- 1Inova Center of Outcomes Research, Inova Heart and Vascular, Fairfax, VA, United States
- 2Section of Cardiovascular Medicine, Yale University School of Medicine, New Haven, CT, United States
- 3Department of Medicine, Division of Cardiology, Johns Hopkins University School of Medicine, Baltimore, MD, United States
The global population of older adults is expanding rapidly resulting in a shift towards managing multiple chronic diseases that coexist and may be exacerbated by cardiovascular illness. Stable ischemic heart disease (SIHD) is a predominant contributor to morbidity and mortality in the older adult population. Although results from clinical trials demonstrate that chronological age is a predictor of poor health outcomes, the current management approach remains suboptimal due to insufficient representation of older adults in randomized trials and the inadequate consideration for the interaction between biological aging, concurrent geriatric syndromes, and patient preferences. A shift towards a more patient-centered approach is necessary for appropriately and effectively managing SIHD in the older adult population. In this review, we aim to demonstrate the distinctive needs of older adults who prioritize holistic health outcomes like functional capacity, cognitive abilities, mental health, and quality of life alongside the prevention of major adverse cardiovascular outcomes reported in cardiovascular clinical trials. An individualized, patient-centered approach that involves shared decision-making regarding outcome prioritization is needed when any treatment strategy is being considered. By prioritizing patients and addressing their unique needs for successful aging, we can provide more effective care to a patient population that exhibits the highest cardiovascular risks.
1. Introduction
According to the United Nations (1), the world population of adults over 75 years of age is expected to rise by 40% within the next decade, growing from 165 million in 2020 to 231 million in 2030. The life expectancy at 80 years of age is projected to reach 7.8 years by 2025, 8 years by 2030, and 9.1 years by 2050 (1). With these projections, the number of people aged 75 or older is expected to double by 2050, accounting for over half of the total demographic of the older adult population.
In the United States, a similar demographic shift is evident. The Centers for Disease Control and Prevention (CDC) estimate that the number of individuals >80 years will grow from 9.3 million in 2000 to 19.5 million in 2030 (2). The life expectancy at 85 is predicted to increase from 7.1 years in 2017 to 8.4 years in 2060, according to the US Census (3). These trends call for urgent action in appropriately managing therapeutic strategies and interventions for favorable outcomes among older adults (4). Through recent decades, there has been a clear shift in the major disease spectrum (2), with chronic cardiovascular diseases now forming the majority of the comorbidity burden.
Currently, stable ischemic heart disease (SIHD) ranks among the top causes of morbidity and mortality in older patient populations (5–7). There is a projected doubling in the incidence of SIHD in the population aged >75 years in both men and women (5–8). The Global Burden of Diseases, Injuries, and Risk Factors study that assessed diseases with the largest impact on disability-adjusted life years, showed that in patients aged >75 years, SIHD remains the highest contributor for the past three decades (9). According to the recent American Heart Association (AHA) statement on the management of acute coronary syndrome (ACS) in the older adult population, the highest proportion of individuals who are hospitalized for ACS are patients aged 75 and above (10). These patients also tend to be sicker and require more frequent escalation of care at presentation (11). Owing to cardiovascular changes with aging, pre-existing geriatric syndromes, multimorbidity, and the scarcity of evidence on management of SIHD in the older population with multiple chronic conditions, their clinical outcomes in practice remain suboptimal (10).
1.1 Aging and SIHD
The initial definition of successful aging by Rowe and Kahn (12) emphasized the concept of disease avoidance and maintaining a disease-free state, thereby excluding most older patients living with multiple chronic conditions. However, the perceptions of successful aging are evolving. Empirical studies demonstrate that older adults frequently equate successful aging with multi-dimensional behavioral and psychosocial factors (13, 14). In a study, Bowling et al. (15) compared a biomedical and a psychosocial model of healthy aging to patient's own perspectives and observed that there was a divergence in what constituted important parameters of healthy aging. The literature consistently demonstrates a misalignment between the physiological or functional model of aging and the perspectives of older patients (16–18), highlighting the importance of incorporating subjective criteria of assessment.
Patient-reported outcomes (PROs) are defined as measures that directly capture patients' perspectives on their health, functional status, symptoms, and QoL (19). PROs encompass multiple domains pertinent to the health of older patients, including but not limited to functional ability and physical health, social and environmental support, religiosity (20), less depressive affective functioning (18), and intact cognition. There is a compelling call to prioritize PRO in cardiovascular care for older patients (21). The American Geriatrics Society (AGS) advocates for clinicians to engage in discussions about health care with patients and caregivers. Decisions should be aligned with patients' health priorities and their health trajectories instead of disease-specific care (22). The Geriatric 5Ms (mind, mobility, medications, multicomplexity, and “matters most to me”) (23) is a communication tool that can be used in the majority of healthcare decisions in older patients.
The number of older patients living with SIHD is rising, but evidence-based therapies in older adult populations remain limited (24). Randomized controlled trials (RCTs) evaluating management strategies have failed to adequately incorporate geriatric syndromes and age-related physical and cognitive confounders in the precepts of care (25, 26). Moreover, these trials have inconsistently defined major adverse cardiovascular events (MACE) and have largely ignored patient preferences while implementing therapeutic management strategies (25, 26). Consequently, relying solely on clinical practice guidelines based on published literature may prove insufficient and may introduce adverse outcomes in the highest-risk populations of older adults (27). To address this, there is a growing need to broaden our focus from preventing MACE outcomes to capturing the heterogeneous aging experiences, redefining health priorities, and evaluating the progression of geriatric syndromes with cardiovascular outcomes (28, 29).
The central objective of this review is to critically evaluate the relationship between PROs and the treatment approach for SIHD in older patients. We will examine the multidimensional aspects of PROs—encompassing functional ability, physical health, social support, mental health, and cognitive status—and their implications on SIHD management. The care objectives of treating older individuals are distinct, and a patient's perspective on these objectives may outweigh the potential benefits of life-extending, evidence-based treatments. Prior to implementing a treatment plan for this patient population, it is essential to evaluate existing body of evidence and conduct a risk-benefit analysis. We also aim to establish a tailored approach to SIHD management by incorporating a patient-centric model that aligns with older patients' specific health priorities, preferences, and unique aging experiences.
Key Takeaway
1. There is a disconnect between traditional biomedical models of aging and older adults' actual experiences and perspectives.
2. Current management strategies for SIHD in older patients are insufficient as they do not account for geriatric syndromes, age-related risks, and patient preferences, necessitating a shift towards an individualized, patient-centered approach that reflects the diverse aging experience of these patients.
2. Definition of older adults
In the foundational framework of active aging, the World Health Organization (WHO) proposed that older adults be defined as individuals over the age of 60 years (30). Despite acknowledging the potential ambiguity of this definition, it became an anchor for the initial age-related frameworks (31). In contemporary times, for both national and international population demographics, the definition of older adults has been extended to include those above 65 years of age consistent with Medicare eligibility (32, 33). Yet, in recent years, as life expectancy has increased, simultaneously with improvements in QoL, there has been an emerging consensus on the need to reevaluate and redefine the definition of older adults. In the Journal of Aging and Physical Activity, there was advocacy for adopting a more nuanced and stratified classification of the older patients (34). This proposed classification by Spirduso et al. subdivides the older population into the “young old” (ages 65–74), the “old” (ages 75–84), the “old-old” (ages 85–99), and the “oldest old” (ages 100+). This approach helps to capture the heterogeneity within the older adult population, acknowledging that a 65-year-old and an 80-year-old are likely to have markedly different health and functional profiles and hence have different biological ages.
The concept of biological aging can significantly diverge from chronological aging, which follows a fixed and linear pattern. The aging process is inherently heterogeneous, impacting cellular structures, molecular pathways, and entire organ systems in diverse ways. This complexity arises from various factors, including immune aging, accumulative metabolic damage at the cellular level (35), and inflammageing (increasingly recognized as both a symptom and a cause of age-associated illnesses) (36–38). The emerging understanding of cellular senescence further contributes to this multifaceted process (39). Lipsitz's review encapsulates this dynamic interplay by elucidating that aging is not only associated with increased complexity within anatomic structures and physiological functions, but also with heightened variability in physiological responses (40). This increased variability, coupled with a concurrent decline in adaptive capacity, is a distinctive hallmark of the aging process (40).
The molecular intricacies of aging are further complicated by the impact of diverse factors, including genetics, lifestyle choices, disease burden, and the presence or absence of geriatric syndromes. Geriatric syndromes, such as frailty, multimorbidity, and functional disability, can significantly accelerate the biological and, subsequently, cardiovascular aging processes (41, 42). They also shape the individual's physical resilience or susceptibility to various stressors and diseases. Frailty, a clinical syndrome reflecting a decline in physiological and functional reserve, increases with age and is a precursor of disability (43, 44). Frailty not only contributes to accelerated physiological aging but is independently associated with poor health outcomes regardless of the presence or absence of a specific disease state (45, 46). Another geriatric syndrome, multimorbidity, is highly prevalent in the older population (47–49). It is characterized by the coexistence of diseases that are functionally and physiologically independent but may synergistically contribute to physical dysfunction and functional decline (50, 51). This concept differs from comorbidity, which refers to a condition where one disease state is the chronological successor of multiple interacting conditions (52). As Calderón-Larrañaga et al. discuss comprehensively (53), multimorbidity involves a deleterious cycle wherein co-existing diseases interact, thereby undermining compensatory mechanisms and leading to physical and cognitive decline. Conversely, physical and cognitive impairments exacerbate the severity and burden of multimorbidity, thus establishing a bidirectional dysfunction (46). Much like frailty, the presence of multimorbidity is associated with poor functional ability, adverse health outcomes, and increased mortality (48, 49). Taken together, understanding and accounting for these differences is important in delivering effective care to older patients (54). Owing to the significant differences that exist in biological aging with every decade, it is important to revise the definition of “older adult” to encompass a more nuanced approach to aging. In this review, we refer to older individuals as those above the age of 75 and limit our discussion to this cohort.
Key Takeaway
1. The conventional definition of older adults as individuals over 60 or 65 is overly simplistic because it fails to take into account the complexities of biological aging.
2. Geriatric syndromes can influence the biological and cardiovascular aging process, impact resilience, and alter an individual's functional status. Hence, a tailored healthcare approach that accounts for these complexities is important.
3. Cardiovascular physiology and aging
A constellation of molecular, biological, and clinical changes constitute the hallmarks of aging that increase the susceptibility of older patients to the spectrum of cardiovascular diseases (55, 56). At the cellular level, multiple interdependent mechanisms and processes are observed that facilitate cardiovascular aging (55, 56). This includes the superoxide-driven upsurge in oxidative stress, chronic low-grade inflammation (57), and the increased expression of pro-inflammatory cytokines (58). Endothelial damage further leads to a dysregulated response to vascular injuries and stressors, impaired vasodilatory mechanisms, and increased intimal thickness (58–61). This detrimental sequence of events leads to a distinctive phenomenon of vascular aging (61–63). Simultaneously, aging also impairs the compensatory mechanism of the cardiovascular system to both internal and external stressors (59). For instance, older patients have impaired myocardial reperfusion post-acute myocardial infarction (AMI), which prolongs the recovery process (60). These cumulative maladaptive structural and functional transformations not only amplify the incidence of SIHD and AMI in older populations but also contribute to poor health outcomes (59). This has been corroborated by observations from several clinical studies. Early research from the GUSTO-I (64) trial highlighted age as a key determinant of outcomes in STEMI patients, a finding supported by the PURSUIT Trial of NSTEMI patients (65). Subsequently, both the Thrombolysis in Myocardial Infarction (TIMI) score (66) and the GRACE score (67) incorporated older age as an important factor that predicts death and cardiac ischemic events. More recently, Luca et al. recognized age as the strongest predictor of poor outcomes, even when adjusted for other risk factors (68). Both Rosengren et al. (69), and APEX-AMI (70) showed that in patients with ACS and STEMI, respectively, there was an increased likelihood of heart failure, cardiogenic shock, atrial fibrillation, and recurrent ischemia in older patients. In-hospital death rates also markedly increased in proportion to higher age for patients over 75 years. This understanding is important because not only the mechanism of SIHD development is significantly different in older patients, but also the trajectory it follows.
The second confounder of outcomes is the presence of geriatric syndromes, such as frailty, cognitive impairment, and multimorbidity, which amplifies the risk of systemic diseases, including SIHD, in older patients. Geriatric syndromes are associated with poor health outcomes across the spectrum of SIHD severity. The prevalence of frailty in older patients presenting with coronary disease is estimated to be as high as 19% (71). Frailty has been linked to sub-optimal/detrimental cardiovascular and all-cause morbidity and mortality outcomes in older patients with SIHD (54, 71–78). Adding to this burden is the presence of comorbidities which exacerbates the risk of poor health outcomes. Mortality risk in older patients has been shown to increase in a proportional pattern with the increase in comorbidity burden (79–81), and the benefits of revascularization, while generally improving survival after ACS event, show progressive less benefit with increasing comorbidity and frailty burden (79, 82).
Cognitive impairment, which is shown to increase in prevalence with increasing age (83), further complicates the prognosis in older patients with SIHD (46). Even mild cognitive impairment has been associated with poor cardiovascular outcomes (84). A higher 30-day mortality rate and an increased risk of admission at one year were reported among those with dementia or advanced cognitive decline (85). A recent meta-analysis reinforced this concept by demonstrating a higher short-term (30-day) and long-term mortality in older patients with cognitive impairment compared to their cognitively intact peers (86). Hence, an improved understanding of the influence of age-associated factors on MACE outcomes can help improve the clinical management of the older patient populations.
Key Takeaways
1. Chronological age influences SIHD outcome. There is a higher risk of cardiac ischemic events, heart failure, atrial fibrillation, and cardiogenic shock in patients aged 75 years and older.
2. The spectrum of changes associated with biological aging and geriatric syndromes, such as frailty, cognitive impairment, and multimorbidity, contribute to poor health outcomes in older patients with SIHD and should be taken into account when planning for any revascularization strategy.
4. Older adults in SIHD trials
The establishment of clinical practice guidelines and their general applicability necessitates evidence based on a well-represented study population. The lack of age-appropriate safety data, adverse event profiles, and insights into real-world effectiveness can, and does, lead to poor clinical outcomes (87). Up until the last decade, the majority of clinical practice guidelines on the prevention, diagnosis, and management of SIHD in the older adults have been extrapolation from clinical trials from the much younger patient cohorts. In fact, a recent systematic review of all clinical trials focusing on ACS management revealed that a meager 12.9% of over 1 million patients enrolled were aged 75 and above (88). Despite efforts to improve enrollment of representative older populations by removing chronologic age cutoffs, progress has remained modest (89).
Several factors contribute to the poor representation of older adults in clinical trials, which can be broadly classified into three categories: restrictive study design, recruitment difficulties, and retention challenges (90). Geriatric syndromes such as frailty, multimorbidity, and cognitive impairment pose significant challenges at each of these stages. The presence of geriatric syndromes is frequently encountered as a study exclusion criteria itself. Multimorbidity poses a challenge for both the internal and external validity of clinical trials (91). It can affect appropriate treatment selection as well as lead to confounding of treatment outcomes (91). Furthermore, polypharmacy is a common occurrence in older adult individuals due to the prevalence of multimorbidity. Polypharmacy complicates the investigation of the efficacy and safety of new drugs due to the higher potential for drug-drug interactions (92), which can lead to ineffectiveness and increased adverse events. Beyond the biological model, geriatric syndromes also impact recruitment and compound retention problems due to various logistical hurdles. These issues span a wide spectrum: transportation and mobility difficulties arising from functional dependence, economic constraints, and limited understanding or access to digital technology, all of which could curtail participation or heighten dropout rates (90). All these factors lead to under-representation, not only by direct causal effects but also by selection biases, as clinicians and researchers hesitate to recruit these complex patients in clinical trials (93).
Key Takeaways
1. Most clinical guidelines on prevention, diagnosis, and management of SIHD have been based on clinical trials primarily involving younger patient cohorts, and hence there is inadequate representation of older adults.
2. Selection bias, restrictive study design, recruitment difficulties, retention challenges, and logistical issues have all contributed to the underrepresentation of older adults in cardiovascular clinical trials
3. Geriatric syndromes such as multimorbidity, polypharmacy, frailty, and cognitive impairment further complicate clinical trials due to potential interactions.
5. Current outcomes measures in clinical trials
5.1. Traditional MACE vs. patient-centered outcomes
Following on the initiatives from the National Institutes of Health, including the Inclusion Across the Lifespan policy (94), there has been a deliberate shift towards inclusion of older patients in cardiovascular trials. However, many of the more recent pivotal trials focused on traditional MACE outcomes such as mortality, rehospitalization, repeat revascularization, stroke, and peripheral vascular disease (68). These outcomes are seldom standardized or take into account the complexity of age-associated conditions that coexist in the older population. Table 1 shows the major landmark trials and registries that are currently inclusive of the older adult population.
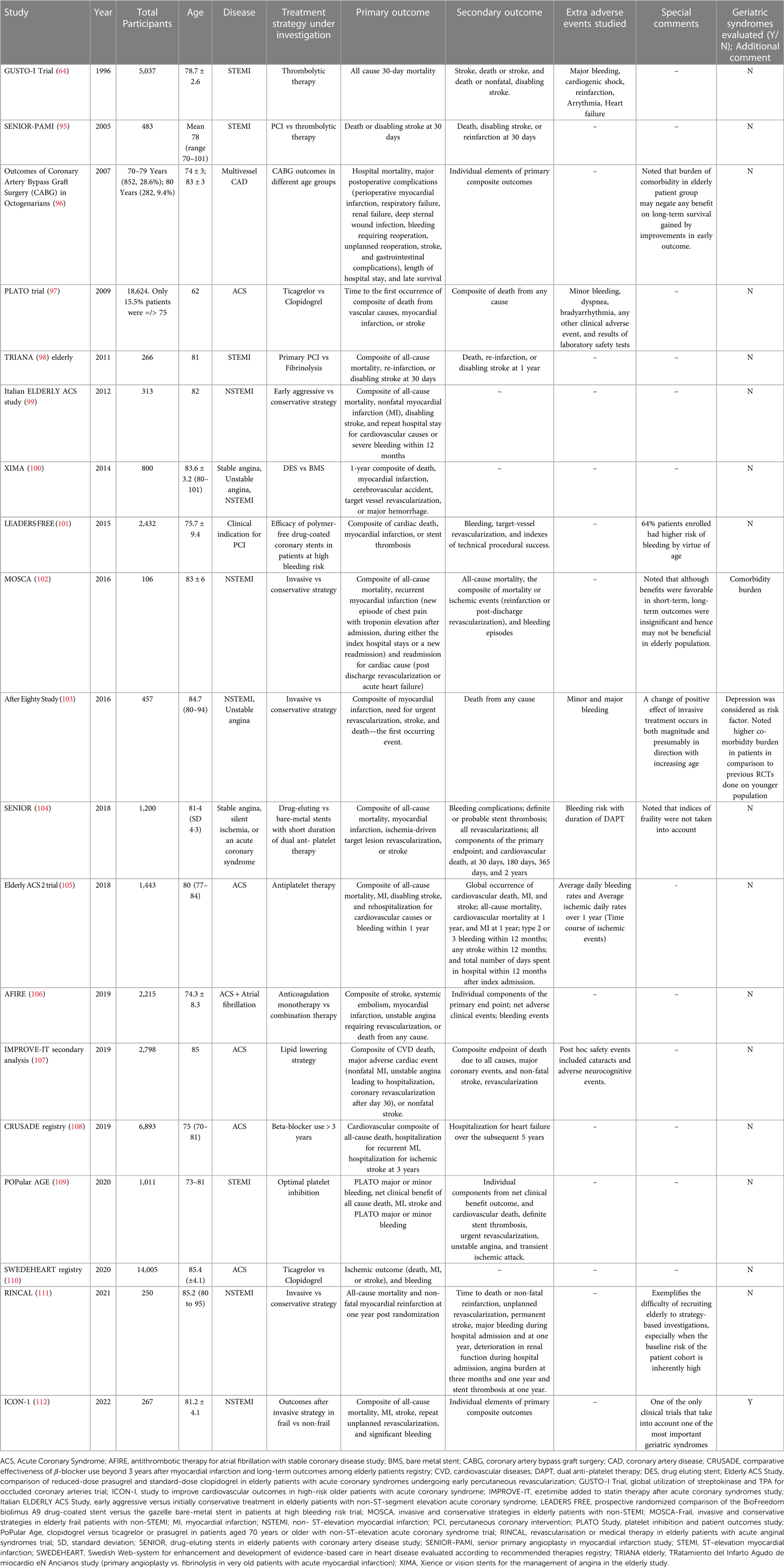
Table 1. Major randomized clinical trials and prospective cohort studies that enrolled older adult populations with coronary artery disease.
When we examine these trials, it is strikingly clear that the participation of older patients in clinical trials remains unsatisfactory. This underrepresentation is disproportional to the prevalence of SIHD in this population. The definition of MACE as a primary or co-primary outcome also exhibits considerable variability across the trials, and the follow-up varies significantly. These inconsistencies pose a significant challenge in pooling the data and comparing the results for generalizability. Moreover, geriatric syndromes have rarely been evaluated in landmark trials, except After Eighty Study (103) and ICON-1 (112). These parameters significantly impact the prognosis and management of SIHD for older individuals.
Even with recent strides that have been made to better understand and address the health outcomes pertinent to older patients, an essential facet that remains inadequately addressed is considering patient-centric preferences. These preferences, shaped by their own perception of successful or healthy aging, can deviate from the traditionally disease-oriented view of clinicians, researchers, and/or policymakers. For instance, von Faber et al. (113) presented a model of successful aging that includes optimal physical and social functioning as well as the subjective state of well-being. In their study cohort of individuals aged over 85 years, while only 10% of patients met the traditional health metrics of successful aging, a striking 80% subjectively reported successful aging. This perspective highlights that older patients often view successful aging as an adaptive process that consists of physical and social functioning and may not ascertain all benefits in traditional terms. Strawbridge et al. (114) compared patients' self-rating with Rowe and Kahn's criteria of successful aging (12). Only 18% of patients met the criteria as defined by Rowe, but >50% rated themselves as successful aged. They found that the self-rated model of successful aging demonstrated stronger associations with most well-being measures when compared to the Rowe and Kahn model.
Montgomery and Fahey (115) noted the divergence between patients' and physicians' treatment preferences in the importance of healthcare outcomes. Patients were more likely to select an additional therapeutic intervention if they perceived a significantly elevated disease-related risk, although the focus was on a single disease-related outcome. For older SIHD patients, this decision-making process becomes even more complex due to the multimorbidity (116) and polypharmacy (117). Nanna et al. identified that age influences treatment goals, willingness to consider invasive cardiac procedures, and risk tolerance among hospitalized older patients with SIHD. As patients age, they tend to more frequently prioritize goals, such as maintaining independence and mental capabilities, while being concerned with the loss of physical abilities and mental capacity (118). The AGS has acknowledged the importance of “preference-sensitive” decisions (117), stating that outcomes valued by older patients may deviate from disease-focused clinical practice guidelines and, in fact, may be in conflict with their individual health preferences. Fried et al. (119) examined the concept of competing outcomes of significant relevance in the older population. When queried about single-disease treatment preferences, patients would initially strongly align with evidence-based guidelines. However, a notable shift was observed when their global health was considered: they prioritized avoiding significant adverse effects secondary to therapy over disease-focused treatment guidelines. This was especially pronounced when treatment had marginal effects on disease-specific outcomes and/or failed to improve their QoL. This trend was in alignment with a subsequent study wherein more than 90% of the patients would decline even a low-burden evidence-based therapy if it potentially led to functional or cognitive impairment, irrespective of disease-specific benefits (120). Examining cardiovascular outcomes specifically, Tinetti et al. (121) reported that nearly half of their older cohort prioritized mitigating the risk of fall injuries and medication-related symptoms over curtailing the future risk of cardiovascular events in hypertension management. In a similar way, Caughey et al. (122) found that the initial patient preference for taking a disease-specific medication dropped dramatically when potential adverse events or competing health outcomes were considered. The inclusion of these preferences enables clinicians and investigators to evaluate the comprehensive impact of any treatment strategy on the overall health and QoL of older adults from their own perspective. Nanna et al. have recommended a “Consider, Listen, Decide” approach to complex decision-making in older adults with SIHD that incorporates these concepts (24, 123). We discuss the important patient-reported outcomes and their relevance in managing CAD in the older patient population below (Figure 1).
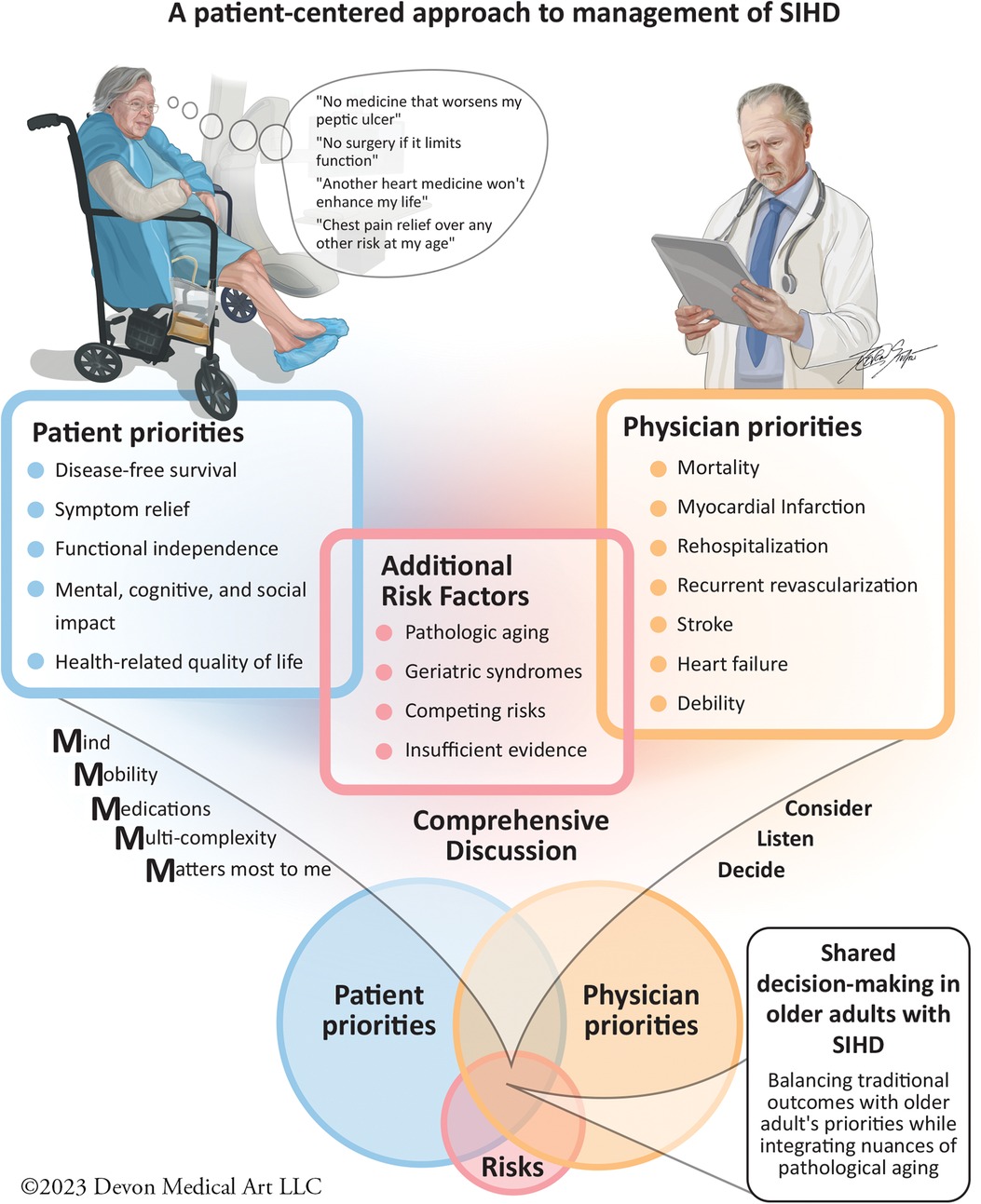
Figure 1. In older adults presenting with SIHD symptoms, it is essential to first identify their expected outcomes and set priorities in the context of potential competing results. Physicians then must take into account the additional risk factors, such as age, concomitant geriatric syndromes, and evaluate the evidence supporting specific therapies. Physicians then present management options and highlight their associated traditional outcomes, such as MACE to the patient. Using the “Geriatric 5 M's” and the “Consider, Listen, and Decide” Approach, can help to promote shared decision-making. This approach ensures we develop a strategy that respects patient-reported outcomes and aligns with evidence-based therapy for SIHD management.
Key Takeaways
1. Traditional MACE outcomes, such as rehospitalization, stroke, and mortality, that are often used in clinical trials are seldom standardized in accordance with pre-existing conditions in older adults.
2. Older patients often view successful aging as an adaptive process that includes optimal physical and social functioning rather than meeting traditional health metrics. Older adults may prioritize avoiding significant adverse effects secondary to therapy over disease-focused treatment guidelines, especially when the treatment has marginal effects on improving their QoL.
3. Competing health outcomes is an important consideration for older patients with multimorbidity and polypharmacy.
5.2. Patient-reported outcomes
5.2.1 Quality of life
The WHO defines QoL as “an individual's perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards, and concerns” (124). Bowling et al. defined QoL as a concept that is a collection of interactive objective and subjective dimensions (125). Bowling further refined the definition as a measure that reflects not just macro-societal influences but also delves into the nuances of an individual's personal experiences, circumstances, well-being, values, perceptions, and self-assessment of health (125).
One of the earliest large-scale oL assessments that involved patients' own perspectives was done by posing open-ended questions to patients eliciting a multifaceted range of responses that spanned several domains: independence, social relationships, social roles and activities, health, psychological well-being, perspective about home and neighborhood, and financial circumstances (125). In addition to identifying the core values that defined the meaning of QoL for older patients, this study also demonstrated the wide array of responses elicited by similar questions of integrating individualistic preferences. A public survey conducted by Brown et al. (126) identified the most important components of QoL for older patients—family and social relationships, emotional well-being, spirituality, functional independence, social engagement, standard of living, and health maintenance. Multiple studies focusing on older men and women further corroborated these QoL facets (121, 127, 128).
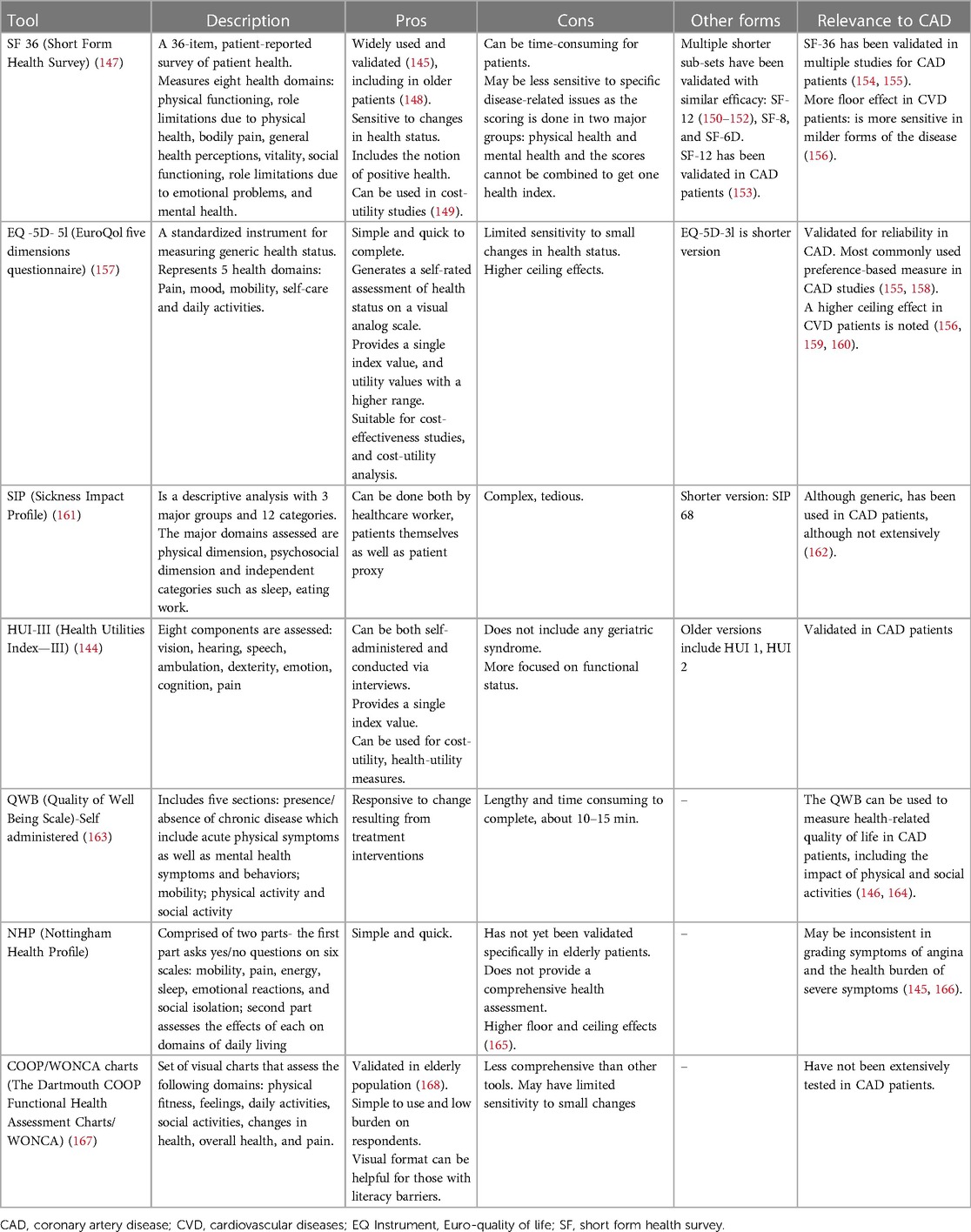
The QoL outcomes tie into what constitutes successful aging for older cardiovascular patients. This also makes it important to factor in the phenomenon of response shift, a concept that elucidates changes in an individual's QoL perception based on alterations in their internal standards, values, or conceptualizations (129). Patients' present QoL preferences might evolve over time in line with their health and life trajectory. Hence, the emphasis on long-term QoL outcomes is critical in concordance with the discussion of short-term benefits. Owing to these insights, the focus has appropriately shifted toward QoL measures in older patients, and numerous global policies and decisions are being implemented to enhance QoL with a focus on long-term care (21, 22, 32). In the context of SIHD management, the inclusion of QoL outcomes remains critical. The symptoms and impacts of the disease—such as chest pain, shortness of breath, and fatigue—can greatly influence both QoL and Health-related quality of life (HRQoL), as these factors often limit social activities, induce emotional distress, and affect overall well-being. Accurately assessing QoL outcomes in the context of cardiovascular treatment therapeutics improves patient selection, helps provide a roadmap for discussions regarding the risks and benefits of treatment, and solidifies a process of shared medical decision-making, particularly for invasive cardiovascular procedures. The instruments used to measure patients' preference for QoL that are validated in older patients are described in Table 2.
Key Takeaways
1. QoL is a multidimensional concept that includes macro societal influences as well as individual experiences, circumstances, well-being, values, and perceptions. This encompasses components such as family, social relationships, emotional wellbeing, spirituality, functional dependence, the standard of living, and health maintenance.
2. The concept of QoL aligns with the self-perception of successful aging for older cardiovascular patients and hence is an important outcome to factor in for any management strategy.
3. There is a lack of standardization and validation of tools to measure patient-centered QoL in older CAD patients.
5.2.2. Health-related quality of life
The CDC defines HRQoL at the individual level as perceptions of physical and mental health, such as energy levels, mood, and their correlates (137). For all individuals, especially older people, health impacts not only their functioning but their global QoL, and hence, maintaining good health and minimizing its disease impacts is one of the most important preferences (138). There exists a bidirectional interaction between unrelated symptoms, functionality, and the resultant QoL, which can help in HRQoL assessment (139).
The critical role of HRQoL assessment is its ability to influence disease outcomes positively and guide treatment strategies (140, 141). To address HRQoL assessment, the CDC proposed four core values: patients' perspectives on general and physical health, mental health, and the impact of poor physical or mental health on their usual activities (137, 142). These core values are foundational for the creation of the Patient-Reported Outcomes Measurement Information System (PROMIS) database, which was a landmark step towards incorporating patient perspectives in HRQoL measurement and laid the groundwork for validating all patient-reported outcomes (143).
However, when measuring HRQoL, we must consider the heterogeneity of the tools used for measurement. While tools like SF-36 and EQ-5D-5l offer a broad, encompassing perspective on HRQoL, they may lack the sensitivity to capture disease-specific subtleties. In contrast, the HUI-III, despite its less widespread use, can provide nuanced functional status and coping assessments, which could provide valuable insights in the context of SIHD (144, 145). An early assessment comparing six widely used generic instruments against SIHD-specific HRQoL tools found that generic tools were capable of measuring both SIHD-specific symptoms and their impact on global health along with patients' overall health status (146). Table 3 discusses various generic and SIHD-specific/validated HRQoL tools.
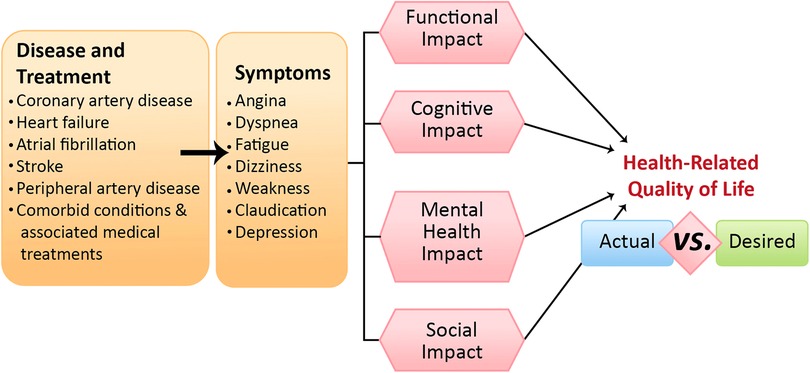
Figure 2. Cardiovascular diseases, associated comorbidities, and multimorbidities can lead to a spectrum of symptoms that may or may not directly reflect direct causation. These initial symptoms are the more obvious cardiovascular symptoms, such as angina, dyspnea, fatigue, etc., but these symptoms, in turn, exert distinct impacts on four key areas: functional, cognitive, mental health, and social domains. Collectively, these influences shape older adults’ health-related quality of life, highlighting a potential disparity between clinical observations and patients’ desires.
These instruments have inherent limitations, often involving trade-offs between comprehensiveness, feasibility, disease specificity, and global health. From a broader perspective, investigators and clinicians must carefully select the most appropriate instrument taking into account their unique strengths and weaknesses. Owing to the diverse study populations with SIHD and the numerous tools of HRQoL utilized in practice, standardization and comparisons of existing instruments are challenging (158, 169). A disease-specific and a generic HRQoL tool together may be required in tailoring treatment plans that prioritize patient preferences and improve overall outcomes in the management of SIHD. As the field evolves, there is a pressing need for robust, replicable, and standardized tools for HRQoL measurement in the context of older patients with SIHD.
Key Takeaways
1. HRQoL determines the impact of health on an individual's perceived well-being, with physical and mental health perceptions as critical components.
2. HRQoL assessment can positively impact SIHD outcomes and guide treatment strategies that align with patient preferences.
3. There is significant heterogeneity in current HRQoL tools, and standardization is required. Generic tools may not capture SIHD-specific nuances, and hence comprehensive understanding is needed before implementing them in clinical trials and clinical practice.
5.2.3. Functional status
Age-related functional decline is a widely recognized phenomenon in geriatric medicine (170). As individuals advance in age, their functional capacity, or their ability to conduct daily tasks and activities vital for maintaining independence, tends to diminish. This capacity encompasses both activities of daily living (ADLs), and instrumental activities of daily living (IADLs). In the context of successful aging, preservation of functional ability is often prioritized as a key health outcome by older patients (171–174). Functional independence was identified as an important domain for maintaining the QoL in the Delphi consensus study (175), reflecting the findings of a comprehensive review by van Leeuwen which identified functional independence as the most common domain in patients' perspectives on healthy aging (176). The importance of functional status is further emphasized in settings of multiple diseases and limitations. Any health problem or outcome that hinders an individual's capacity to carry out desired or necessary tasks leads to poor functional outcomes (177). Furthermore, health conditions that limit a patient's daily activities are typically prioritized as severe or urgent among all comorbidities (178). This desire for independence extends beyond basic functional activities and encompasses maintaining adequate mobility, living independently, and continuing work-related activities (173, 179). Functional independence also carries significant protective effects on health. Functional limitations exacerbate feelings of social isolation and can negatively affect HRQoL.
Older patients, particularly those who have suffered from acute health conditions such as MI, often experience a significant decrease in their functional capacity (180). SIHD and related conditions, such as angina pectoris, markedly impair physical activity in older patients. This impairment consequently leads to reduced QoL and further diminishes their functional independence. It's important to note that these negative impacts on functional ability and independence can, in turn, adversely affect PROs, adding another layer of complexity to the management of older patients with SIHD. Therefore, the inclusion of functional status assessment and outcomes in SIHD management strategies for the older adult population is important in aligning care with the patient's health goals.
Key Takeaways
1. Preserving functional ability is one of the most important PROs across older adults' cohort.
2. SIHD can impose restriction in functional abilities, which in turn can negatively affect social and mental domains and significantly affect the HRQoL.
3. Revascularization strategy that improves functional ability and outcomes that correlate with better functional health, may be prioritized in older adults living with debilitating SIHD.
5.2.4. Symptoms
In the older population, the burden of symptoms is complex and multifaceted, given the prevalence of multiple chronic conditions. Findings from the National Health and Aging Trends study found that at least 20% of community-dwelling older adults experienced two symptoms concurrently, including pain, fatigue, breathing difficulty, anxiety, depressed mood, and sleep disturbance (181). A significant portion of approximately 14%, reported an even higher symptom burden, with three or more coexisting symptoms. Two of the most prevalent symptoms reported by this population, regardless of gender, were pain and fatigue (182). Somatic symptoms, referring to physical manifestations of discomfort, pain, or other physically distressing conditions that hinder a patient's functional capacity, are important outcomes that patients wish to address (175). Any symptom that triggers a loss of functional ability is associated with poor functional health and remains a top priority from the patient's perspective (177). Unmanaged, persistent symptoms can significantly compromise the HRQoL of patients (183). The impact of persistent pain has been shown to be profound as it poses the most significant obstacle to performing ADLs and IADLs (182). Pain management is often a recurring theme in patients' discussions of physical health, and thus, it stands as a high-priority health outcome (184).
Chronic conditions such as SIHD and stable angina are examples of high-burden symptoms that significantly impact all aspects of a patient's QoL (185). In a comparative study of more than ten diseases, symptoms associated with CAD were found to exert the second highest impact on functional disability (186). A recent analysis showed that patients with typical angina had poor scores in the physical health component of SF score, as well as patients had much higher anxiety than those without typical angina (187). Taken together, symptom relief should routinely be assessed and managed appropriately in SIHD patients and is a critical outcome that needs to be incorporated in future research that targets SIHD in the older adult population.
Key Takeaways
1. The symptom burden in older adults is multifaceted due to the concomitant presence of multiple chronic conditions. Older adults experience multiple symptoms, which may or may not overlap with SIHD symptoms, such as pain, fatigue, dyspnea, and mood disturbances.
2. Symptoms that interfere with the functional ability of older patients are emphasized as a high priority when discussing preferred health outcomes.
3. SIHD is a disease that results in high-burden of symptoms that significantly impacts all aspects of older patients’ QoL. Symptoms associated with SIHD considerably impact functional disability, making their management critical in care for older adults.
5.2.5. Mental health
Mental health serves as an important component in the context of successful aging (171). This is understood not merely as the absence of depressed or negative feelings but also incorporates the presence of positive mental outlooks and robust coping mechanisms (173, 175). These findings are corroborated throughout the literature. For instance, a study assessing the correlation between exercise tolerance and age discovered a robust independent association between high depression scores and age-associated exercise intolerance (180). A comprehensive review by Pressman et al. found that patients with a more pronounced negative affect reported their physical symptoms as disproportionately severe relative to their actual disease (188). Conversely, a positive mental outlook was linked with improved health outcomes (189), higher subjective QoL (188), and successful aging (173).
It was proposed that any acute illness might trigger a stress response in older patients, leading to a spectrum of adjustment disorders (190). This captures the maladaptive psychological responses prompted by changes in life circumstances, with diseases playing a significant role. The concept is unequivocally illustrated in the AHRQ evidence report, which found a strong association between developing MI and increased depressive symptoms (191). Typical angina has been shown to elicit higher anxiety in older adults when compared to their counterparts without these symptoms (187). Importantly, older individuals often demonstrate a robust positive reaction to mental adaptability, which encompasses accepting their life circumstances while maintaining a positive outlook on life (176). Such resilience underscores the pivotal role mental health plays in their overall well-being, particularly in the context of managing chronic disease. Consequently, mental health emerges as a significant PRO that should be prioritized in the management of SIHD in the older adult population to ensure adequate QoL. When adverse events occur, interventions to help cope with the stressor should be provided to older patients to ensure adequate recovery of functional abilities.
Key Takeaways
1. Mental health is a critical component of successful aging, encompassing not only the absence of negative feelings but also the presence of positive mental outlooks and robust coping mechanisms.
2. Patients with more pronounced negative affect report their physical symptoms as disproportionately severe relative to their actual disease, impacting the perception of health status.
3. SIHD has been independently associated with increased depressive and anxiety symptoms as well as acute stress response, which can cause maladaptive psychological responses.
5.2.6. Cognitive function
There is a known trend of cognitive functional decline with pathologic aging, impacting all facets of cognitive functioning (192). Cognitive functioning serves as a critical pillar of achieving functional independence among older patients and hence maintaining their sense of well-being as well as perceptions of successful aging. Adequate cognitive functioning includes the preservation of memory, the ability to engage in cognitive activities within their community, and the capacity to acquire new skills or experiences (173). In the older population, cognitive dysfunction can span a spectrum that ranges from mild cognitive impairment to more severe forms such as dementia, including Alzheimer's disease (193). Even mild cognitive impairment can notably undermine the ability of older adults to maintain their independence (192, 194). Moreover, the implications of cognitive impairment extend to HRQoL, with both subjective and mild cognitive impairment correlating with poor HRQoL outcomes (195, 196).
An important concept in gerontology is cognitive frailty, which is defined by the International Academy on Nutrition and Aging (I.A.N.A) and the International Association of Gerontology and Geriatrics (I.A.G.G) consensus group as the simultaneous presence of physical frailty and mild cognitive impairment in the absence of dementia or other pre-existing brain disorders. This state of cognitive frailty contributes to increased disease burden and is associated with poorer outcomes (46, 197). When we consider the impact of SIHD or ACS on cognitive function, several studies show that these conditions can indeed have a detrimental impact. For instance, individuals with SIHD often demonstrate poorer cognitive function compared to their counterparts, affecting domains such as memory, attention, and executive function. The exact mechanism of this interaction is multifaceted, with potential contributions from cerebrovascular disease, shared risk factors (198), and the effects of chronic systemic inflammation. Moreover, post-ACS patients may experience a decline in cognitive function, which can influence their functional independence and HRQoL. This highlights the importance of routine cognitive assessment and appropriate management in the context of SIHD, which could subsequently lead to improved outcomes and QoL in these patients.
Key Takeaways
1. Cognitive abilities, including memory, the ability to engage in cognitive activities, and the capacity to acquire new skills, are frequently emphasized in older individuals' perceptions of successful aging.
2. Studies have shown that both SIHD and ACS can have a detrimental impact on cognitive function, which in turn can cause functional impedance. Hence, routine cognitive assessment and appropriate management in this patient cohort is a necessity.
5.2.7. Social support
Social support and meaningful interpersonal relationships are fundamental for older patients to maintain a QoL that is personally fulfilling. This encompasses not only the avoidance of loneliness but also the establishment and continued cultivation of positive connections. Receiving emotional and psychological support from family, friends, colleagues, and others in their social circles, being contributing members of society, and feeling a sense of belonging is essential to their well-being (175, 176, 179, 199). These relationships provide instrumental and emotional support, fostering resilience and adaptability in this population (200). Evidence shows that the social dynamics of the older individual's life can directly impact their HRQoL (183, 201, 202), and subjective social aspects are associated with both subjective well-being as well as positive health affect (173, 203). These findings make it clear that subjective health parameters are interconnected and can mutually influence the overall well-being of the older patient. These complex interactions between social relationships, subjective well-being, and health-related outcomes suggest a need for an integrative and comprehensive approach to the management of CAD in older patients.
Key Takeaways
1. Social support, strong interpersonal relationships, and maintaining roles within the community are important for ensuring adaptability in older adults.
2. Social dynamics of an older individual's life can directly impact both the mental health component as well as HRQoL and hence should be taken into perspective when deciding any management strategies.
5.3. Gaps in knowledge
Understanding and acknowledging the need for a patient-centered approach in managing SIHD in the older adult population, several gaps in the current body of research need to be addressed:
1. The categorization of “older adults” at a threshold age of 65 years, might not be an accurate representation of today's older adult populations due to improving health care and increased life expectancy. Studies examining the “old-old” population, those above 85 years and older, and incorporating biologic or physiologic aging are required to understand cardiovascular aging.
2. Despite the acknowledgment of the significance of PROs in geriatric cardiology, they are still under-utilized in research and practice. Systematic reviews or meta-analyses investigating patient-preferred outcomes such as functional independence, cognitive abilities, and mental health in SIHD management, are limited and not externally validated.
3. Clinical trials aimed at enrolling older patients with SIHD must incorporate patient-reported outcomes in their methodology.
4. The development and validation of novel outcome measures that capture older adults' priorities are needed. Current outcome measures may not fully capture the range of patients' experiences and concerns, particularly in the realm of mental health and social functioning.
6. Conclusion
The National Academy of Medicine, the European Society of Cardiology, the American College of Cardiology, the American Heart Association, and the American Geriatric Society, strongly advocate for patient-centered care and propose personalized strategies for managing older patients living with SIHD. To optimize cardiovascular care for older patients with SIHD, research evaluating therapeutic outcomes must consider patient preferences and their perceptions of successful aging. These factors should be evaluated within each patient's unique cultural, social, and physical contexts, and weighed against the risks and benefits concerning mortality and morbidity (Figure 2). Geriatric syndromes should be recognized for their significant prognostic implications, and therapeutic interventions should be combined with both preventative and long-term care plans to mitigate these. Discussing therapeutic interventions for SIHD necessitates a comprehensive dialogue about the burden of treatment on the patient, balancing short- and long-term goals identified by the individuals themselves. To ensure a comprehensive and accurate assessment, standardized definitions for patient-reported outcomes in the older population should be the next frontier in clinical research. Taken together, guidelines for chronic coronary disease should not solely focus on managing hard clinical outcomes of SIHD, but rather reflect a more comprehensive person-centered care by incorporating PROs in the approach to management.
Author contributions
KK: Conceptualization, Investigation, Visualization, Writing – original draft, Writing – review & editing. MM: Writing – original draft, Writing – review & editing. MN: Writing – review & editing, Data curation, Methodology, Supervision, Visualization. AD: Data curation, Methodology, Supervision, Visualization, Writing – review & editing, Conceptualization, Formal Analysis, Funding acquisition, Investigation, Project administration, Resources, Software, Validation, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
MN reports current research support from the American College of Cardiology Foundation supported by the George F. and Ann Harris Bellows Foundation, the Patient-Centered Outcomes Research Institute (PCORI), the Yale Claude D. Pepper Older Americans Independence Center (P30AG021342), and the National Institute on Aging/National Institutes of Health from R03AG074067 (GEMSSTAR award). AD receives research funding from the Pepper Scholars Program of the Johns Hopkins University Claude D. Pepper Older Americans Independence Center funded by the National Institute on Aging P30-AG021334 and receives mentored patient-oriented research career development award from the National Heart, Lung, and Blood Institute K23-HL153771-04.
Conflict of interest
MN receives consulting fees from Heartflow, Inc, Merck.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. United Nations, Department of Economic and Social Affairs, Population Division. World population prospects 2022 (2022). Online Edition.
2. Centers for Disease Control and Prevention (CDC). Trends in aging–United States and worldwide. MMWR Morb Mortal Wkly Rep. (2003) 52:101–4, 106.12645839
3. Medina L, Sabo S, Vespa J. Living longer: historical and projected life expectancy in the United States, from 1960 to 2060).
4. Organization WH. Ageing and health (2022). Available at: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (Accessed April 15, 2023).
5. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics—2022 update: a report from the American heart association. Circulation. (2022) 145(8):e153–639. doi: 10.1161/CIR.0000000000001052
6. Odden MC, Coxson PG, Moran A, Lightwood JM, Goldman L, Bibbins-Domingo K. The impact of the aging population on coronary heart disease in the United States. Am J Med. (2011) 124:827–33.e825. doi: 10.1016/j.amjmed.2011.04.010
7. Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. (2017) 70:1–25. doi: 10.1016/j.jacc.2017.04.052
8. Weiss CO, Boyd CM, Yu Q, Wolff JL, Leff B. Patterns of prevalent major chronic disease among older adults in the United States. Jama. (2007) 298:1160–2. doi: 10.1001/jama.298.10.1160-b
9. Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. (2020) 396:1204–22. doi: 10.1016/S0140-6736(20)30925-9
10. Damluji AA, Forman DE, Wang TY, Chikwe J, Kunadian V, Rich MW, et al. Management of acute coronary syndrome in the older adult population: a scientific statement from the American heart association. Circulation. (2023) 147:e32–62. doi: 10.1161/CIR.0000000000001112
11. Leonardo De L, Zoran O, Leonardo B, Donata L, Lucio G, Antonio Di C, Gianni C, Francesco C, Alessandro B, Giuseppe Di P, et al. A decade of changes in clinical characteristics and management of elderly patients with non-ST elevation myocardial infarction admitted in Italian cardiac care units. Open Heart. 2014;1:e000148. doi: 10.1136/openhrt-2014-000148
12. Rowe JW, Kahn RL. Successful aging. Gerontologist. (1997) 37:433–40. doi: 10.1093/geront/37.4.433
14. Strawbridge WJ, Cohen RD, Shema SJ, Kaplan GA. Successful aging: predictors and associated activities. Am J Epidemiol. (1996) 144:135–41. doi: 10.1093/oxfordjournals.aje.a008900
15. Bowling A, Iliffe S. Which model of successful ageing should be used? Baseline findings from a British longitudinal survey of ageing. Age Ageing. (2006) 35:607–14. doi: 10.1093/ageing/afl100
16. Hung L-W, Kempen GIJM, De Vries NK. Cross-cultural comparison between academic and lay views of healthy ageing: a literature review. Ageing Soc. (2010) 30:1373–91. doi: 10.1017/S0144686X10000589
17. Poon LW, Gueldner SH, Sprouse BM. Successful aging and adaptation with chronic diseases. Springer Series on Lifestyles and Issues in Aging. New York, NY: Springer Publishing Company (2003).
18. Cernin PA, Lysack C, Lichtenberg PA. A comparison of self-rated and objectively measured successful aging constructs in an urban sample of African American older adults. Clin Gerontol. (2011) 34:89–102. doi: 10.1080/07317115.2011.539525
19. Acquadro C, Berzon R, Dubois D, Leidy NK, Marquis P, Revicki D, et al. Incorporating the patient’s perspective into drug development and communication: an ad hoc task force report of the patient-reported outcomes (PRO) harmonization group meeting at the food and drug administration, February 16, 2001. Value Health. (2003) 6:522–31. doi: 10.1046/j.1524-4733.2003.65309.x
20. Pruchno RA, Wilson-Genderson M, Rose M, Cartwright F. Successful aging: early influences and contemporary characteristics. Gerontologist. (2010) 50:821–33. doi: 10.1093/geront/gnq041
21. Institute of Medicine (US) Committee on Quality of Health Care in America. Crossing the quality chasm: A new health system for the 21st century. Chapter 1. Washington (DC): National Academies Press (US) (2001). ISBN-10: 0-309-07280-8
22. Boyd C, Smith CD, Masoudi FA, Blaum CS, Dodson JA, Green AR, et al. Decision making for older adults with multiple chronic conditions: executive summary for the American geriatrics society guiding principles on the care of older adults with multimorbidity. J Am Geriatr Soc. (2019) 67:665–73. doi: 10.1111/jgs.15809
23. Tinetti M, Huang A, Molnar F. The geriatrics 5M’s: a new way of communicating what we do. J Am Geriatr Soc. (2017) 65:2115. doi: 10.1111/jgs.14979
24. Nanna MG, Wang SY, Damluji AA. Management of stable angina in the older adult population. Circ Cardiovasc Interv. (2023) 16:e012438. doi: 10.1161/circinterventions.122.012438
25. Nanna Michael G, Sutton Nadia R, Kochar A, Rymer Jennifer A, Lowenstern Angela M, Gackenbach G, et al. Assessment and management of older adults undergoing PCI, part 1. JACC Adv. (2023) 2:100389. doi: 10.1016/j.jacadv.2023.100389
26. Nanna Michael G, Sutton Nadia R, Kochar A, Rymer Jennifer A, Lowenstern Angela M, Gackenbach G, et al. A geriatric approach to percutaneous coronary interventions in older adults, part II. JACC Adv. (2023) 2:100421. doi: 10.1016/j.jacadv.2023.100421
27. Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid DiseasesImplications for pay for performance. JAMA. (2005) 294:716–24. doi: 10.1001/jama.294.6.716
28. Nanna MG, Sutton NR, Kochar A, Rymer JA, Lowenstern AM, Gackenbach G, et al. Assessment and management of older adults undergoing PCI, part 1: a JACC: advances expert panel. JACC Adv. (2023) 2:100389. doi: 10.1016/j.jacadv.2023.100389
29. Tinetti ME, Esterson J, Ferris R, Posner P, Blaum CS. Patient priority-directed decision making and care for older adults with multiple chronic conditions. Clin Geriatr Med. (2016) 32:261–75. doi: 10.1016/j.cger.2016.01.012
30. Report of the World Health Organization. Active ageing: a policy framework. Aging Male. (2002) 5:1–37. doi: 10.1080/tam.5.1.1.37
31. Damluji AA, Rymer JA, Nanna MG. The heterogeneity of old age: healthy aging in older adults undergoing TAVR. JACC Cardiovasc Interv. (2023) 16:189–92. doi: 10.1016/j.jcin.2022.12.008
34. Whaley DE. An argument for a developmental approach in studying older adults’ physical activity. J Aging Phys Act. (2014) 22:i–iv. doi: 10.1123/japa.2014-0133
35. Gladyshev VN. The free radical theory of aging is dead. Long live the damage theory! Antioxid Redox Signal. (2014) 20:727–31. doi: 10.1089/ars.2013.5228
36. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. (2018) 15:505–22. doi: 10.1038/s41569-018-0064-2
38. Hamczyk MR, Nevado RM, Barettino A, Fuster V, Andrés V. Biological versus chronological aging: jACC focus seminar. J Am Coll Cardiol. (2020) 75:919–30. doi: 10.1016/j.jacc.2019.11.062
39. Zhu X, Chen Z, Shen W, Huang G, Sedivy JM, Wang H, et al. Inflammation, epigenetics, and metabolism converge to cell senescence and ageing: the regulation and intervention. Signal Transduc Target Ther. (2021) 6:245. doi: 10.1038/s41392-021-00646-9
40. Lipsitz LA. Dynamics of stability: the physiologic basis of functional health and frailty. J Gerontol A Biol Sci Med Sci. (2002) 57:B115–125. doi: 10.1093/gerona/57.3.b115
41. O'Neill DE, Forman DE. Cardiovascular care of older adults. Br Med J. (2021) 374:n1593. doi: 10.1136/bmj.n1593
42. Damluji AA, Huang J, Bandeen-Roche K, Forman DE, Gerstenblith G, Moscucci M, et al. Frailty among older adults with acute myocardial infarction and outcomes from percutaneous coronary interventions. J Am Heart Assoc. (2019) 8:e013686. doi: 10.1161/jaha.119.013686
43. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. (2001) 56:M146–156. doi: 10.1093/gerona/56.3.m146
44. Damluji AA, Chung SE, Xue QL, Hasan RK, Moscucci M, Forman DE, et al. Frailty and cardiovascular outcomes in the national health and aging trends study. Eur Heart J. (2021) 42:3856–65. doi: 10.1093/eurheartj/ehab468
45. Chen X, Mao G, Leng SX. Frailty syndrome: an overview. Clin Interv Aging. (2014) 9:433–41. doi: 10.2147/cia.S45300
46. Damluji AA, Ijaz N, Chung S-E, Xue Q-L, Hasan RK, Batchelor WB, et al. Hierarchical development of physical frailty and cognitive impairment and their association with incident cardiovascular disease. JACC: Advances. (2023) 2:100318. doi: 10.1016/j.jacadv.2023.100318
47. Marengoni A, Angleman S, Melis R, Mangialasche F, Karp A, Garmen A, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. (2011) 10:430–9. doi: 10.1016/j.arr.2011.03.003
48. Nunes BP, Flores TR, Mielke GI, Thumé E, Facchini LA. Multimorbidity and mortality in older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr. (2016) 67:130–8. doi: 10.1016/j.archger.2016.07.008
49. Salive ME. Multimorbidity in older adults. Epidemiol Rev. (2013) 35:75–83. doi: 10.1093/epirev/mxs009
50. Almirall J, Fortin M. The coexistence of terms to describe the presence of multiple concurrent diseases. J Comorb. (2013) 3:4–9. doi: 10.15256/joc.2013.3.22
51. van den Akker M, Buntinx F, Knottnerus JA. Comorbidity or multimorbidity. Eur J Gen Pract. (1996) 2:65–70. doi: 10.3109/13814789609162146
52. Valderas JM, Starfield B, Sibbald B, Salisbury C, Roland M. Defining comorbidity: implications for understanding health and health services. Ann Fam Med. (2009) 7:357–63. doi: 10.1370/afm.983
53. Calderón-Larrañaga A, Vetrano DL, Ferrucci L, Mercer SW, Marengoni A, Onder G, et al. Multimorbidity and functional impairment-bidirectional interplay, synergistic effects and common pathways. J Intern Med. (2019) 285:255–71. doi: 10.1111/joim.12843
54. Damluji AA, Chung S-E, Xue Q-L, Hasan RK, Moscucci M, Forman DE, et al. Frailty and cardiovascular outcomes in the national health and aging trends Study. Eur Heart J. (2021) 42:3856–65. doi: 10.1093/eurheartj/ehab468
55. Seals DR, Brunt VE, Rossman MJ. Keynote lecture: strategies for optimal cardiovascular aging. Am J Physiolo Heart Circ Physiol. (2018) 315:H183–8. doi: 10.1152/ajpheart.00734.2017
56. Triposkiadis F, Xanthopoulos A, Butler J. Cardiovascular aging and heart failure. J Am Coll Cardiol. (2019) 74:804–13. doi: 10.1016/j.jacc.2019.06.053
57. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. (2018) 15:505–22. doi: 10.1038/s41569-018-0064-2
58. North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res. (2012) 110:1097–108. doi: 10.1161/circresaha.111.246876
59. Singam NSV, Fine C, Fleg JL. Cardiac changes associated with vascular aging. Clin Cardiol. (2020) 43:92–8. doi: 10.1002/clc.23313
60. Paneni F, Diaz Cañestro C, Libby P, Lüscher TF, Camici GG. The aging cardiovascular system: understanding it at the cellular and clinical levels. J Am Coll Cardiol. (2017) 69:1952–67. doi: 10.1016/j.jacc.2017.01.064
61. Madhavan MV, Gersh BJ, Alexander KP, Granger CB, Stone GW. Coronary artery disease in patients ≥80 years of age. J Am Coll Cardiol. (2018) 71:2015–40. doi: 10.1016/j.jacc.2017.12.068
62. Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Circulation. (2003) 107:490–7. doi: 10.1161/01.CIR.0000048894.99865.02
63. Strait JB, Lakatta EG. Aging-associated cardiovascular changes and their relationship to heart failure. Heart Fail Clin. (2012) 8:143–64. doi: 10.1016/j.hfc.2011.08.011
64. White HD, Barbash GI, Califf RM, Simes RJ, Granger CB, Weaver WD, et al. Age and outcome with contemporary thrombolytic therapy. Results from the GUSTO-I trial. Global utilization of streptokinase and TPA for occluded coronary arteries trial. Circulation. (1996) 94:1826–33. doi: 10.1161/01.cir.94.8.1826
65. Boersma E, Pieper KS, Steyerberg EW, Wilcox RG, Chang WC, Lee KL, et al. Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation. Results from an international trial of 9461 patients. The PURSUIT investigators. Circulation. (2000) 101:2557–67. doi: 10.1161/01.cir.101.22.2557
66. Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. (2000) 284:835–42. doi: 10.1001/jama.284.7.835
67. Fox KA, Dabbous OH, Goldberg RJ, Pieper KS, Eagle KA, Van de Werf F, et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). Br Med J. (2006) 333:1091. doi: 10.1136/bmj.38985.646481.55
68. Luca LD, Marini M, Gonzini L, Boccanelli A, Casella G, Chiarella F, et al. Contemporary trends and age; specific sex differences in management and outcome for patients with ST-segment elevation myocardial infarction. J Am Heart Assoc. (2016) 5:e004202. doi: 10.1161/JAHA.116.004202
69. Rosengren A, Wallentin L, Simoons M, Gitt AK, Behar S, Battler A, et al. Age, clinical presentation, and outcome of acute coronary syndromes in the euroheart acute coronary syndrome survey. Eur Heart J. (2006) 27:789–95. doi: 10.1093/eurheartj/ehi774
70. Gharacholou SM, Lopes RD, Alexander KP, Mehta RH, Stebbins AL, Pieper KS, et al. Age and outcomes in ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention: findings from the APEX-AMI trial. Arch Intern Med. (2011) 171:559–67. doi: 10.1001/archinternmed.2011.36
71. Damluji AA, Huang J, Bandeen-Roche K, Forman DE, Gerstenblith G, Moscucci M, et al. Frailty among older adults with acute myocardial infarction and outcomes from percutaneous coronary Interventions. J Am Heart Assoc. (2019) 8:e013686. doi: 10.1161/JAHA.119.013686
72. Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol. (2009) 103:1616–21. doi: 10.1016/j.amjcard.2009.01.375
73. Gharacholou SM, Roger VL, Lennon RJ, Rihal CS, Sloan JA, Spertus JA, et al. Comparison of frail patients versus nonfrail patients ≥65 years of age undergoing percutaneous coronary intervention. Am J Cardiol. (2012) 109:1569–75. doi: 10.1016/j.amjcard.2012.01.384
74. Ekerstad N, Swahn E, Janzon M, Alfredsson J, Löfmark R, Lindenberger M, et al. Frailty is independently associated with 1-year mortality for elderly patients with non-ST-segment elevation myocardial infarction. Eur J Prev Cardiol. (2014) 21:1216–24. doi: 10.1177/2047487313490257
75. Ekerstad N, Javadzadeh D, Alexander KP, Bergström O, Eurenius L, Fredrikson M, et al. Clinical frailty scale classes are independently associated with 6-month mortality for patients after acute myocardial infarction. Eur Heart J Acute Cardiovasc Care. (2022) 11:89–98. doi: 10.1093/ehjacc/zuab114
76. Patel A, Goodman SG, Yan AT, Alexander KP, Wong CL, Cheema AN, et al. Frailty and outcomes after myocardial infarction: insights from the CONCORDANCE registry. J Am Heart Assoc. (2018) 7:e009859. doi: 10.1161/jaha.118.009859
77. Bebb O, Smith FG, Clegg A, Hall M, Gale CP. Frailty and acute coronary syndrome: a structured literature review. Eur Heart J Acute Cardiovasc Care. (2018) 7:166–75. doi: 10.1177/2048872617700873
78. Qayyum S, Rossington JA, Chelliah R, John J, Davidson BJ, Oliver RM, et al. Prospective cohort study of elderly patients with coronary artery disease: impact of frailty on quality of life and outcome. Open Heart. (2020) 7. doi: 10.1136/openhrt-2020-001314
79. Sanchis J, García Acuña JM, Raposeiras S, Barrabés JA, Cordero A, Martínez-Sellés M, et al. Comorbidity burden and revascularization benefit in elderly patients with acute coronary syndrome. Rev Esp Cardiol (Engl Ed). (2021) 74:765–72. doi: 10.1016/j.rec.2020.06.015
80. Beska B, Mills GB, Ratcovich H, Wilkinson C, Damluji AA, Kunadian V. Impact of multimorbidity on long-term outcomes in older adults with non-ST elevation acute coronary syndrome in the north east of England: a multi-centre cohort study of patients undergoing invasive care. BMJ Open. (2022) 12:e061830. doi: 10.1136/bmjopen-2022-061830
81. Rashid M, Kwok CS, Gale CP, Doherty P, Olier I, Sperrin M, et al. Impact of co-morbid burden on mortality in patients with coronary heart disease, heart failure, and cerebrovascular accident: a systematic review and meta-analysis. Eur Heart J Qual Care Clin Outcomes. (2017) 3:20–36. doi: 10.1093/ehjqcco/qcw025
82. Chuang A, Hancock DG, Halabi A, Horsfall M, Vaile J, De Pasquale C, et al. Invasive management of acute coronary syndrome: interaction with competing risks. Int J Cardiol. (2018) 269:13–8. doi: 10.1016/j.ijcard.2018.07.078
83. Unverzagt FW, Gao S, Baiyewu O, Ogunniyi AO, Gureje O, Perkins A, et al. Prevalence of cognitive impairment: data from the Indianapolis study of health and aging. Neurology. (2001) 57:1655–62. doi: 10.1212/wnl.57.9.1655
84. Bae JB, Han JW, Kwak KP, Kim BJ, Kim SG, Kim JL, et al. Impact of mild cognitive impairment on mortality and cause of death in the elderly. J Alzheimers Dis. (2018) 64:607–16. doi: 10.3233/jad-171182
85. Sloan FA, Trogdon JG, Curtis LH, Schulman KA. The effect of dementia on outcomes and process of care for medicare beneficiaries admitted with acute myocardial infarction. J Am Geriatr Soc. (2004) 52:173–81. doi: 10.1111/j.1532-5415.2004.52052.x
86. Prasitlumkum N, Doyle KS, Ding KR, Natarajan B, Mukherjee A, Varadarajan P, et al. The impact of cognitive impairment in patients with acute coronary syndrome undergoing percutaneous revascularization: a systematic review and meta-analysis. Coron Artery Dis. (2022) 31:e59–66. doi: 10.1097/mca.0000000000001049
87. Vitale C, Fini M, Spoletini I, Lainscak M, Seferovic P, Rosano GM. Under-representation of elderly and women in clinical trials. Int J Cardiol. (2017) 232:216–21. doi: 10.1016/j.ijcard.2017.01.018
88. Tahhan AS, Vaduganathan M, Greene SJ, Alrohaibani A, Raad M, Gafeer M, et al. Enrollment of older patients, women, and racial/ethnic minority groups in contemporary acute coronary syndrome clinical trials: a systematic review. JAMA Cardiol. (2020) 5:714–22. doi: 10.1001/jamacardio.2020.0359
89. Nanna MG, Chen ST, Nelson AJ, Navar AM, Peterson ED. Representation of older adults in cardiovascular disease trials since the inclusion across the lifespan policy. JAMA Intern Med. (2020) 180:1531–3. doi: 10.1001/jamainternmed.2020.2750
90. Buttgereit T, Palmowski A, Forsat N, Boers M, Witham MD, Rodondi N, et al. Barriers and potential solutions in the recruitment and retention of older patients in clinical trials—lessons learned from six large multicentre randomized controlled trials. Age Ageing. (2021) 50:1988–96. doi: 10.1093/ageing/afab147
91. Weiss CO, Varadhan R, Puhan MA, Vickers A, Bandeen-Roche K, Boyd CM, et al. Multimorbidity and evidence generation. J Gen Intern Med. (2014) 29:653–60. doi: 10.1007/s11606-013-2660-5
92. Fialová D, Laffon B, Marinković V, Tasić L, Doro P, Sόos G, et al. Medication use in older patients and age-blind approach: narrative literature review (insufficient evidence on the efficacy and safety of drugs in older age, frequent use of PIMs and polypharmacy, and underuse of highly beneficial nonpharmacological strategies). Eur J Clin Pharmacol. (2019) 75:451–66. doi: 10.1007/s00228-018-2603-5
93. Crome P, Lally F, Cherubini A, Oristrell J, Beswick AD, Clarfield AM, et al. Exclusion of older people from clinical trials: professional views from nine European countries participating in the PREDICT study. Drugs Aging. (2011) 28:667–77. doi: 10.2165/11591990-000000000-00000
94. Bernard MA, Clayton JA, Lauer MS. Inclusion across the lifespan: nIH policy for clinical research. Jama. (2018) 320:1535–6. doi: 10.1001/jama.2018.12368
95. CG. Senior primary angioplasty in myocardial infarction study—sENIOR PAMI. In: TCT 2005. Washinton DC: American Collegel of Cardiology (2005).
96. Filsoufi F, Rahmanian PB, Castillo JG, Chikwe J, Silvay G, Adams DH. Results and predictors of early and late outcomes of coronary artery bypass graft surgery in octogenarians. J Cardiothorac Vasc Anesth. (2007) 21:784–92. doi: 10.1053/j.jvca.2007.08.007
97. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. (2009) 361:1045–57. doi: 10.1056/NEJMoa0904327
98. Bueno H, Betriu A, Heras M, Alonso JJ, Cequier A, García EJ, et al. Primary angioplasty vs. Fibrinolysis in very old patients with acute myocardial infarction: tRIANA (TRatamiento del infarto agudo de miocardio eN ancianos) randomized trial and pooled analysis with previous studies. Eur Heart J. (2011) 32:51–60. doi: 10.1093/eurheartj/ehq375
99. Savonitto S, Cavallini C, Petronio AS, Murena E, Antonicelli R, Sacco A, et al. Early aggressive versus initially conservative treatment in elderly patients with non-ST-segment elevation acute coronary syndrome: a randomized controlled trial. JACC Cardiovasc Interv. (2012) 5:906–16. doi: 10.1016/j.jcin.2012.06.008
100. de Belder A, de la Torre Hernandez JM, Lopez-Palop R, O'Kane P, Hernandez Hernandez F, Strange J, et al. A prospective randomized trial of everolimus-eluting stents versus bare-metal stents in octogenarians: the XIMA trial (Xience or vision stents for the management of angina in the elderly). J Am Coll Cardiol. (2014) 63:1371–5. doi: 10.1016/j.jacc.2013.10.053
101. Urban P, Meredith IT, Abizaid A, Pocock SJ, Carrié D, Naber C, et al. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med. (2015) 373:2038–47. doi: 10.1056/NEJMoa1503943
102. Sanchis J, Núñez E, Barrabés JA, Marín F, Consuegra-Sánchez L, Ventura S, et al. Randomized comparison between the invasive and conservative strategies in comorbid elderly patients with non-ST elevation myocardial infarction. Eur J Intern Med. (2016) 35:89–94. doi: 10.1016/j.ejim.2016.07.003
103. Tegn N, Abdelnoor M, Aaberge L, Endresen K, Smith P, Aakhus S, et al. Invasive versus conservative strategy in patients aged 80 years or older with non-ST-elevation myocardial infarction or unstable angina pectoris (after eighty study): an open-label randomised controlled trial. Lancet. (2016) 387:1057–65. doi: 10.1016/s0140-6736(15)01166-6
104. Varenne O, Cook S, Sideris G, Kedev S, Cuisset T, Carrié D, et al. Drug-eluting stents in elderly patients with coronary artery disease (SENIOR): a randomised single-blind trial. Lancet. (2018) 391:41–50. doi: 10.1016/S0140-6736(17)32713-7
105. Savonitto S, Ferri LA, Piatti L, Grosseto D, Piovaccari G, Morici N, et al. Comparison of reduced-dose prasugrel and standard-dose clopidogrel in elderly patients with acute coronary syndromes undergoing early percutaneous revascularization. Circulation. (2018) 137:2435–45. doi: 10.1161/CIRCULATIONAHA.117.032180
106. Yasuda S, Kaikita K, Akao M, Ako J, Matoba T, Nakamura M, et al. Antithrombotic therapy for atrial fibrillation with stable coronary disease. N Engl J Med. (2019) 381:1103–13. doi: 10.1056/NEJMoa1904143
107. Bach RG, Cannon CP, Giugliano RP, White JA, Lokhnygina Y, Bohula EA, et al. Effect of simvastatin-ezetimibe compared with simvastatin monotherapy after acute coronary syndrome among patients 75 years or older: a secondary analysis of a randomized clinical trial. JAMA Cardiol. (2019) 4:846–54. doi: 10.1001/jamacardio.2019.2306
108. Shavadia JS, Holmes DN, Thomas L, Peterson ED, Granger CB, Roe MT, et al. Comparative effectiveness of β-blocker use beyond 3 years after myocardial infarction and long-term outcomes among elderly patients. Circ Cardiovasc Qual Outcomes. (2019) 12:e005103. doi: 10.1161/circoutcomes.118.005103
109. Gimbel M, Qaderdan K, Willemsen L, Hermanides R, Bergmeijer T, de Vrey E, et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): the randomised, open-label, non-inferiority trial. Lancet. (2020) 395:1374–81. doi: 10.1016/s0140-6736(20)30325-1
110. Szummer K, Montez-Rath ME, Alfredsson J, Erlinge D, Lindahl B, Hofmann R, et al. Comparison between ticagrelor and clopidogrel in elderly patients with an acute coronary syndrome: insights from the SWEDEHEART registry. Circulation. (2020) 142:1700–8. doi: 10.1161/circulationaha.120.050645
111. de Belder A, Myat A, Blaxill J, Haworth P, O'Kane PD, Hatrick R, et al. Revascularisation or medical therapy in elderly patients with acute anginal syndromes: the RINCAL randomised trial. EuroIntervention. (2021) 17:67–74. doi: 10.4244/eij-d-20-00975
112. Ratcovich H, Beska B, Mills G, Holmvang L, Adams-Hall J, Stevenson H, et al. Five-year clinical outcomes in patients with frailty aged ≥75 years with non-ST elevation acute coronary syndrome undergoing invasive management. Eur Heart J Open. (2022) 2. doi: 10.1093/ehjopen/oeac035
113. von Faber M, Bootsma-van der Wiel A, van Exel E, Gussekloo J, Lagaay AM, van Dongen E, et al. Successful aging in the oldest old: who can be characterized as successfully aged? Arch Intern Med. (2001) 161:2694–700. doi: 10.1001/archinte.161.22.2694
114. Strawbridge WJ, Wallhagen MI, Cohen RD. Successful aging and well-being: self-rated compared with Rowe and Kahn. Gerontologist. (2002) 42:727–33. doi: 10.1093/geront/42.6.727
115. Montgomery AA, Fahey T. How do patients’ treatment preferences compare with those of clinicians? Qual Health Care. (2001) 10(Suppl 1):i39–43. doi: 10.1136/qhc.0100039.
116. Tinetti ME, Bogardus ST Jr, Agostini JV. Potential pitfalls of disease-specific guidelines for patients with multiple conditions. N Engl J Med. (2004) 351:2870–4. doi: 10.1056/NEJMsb042458
117. Guiding principles for the care of older adults with multimorbidity: an approach for clinicians. Guiding principles for the care of older adults with multimorbidity: an approach for clinicians: american geriatrics society expert panel on the care of older adults with multimorbidity. J Am Geriatr Soc. (2012) 60:E1–e25. doi: 10.1111/j.1532-5415.2012.04188.x
118. Nanna MG, Peterson ED, Wu A, Harding T, Galanos AN, Wruck L, et al. Age, knowledge, preferences, and risk tolerance for invasive cardiac care. Am Heart J. (2020) 219:99–108. doi: 10.1016/j.ahj.2019.09.008
119. Fried TR, McGraw S, Agostini JV, Tinetti ME. Views of older persons with multiple morbidities on competing outcomes and clinical decision-making. J Am Geriatr Soc. (2008) 56:1839–44. doi: 10.1111/j.1532-5415.2008.01923.x
120. Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. (2002) 346:1061–6. doi: 10.1056/NEJMsa012528
121. Tinetti ME, McAvay GJ, Fried TR, Allore HG, Salmon JC, Foody JM, et al. Health outcome priorities among competing cardiovascular, fall injury, and medication-related symptom outcomes. J Am Geriatr Soc. (2008) 56:1409–16. doi: 10.1111/j.1532-5415.2008.01815.x
122. Caughey GE, Tait K, Vitry AI, Shakib S. Influence of medication risks and benefits on treatment preferences in older patients with multimorbidity. Patient Prefer Adher. (2017) 11:131–40. doi: 10.2147/ppa.S118836
123. Goyal P, Nanna MG. Stable coronary artery disease in the age of geriatric cardiology. J Am Coll Cardiol. (2023) 81:1710–3. doi: 10.1016/j.jacc.2023.03.378
125. Bowling A, Gabriel Z, Dykes J, Dowding LM, Evans O, Fleissig A, et al. Let’s ask them: a national survey of definitions of quality of life and its enhancement among people aged 65 and over. Int J Aging Hum Dev. (2003) 56:269–306. doi: 10.2190/bf8g-5j8l-ytrf-6404
126. Brown J, Bowling A, Flynn TN. Models of quality of life: a taxonomy, overview and systematic review of the literature. Paper/Poster presented (2004).
127. Gabriel Z, Bowling ANN. Quality of life from the perspectives of older people. Ageing Soc. (2004) 24:675–91. doi: 10.1017/S0144686X03001582
128. Bergland A, Narum I. Quality of life demands comprehension and further exploration. J Aging Health. (2007) 19:39–61. doi: 10.1177/0898264306296766
129. Sprangers MA, Schwartz CE. Integrating response shift into health-related quality of life research: a theoretical model. Soc Sci Med. (1999) 48:1507–15. doi: 10.1016/s0277-9536(99)00045-3
130. Power M, Quinn K, Schmidt S. Development of the WHOQOL-old module. Qual Life Res. (2005) 14:2197–214. doi: 10.1007/s11136-005-7380-9
132. Caballero FF, Miret M, Power M, Chatterji S, Tobiasz-Adamczyk B, Koskinen S, et al. Validation of an instrument to evaluate quality of life in the aging population: wHOQOL-AGE. Health Qual Life Outcomes. (2013) 11:177. doi: 10.1186/1477-7525-11-177
133. Netten A, Burge P, Malley J, Potoglou D, Towers AM, Brazier J, et al. Outcomes of social care for adults: developing a preference-weighted measure. Health Technol Assess. (2012) 16:16. doi: 10.3310/hta16160
134. Grewal I, Lewis J, Flynn T, Brown J, Bond J, Coast J. Developing attributes for a generic quality of life measure for older people: preferences or capabilities? Soc Sci Med. (2006) 62:1891–901. doi: 10.1016/j.socscimed.2005.08.023
135. Coast J, Flynn TN, Natarajan L, Sproston K, Lewis J, Louviere JJ, et al. Valuing the ICECAP capability index for older people. Soc Sci Med. (2008) 67:874–82. doi: 10.1016/j.socscimed.2008.05.015
136. van Leeuwen KM, Bosmans JE, Jansen AP, Hoogendijk EO, van Tulder MW, van der Horst HE, et al. Comparing measurement properties of the EQ-5D-3l, ICECAP-O, and ASCOT in frail older adults. Value Health. (2015) 18:35–43. doi: 10.1016/j.jval.2014.09.006
137. Prevention CfDCa. HRQOL Concepts (2018). Available at: https://www.cdc.gov/hrqol/concept.htm (Accessed April 10, 2023).
138. Black K, Dobbs D. Community-Dwelling older Adults’ perspectives on what matters most: findings from an exploratory inquiry. Act Adapt Aging. (2015) 39:133–52. doi: 10.1080/01924788.2015.1025674
139. Osoba D. Translating the science of patient-reported outcomes assessment into clinical practice. JNCI Monographs. (2007) 2007:5–11. doi: 10.1093/jncimonographs/lgm002
140. Gutteling JJ, Darlington AS, Janssen HL, Duivenvoorden HJ, Busschbach JJ, de Man RA. Effectiveness of health-related quality-of-life measurement in clinical practice: a prospective, randomized controlled trial in patients with chronic liver disease and their physicians. Qual Life Res. (2008) 17:195–205. doi: 10.1007/s11136-008-9308-7
141. Detmar SB, Muller MJ, Schornagel JH, Wever LD, Aaronson NK. Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA. (2002) 288:3027–34. doi: 10.1001/jama.288.23.3027
142. Centers for Disease Control and Prevention. Measuring healthy days. Atlanta, Georgia: CDC (2000).
143. Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res. (2009) 18:873–80. doi: 10.1007/s11136-009-9496-9
144. Furlong WFD, Torrance GW. Multiplicative multi-attribute utility function for the Health Utilities Index Mark 3 (HUI) System: A technical report. (1998).
145. Coons SJ, Rao S, Keininger DL, Hays RD. A comparative review of generic quality-of-life instruments. Pharmacoeconomics. (2000) 17:13–35. doi: 10.2165/00019053-200017010-00002
146. Garster NC, Palta M, Sweitzer NK, Kaplan RM, Fryback DG. Measuring health-related quality of life in population-based studies of coronary heart disease: comparing six generic indexes and a disease-specific proxy score. Qual Life Res. (2009) 18:1239–47. doi: 10.1007/s11136-009-9533-8
147. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. (1992) 30:473–83. doi: 10.1097/00005650-199206000-00002
148. McHorney CA, Ware JE Jr, Lu JF, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): iII. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. (1994) 32:40–66. doi: 10.1097/00005650-199401000-00004
149. Aaronson NK, Acquadro C, Alonso J, Apolone G, Bucquet D, Bullinger M, et al. International quality of life assessment (IQOLA) project. Qual Life Res. (1992) 1:349–51. doi: 10.1007/bf00434949
150. Ware J Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. (1996) 34:220–33. doi: 10.1097/00005650-199603000-00003
151. Jenkinson C, Layte R, Jenkinson D, Lawrence K, Petersen S, Paice C, et al. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health Med. (1997) 19:179–86. doi: 10.1093/oxfordjournals.pubmed.a024606
152. Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, et al. Cross-validation of item selection and scoring for the SF-12 health survey in nine countries: results from the IQOLA project. International quality of life assessment. J Clin Epidemiol. (1998) 51:1171–8. doi: 10.1016/s0895-4356(98)00109-7
153. Müller-Nordhorn J, Roll S, Willich SN. Comparison of the short form (SF)-12 health status instrument with the SF-36 in patients with coronary heart disease. Heart. (2004) 90:523. doi: 10.1136/hrt.2003.013995
154. Rumsfeld JS, Alexander KP, Goff DC, Graham MM, Ho PM, Masoudi FA, et al. Cardiovascular health: the importance of measuring patient-reported health Status. Circulation. (2013) 127:2233–49. doi: 10.1161/CIR.0b013e3182949a2e
155. De Smedt D, Clays E, Doyle F, Kotseva K, Prugger C, Pająk A, et al. Validity and reliability of three commonly used quality of life measures in a large European population of coronary heart disease patients. Int J Cardiol. (2013) 167:2294–9. doi: 10.1016/j.ijcard.2012.06.025
156. Kularatna S, Byrnes J, Chan YK, Ski CF, Carrington M, Thompson D, et al. Comparison of the EQ-5D-3l and the SF-6D (SF-12) contemporaneous utility scores in patients with cardiovascular disease. Qual Life Res. (2017) 26:3399–408. doi: 10.1007/s11136-017-1666-6
157. EuroQol Group. Euroqol–a new facility for the measurement of health-related quality of life. Health Policy. (1990) 16:199–208. doi: 10.1016/0168-8510(90)90421-9
158. Stevanović J, Pechlivanoglou P, Kampinga MA, Krabbe PF, Postma MJ. Multivariate meta-analysis of preference-based quality of life values in coronary heart disease. PLoS One. (2016) 11:e0152030. doi: 10.1371/journal.pone.0152030
159. De Smedt D, Clays E, Annemans L, De Bacquer D. EQ-5D versus SF-12 in coronary patients: are they interchangeable? Value Health. (2014) 17:84–9. doi: 10.1016/j.jval.2013.10.010
160. van Stel HF, Buskens E. Comparison of the SF-6D and the EQ-5D in patients with coronary heart disease. Health Qual Life Outcomes. (2006) 4:20. doi: 10.1186/1477-7525-4-20
161. Bergner M, Bobbitt RA, Carter WB, Gilson BS. The sickness impact profile: development and final revision of a health status measure. Med Care. (1981) 19:787–805. doi: 10.1097/00005650-198108000-00001
162. Visser MC, Koudstaal PJ, Erdman RA, Deckers JW, Passchier J, van Gijn J, et al. Measuring quality of life in patients with myocardial infarction or stroke: a feasibility study of four questionnaires in The Netherlands. J Epidemiol Community Health. (1995) 49:513–7. doi: 10.1136/jech.49.5.513
163. William J, Seiber PD, Erik J, Groessl PD, Kristin M, David M, Theodore G, Ganiats MD, Robert M, Kaplan PD. Quality of well being self-administered (QWB-SA) scale—user’s manual. (2008)
164. Kaplan RM, Anderson JP. A general health policy model: update and applications. Health Serv Res. (1988) 23:203–35.3384669
165. Thompson DR, Roebuck A. The measurement of health-related quality of life in patients with coronary heart disease. J Cardiovasc Nurs. (2001) 16.11587238
166. Dempster M, Donnelly M. Measuring the health related quality of life of people with ischaemic heart disease. Heart. (2000) 83:641–4. doi: 10.1136/heart.83.6.641
167. Landgraf JM, Nelson EC. Summary of the WONCA/COOP international health assessment field trial. The dartmouth COOP primary care network. Aust Fam Physician. (1992) 21:255–7. (260–252, 266–259).1605765
168. Kempen GIJM, Sonderen EV, Sanderman R. Measuring health Status with the dartmouth COOP charts in low-functioning elderly. Do the illustrations affect the outcomes? Qual Life Res. (1997) 6:323–8. doi: 10.1023/A:1018427225299
169. Xiao-Jun Lin I-ML, Fan S-Y. Methodological issues in measuring health-related quality of life. Sci Direct. (2013) 25:8–12. doi: 10.1016/j.tcmj.2012.09.002
170. Freedman VA, Spillman BC, Andreski PM, Cornman JC, Crimmins EM, Kramarow E, et al. Trends in late-life activity limitations in the United States: an update from five national surveys. Demography. (2013) 50:661–71. doi: 10.1007/s13524-012-0167-z
171. Roos NP, Havens B. Predictors of successful aging: a twelve-year study of Manitoba elderly. Am J Public Health. (1991) 81:63–8. doi: 10.2105/ajph.81.1.63
172. Sathanapally H, Sidhu M, Fahami R, Gillies C, Kadam U, Davies MJ, et al. Priorities of patients with multimorbidity and of clinicians regarding treatment and health outcomes: a systematic mixed studies review. BMJ Open. (2020) 10:e033445. doi: 10.1136/bmjopen-2019-033445
173. Laditka SB, Corwin SJ, Laditka JN, Liu R, Tseng W, Wu B, et al. Attitudes about aging well among a diverse group of older Americans: implications for promoting cognitive health. Gerontologist. (2009) 49:S30–9. doi: 10.1093/geront/gnp084
174. Kuluski K, Gill A, Naganathan G, Upshur R, Jaakkimainen RL, Wodchis WP. A qualitative descriptive study on the alignment of care goals between older persons with multi-morbidities, their family physicians and informal caregivers. BMC Fam Pract. (2013) 14:133. doi: 10.1186/1471-2296-14-133
175. Pietersma S, de Vries M, van den Akker-van Marle ME. Domains of quality of life: results of a three-stage delphi consensus procedure among patients, family of patients, clinicians, scientists and the general public. Qual Life Res. (2014) 23:1543–56. doi: 10.1007/s11136-013-0578-3
176. van Leeuwen KM, van Loon MS, van Nes FA, Bosmans JE, de Vet HCW, Ket JCF, et al. What does quality of life mean to older adults? A thematic synthesis. PLoS One. (2019) 14:e0213263. doi: 10.1371/journal.pone.0213263
177. Cheraghi-Sohi S, Morden A, Bower P, Kennedy A, Rogers A, Richardson J, et al. Exploring patient priorities among long-term conditions in multimorbidity: a qualitative secondary analysis. SAGE Open Med. (2013) 1:2050312113503955. doi: 10.1177/2050312113503955
178. Junius-Walker U, Schleef T, Vogelsang U, Dierks M-L. How older patients prioritise their multiple health problems: a qualitative study. BMC Geriatr. (2019) 19:362. doi: 10.1186/s12877-019-1373-y
179. Douma L, Steverink N, Hutter I, Meijering L. Exploring subjective well-being in older age by using participant-generated word clouds. Gerontologist. (2015) 57:229–39. doi: 10.1093/geront/gnv119
180. Marchionni N, Fattirolli F, Fumagalli S, Oldridge NB, Del Lungo F, Bonechi F, et al. Determinants of exercise tolerance after acute myocardial infarction in older persons. J Am Geriatr Soc. (2000) 48:146–53. doi: 10.1111/j.1532-5415.2000.tb03905.x
181. Patel KV, Guralnik JM, Phelan EA, Gell NM, Wallace RB, Sullivan MD, et al. Symptom burden among community-dwelling older adults in the United States. J Am Geriatr Soc. (2019) 67:223–31. doi: 10.1111/jgs.15673
182. Leveille SG, Fried L, Guralnik JM. Disabling symptoms: what do older women report? J Gen Intern Med. (2002) 17:766–73. doi: 10.1046/j.1525-1497.2002.20229.x
183. Christiansen L, Sanmartin Berglund J, Lindberg C, Anderberg P, Skär L. Health-related quality of life and related factors among a sample of older people with cognitive impairment. Nurs Open. (2019) 6:849–59. doi: 10.1002/nop2.265
184. Fried TR, Tinetti ME, Iannone L, O'Leary JR, Towle V, Van Ness PH. Health outcome prioritization as a tool for decision making among older persons with multiple chronic conditions. Arch Intern Med. (2011) 171:1854–6. doi: 10.1001/archinternmed.2011.424
185. Wu J, Han Y, Xu J, Lu Y, Cong H, Zheng J, et al. Chronic stable angina is associated with lower health-related quality of life: evidence from Chinese patients. PLoS One. (2014) 9:e97294. doi: 10.1371/journal.pone.0097294
186. Ettinger WH Jr, Fried LP, Harris T, Shemanski L, Schulz R, Robbins J. Self-reported causes of physical disability in older people: the cardiovascular health study. CHS collaborative research group. J Am Geriatr Soc. (1994) 42:1035–44. doi: 10.1111/j.1532-5415.1994.tb06206.x
187. Rieckmann N, Neumann K, Feger S, Ibes P, Napp A, Preuß D, et al. Health-related qualify of life, angina type and coronary artery disease in patients with stable chest pain. Health Qual Life Outcomes. (2020) 18:140. doi: 10.1186/s12955-020-01312-4
188. Pressman SD, Cohen S. Does positive affect influence health? Psychol Bull. (2005) 131:925–71. doi: 10.1037/0033-2909.131.6.925
189. Giltay EJ, Kamphuis MH, Kalmijn S, Zitman FG, Kromhout D. Dispositional optimism and the risk of cardiovascular death: the zutphen elderly study. Arch Intern Med. (2006) 166:431–6. doi: 10.1001/archinte.166.4.431
190. Maercker A, Forstmeier S, Enzler A, Krüsi G, Hörler E, Maier C, et al. Adjustment disorders, posttraumatic stress disorder, and depressive disorders in old age: findings from a community survey. Compr Psychiatry. (2008) 49:113–20. doi: 10.1016/j.comppsych.2007.07.002
191. AAFP Guideline for the detection and management of post-myocardial infarction depression. Ann Fam Med. (2009) 7:71–9. doi: 10.1370/afm.918
192. Murman DL. The impact of age on cognition. Semin Hear. (2015) 36:111–21. doi: 10.1055/s-0035-1555115
193. Petersen RC. Mild cognitive impairment. Continuum (Minneap Minn). (2016) 22:404–18. doi: 10.1212/con.0000000000000313
194. Ahn IS, Kim JH, Kim S, Chung JW, Kim H, Kang HS, et al. Impairment of instrumental activities of daily living in patients with mild cognitive impairment. Psychiatry Investig. (2009) 6:180–4. doi: 10.4306/pi.2009.6.3.180
195. Pusswald G, Tropper E, Kryspin-Exner I, Moser D, Klug S, Auff E, et al. Health-Related quality of life in patients with subjective cognitive decline and mild cognitive impairment and its relation to activities of daily living. J Alzheimers Dis. (2015) 47:479–86. doi: 10.3233/jad-150284
196. Pan C-W, Wang X, Ma Q, Sun H-P, Xu Y, Wang P. Cognitive dysfunction and health-related quality of life among older Chinese. Sci Rep. (2015) 5:17301. doi: 10.1038/srep17301
197. Dartigues JF, Amieva H. Cognitive frailty: rational and definition from an (I.a.N.a./i.a.g.g.) international consensus group. J Nutr Health Aging. (2014) 18:95. doi: 10.1007/s12603-013-0437-5
198. Leritz EC, McGlinchey RE, Kellison I, Rudolph JL, Milberg WP. Cardiovascular disease risk factors and cognition in the elderly. Curr Cardiovasc Risk Rep. (2011) 5:407–12. doi: 10.1007/s12170-011-0189-x
199. Naik AD, Martin LA, Moye J, Karel MJ. Health values and treatment goals of older, multimorbid adults facing life-threatening illness. J Am Geriatr Soc. (2016) 64:625–31. doi: 10.1111/jgs.14027
200. Sánchez EMBGMCS, Vicario BP. Perception of the quality of life of a group of older people. Revista de Enfermagem Referência. (2014) IV:7–8. doi: 10.12707/RIII1314
201. Unger JB, Johnson CA, Marks G. Functional decline in the elderly: evidence for direct and stress-buffering protective effects of social interactions and physical activity. Ann Behav Med. (1997) 19:152–60. doi: 10.1007/bf02883332
202. Buchman AS, Boyle PA, Wilson RS, Fleischman DA, Leurgans S, Bennett DA. Association between late-life social activity and motor decline in older adults. Arch Intern Med. (2009) 169:1139–46. doi: 10.1001/archinternmed.2009.135
Keywords: quality of life, aging, myocardial ischemia, survey and questionnaire, acute coronary syndrome
Citation: Kalra K, Moumneh MB, Nanna MG and Damluji AA (2023) Beyond MACE: a multidimensional approach to outcomes in clinical trials for older adults with stable ischemic heart disease. Front. Cardiovasc. Med. 10:1276370. doi: 10.3389/fcvm.2023.1276370
Received: 11 August 2023; Accepted: 2 November 2023;
Published: 17 November 2023.
Edited by:
Maurice Enriquez-Sarano, Minneapolis Heart Institute Foundation (MHIF), United StatesReviewed by:
Johannes Tobias Neumann, University Heart and Vascular Center, University Medical Center Hamburg-Eppendorf, GermanyAndrew J Foy, Penn State Milton S. Hershey Medical Center, United States
© 2023 Kalra, Moumneh, Nanna and Damluji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abdulla A. Damluji QWJkdWxsYS5EYW1sdWppQGpodS5lZHU=
Abbreviations ACS, Acute Coronary Syndrome; ADL, activity of daily living; AGS, American Geriatric Society; AHA, American Heart Association; AHRQ, Agency for Healthcare Research Quality; AMI, acute myocardial infarction; APEX-AMI, pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention trial; CABG, coronary artery bypass graft; CAD, coronary artery disease; CCU, cardiac care unit; CDC, centers for disease control and prevention; ED-5D-5l, EuroQol 5 dimension 5 level; GRACE, Global Registry of Acute Coronary Events; GUSTO-1, Global Utilization of Streptokinase and TPA for Occluded coronary arteries trial; HrQoL, health-related quality of life; HUI 3, health utility index 3; IADL, independent activity of daily living; ICON-1, improve cardiovascular outcomes in high-risk older patients with acute coronary syndrome; MACE, major adverse cardiovascular events; MI, myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; PCI, percutaneous coronary intervention; PRO, patient reported outcome; PROMIS, patient-reported outcomes measurement information system; PURSUIT, platelet glycoprotein IIb/IIIa in unstable angina: receptor suppression using integrilin therapy trial; QoL, quality of life; RCT, randomized controlled trial; SF- 36, 36-item short form survey; SIHD, stable ischemic heart disease; STEMI, ST-segment elevation myocardial infarction; TIMI, thrombolysis in myocardial infarction; UA, unstable angina; WHO, World Health Organization.
 Kriti Kalra
Kriti Kalra Mohamad B. Moumneh
Mohamad B. Moumneh Michael G. Nanna2
Michael G. Nanna2 Abdulla A. Damluji
Abdulla A. Damluji