogramming in COVID-19 patients with therapeutic implications. Nat Commun. (2021) 12(1):1618. doi: 10.1038/s41467-021-21907-9
4. Batabyal R, Freishtat N, Hill E, Rehman M, Freishtat R, Koutroulis I. Metabolic dysfunction and immunometabolism in COVID-19 pathophysiology and therapeutics. Int J Obes. (2021) 45(6):1163–9. doi: 10.1038/s41366-021-00804-7
5. Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. (2006) 4(1):13–24. doi: 10.1016/j.cmet.2006.05.011
6. Hayek SS, Roderburg C, Blakely P, Launius C, Eugen-Olsen J, Tacke F, et al. Circulating osteopontin levels and outcomes in patients hospitalized for COVID-19. J Clin Med. (2021) 10(17):3907. doi: 10.3390/jcm10173907
7. Reisner A, Blackwell LS, Sayeed I, Myers HE, Wali B, Heilman S, et al. Osteopontin as a biomarker for COVID-19 severity and multisystem inflammatory syndrome in children: a pilot study. Experimental Biology and Medicine (Maywood, N.J.). (2022) 247(2):145–51. doi: 10.1177/15353702211046835
8. Piersigilli F, Carkeek K, Hocq C, van Grambezen B, Hubinont C, Chatzis O, et al. COVID-19 in a 26-week preterm neonate. Lancet Child Adolesc Health. (2020) 4(6):476–8. doi: 10.1016/S2352-4642(20)30140-1
Keywords: LDL-receptor, COVID-19, myocarditis, NFkapapB, arrhythima, ACE-2 receptor, SARS- coV- 2, ICAM -1
Citation: Moretti AIS, Schreiber R and Wanschel ABA (2023) Editorial: COVID-19 mechanisms on cardio-vascular dysfunction: from membrane receptors to immune response, volume II. Front. Cardiovasc. Med. 10:1278067. doi: 10.3389/fcvm.2023.1278067
Received: 15 August 2023; Accepted: 11 September 2023;
Published: 13 October 2023.
Edited and Reviewed by: Ichiro Manabe, Chiba University, Japan
© 2023 Moretti, Schreiber and Wanschel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amarylis B. A. Wanschel YXdhbnNjaGVAaGlnaHBvaW50LmVkdQ== Ana Iochabel Moretti YWlzbW9yZXR0aUBnbWFpbC5jb20= Roberto Schreiber cm9iZXJ0b3NAdW5pY2FtcC5icg==
†ORCID Ana Iochabel Soares Moretti orcid.org/0000-0003-3402-5778 Roberto Schreiber orcid.org/0000-0002-8930-7556 Amarylis B. A. Wanschel orcid.org/0000-0002-5603-7983
 Ana Iochabel Soares Moretti1*†
Ana Iochabel Soares Moretti1*† Amarylis B. A. Wanschel
Amarylis B. A. Wanschel