- 1Department of Cardiology, King Edward Medical University, Lahore, Pakistan
- 2Clinical Research Development Unit, Alborz University of Medical Sciences, Karaj, Iran
- 3Student Research Committee, School of Medicine, Alborz University of Medical Sciences, Karaj, Iran
- 4School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 5Department of Internal Medicine, Mass General Brigham - Salem Hospital, Salem, MA, United States
- 6Lahey Hospital and Medical Center, Burlington, MA, United States
- 7Department of Medicine, University of Mississippi Medical Center, Jackson, MS, United States
- 8Hamad Medical Corporation, Doha, Qatar
- 9Department of Medicine, Duke University Medical Center, Durham, NC, United States
- 10Duke Clinical Research Institute, Durham, NC, United States
- 11Ahmanson-UCLA Cardiomyopathy Center, Division of Cardiology, University of California Los Angeles, Los Angeles, CA, United States
Aims: We sought to conduct a meta-analysis to evaluate the efficacy and safety of sodium-glucose cotransporter-2 inhibitors (SGLT2i) in patients with heart failure (HF) with preserved ejection fraction (HFpEF) and HF with mildly reduced ejection fraction (HFmrEF).
Methods: We searched the Cochrane Library, MEDLINE (via PubMed), Embase, and ClinicalTrials.gov till March 2023 to retrieve all randomized controlled trials of SGLT2i in patients with HFpEF or HFmrEF. Risk ratios (RRs) and standardized mean differences (SMDs) with their 95% confidence intervals (95% CIs) were pooled using a random-effects model.
Results: We included data from 14 RCTs. SGLT2i reduced the risk of the primary composite endpoint of first HF hospitalization or cardiovascular death (RR 0.81, 95% CI: 0.76, 0.87; I2 = 0%); these results were consistent across the cohorts of HFmrEF and HFpEF patients. There was no significant decrease in the risk of cardiovascular death (RR 0.96, 95% CI: 0.82, 1.13; I2 = 36%) and all-cause mortality (RR 0.97, 95% CI: 0.89, 1.05; I2 = 0%). There was a significant improvement in the quality of life in the SGLT2i group (SMD 0.13, 95% CI: 0.06, 0.20; I2 = 51%).
Conclusion: The use of SGLT2i is associated with a lower risk of the primary composite outcome and a higher quality of life among HFpEF/HFmrEF patients. However, further research involving more extended follow-up periods is required to draw a comprehensive conclusion.
Systematic Review Registration: PROSPERO (CRD42022364223).
Introduction
Heart failure (HF) is a complex clinical syndrome that results from the impaired ability of the ventricle to fill or eject blood (1). Based on ejection fraction (EF), HF is categorized as HF with preserved ejection fraction (HFpEF): EF ≥50%, HF with mildly reduced ejection fraction (HFmrEF): EF of 41%–49%, and HF with reduced ejection fraction (HFrEF): EF <40% (2). Nearly half of the patients with a diagnosis of HF have a normal or near-normal EF (3). HFpEF is a major global public health concern causing substantial morbidity and mortality (4, 5). With the ageing population and increasing prevalence of comorbidities, the prevalence of HFpEF and HFmrEF is estimated to increase (2, 6).
The sodium-glucose cotransporter-2 inhibitors (SGLT2i) have been established as an important component in the management of HFrEF; however, they have a weaker (class 2a) recommendation in the 2022 American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for HFmrEF and HFpEF (7) although in the most recent update of the European guidelines they have gotten a class Ia recommendation for reducing the risk of HF hospitalization or cardiovascular death (8). There is a growing body of literature establishing the efficacy and benefits of these drugs in patients with HFmrEF and HFpEF. The results of the DELIVER trial, the largest trial to date regarding the use of SGLT2i in HFpEF/HFmrEF patients, have recently been published (9). Furthermore, the results of the individual randomized controlled trials (RCTs) are underpowered in some outcomes such as cardiovascular mortality (9). Therefore, we aimed to conduct this systematic review and meta-analysis to evaluate the safety and efficacy of SGLT2i for managing patients with HFpEF and HFmrEF to inform clinical decision-making.
Methods
We conducted our meta-analysis in conformity with the Cochrane Handbook for Systematic Reviews of Intervention (10) and The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (11) (Supplementary Table 1). In addition, we prospectively registered our protocol with PROSPERO (CRD42022364223).
Search strategy
We searched the following databases and registries from their inception to March 2023: Cochrane Central Register of Controlled Trials (CENTRAL, via The Cochrane Library), MEDLINE (via PubMed), Embase, and ClinicalTrials.gov. The search was conducted using different combinations of the following keywords: (“Sodium-Glucose Transporter 2 Inhibitors” OR “Canagliflozin” OR “dapagliflozin” OR “Empagliflozin” OR “Ipragliflozin” OR “Luseogliflozin” OR “Sotagliflozin” OR “Ertugliflozin”) AND (“Heart Failure”) (Supplementary Table 2). Our search also included bibliographies of identified articles.
Study selection and data extraction
Inclusion criteria for eligible articles were defined as: (1) RCTs only; (2) patient population with HF with preserved/mildly reduced ejection fraction; (3) treatment with any SGLT2i vs. placebo or usual treatment. Trials that were conducted in diabetes mellitus patients but provided data as subgroup or secondary analyses on HFpEF/HFmrEF patients were also included. Meanwhile, observational studies, and animal studies were excluded.
Two reviewers performed the screening process in EndNote X9, including duplication removal, screening via titles and abstracts, and finally, full texts were examined. A third reviewer was asked to assess to decrease the screening bias and resolve the disagreements.
Data were obtained from text, tables, figures, and supplementary materials. Two reviewers independently extracted data and classified them as follows: information about study characteristics (trial name, author name, year of publication, country of the region, type of study), population [the total number of participants, intervention and control descriptions, age, gender, left ventricular ejection fraction (LVEF) and diabetes prevalence], and interventions (diagnostic threshold, duration, and dose of intervention).
Quality assessment and certainty of evidence
Two reviewers independently assessed included studies using the revised Cochrane Risk of Bias tool (RoB 2.0). RoB 2.0 was used for the assessment of the following five domains: (1) selection bias, (2) performance bias, (3) detection bias, (4) attrition bias, and (5) reporting bias. Studies were classified into “high risk,” unclear risk,” and low risk based on the ROB-2 checklist.
To assess the certainty of evidence for each of our outcomes, the five Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) were utilized to assess the certainty of the body of evidence (12).
Outcomes
Primary outcomes included a composite endpoint of cardiovascular death or first HF hospitalization/urgent hospital visit due to heart failure, the incidence of cardiovascular death, and the risk of hospitalization. Secondary outcomes included risk of all-cause mortality, quality of life (using The Kansas City Cardiomyopathy Questionnaire (KCCQ) and Minnesota Living with Heart Failure (MLHF) scales), any adverse events (AEs), and serious adverse events (SAEs). Hypotension, hypoglycemia, ketoacidosis, drug discontinuation, and urinary tract infection were defined as specific adverse events of interest.
Statistical analysis
We summarized the pooled effect size of dichotomous outcomes using the risk ratio (RR) and 95% confidence intervals (95% CI). The standardized mean difference (SMD) was utilized for reporting the results of continuous outcomes. Study heterogeneity was assessed using the Chi-square test and I2 statistic. P < 0.10 was considered statistically significant for the Chi-square Test. A DerSimonian and Laird random-effects approach was used in our meta-analysis. To investigate any potential effects of the individual moderators, subgroup analyses were carried out based on the diabetes status of patients, the type of study (whether an HFpEF-specific trial or a subgroup/post-hoc analysis), the EF diagnostic threshold used as inclusion criteria or cutoff for subgroup analysis by the studies (EF >40%, 45% or 50%), and the baseline LVEF of patients (40%-50% vs. >50%) for the primary outcomes. Publication bias was not assessed since there were fewer than 10 studies for all outcomes. All statistical analyses were carried out using RevMan version 5.4.
Results
Search results and study characteristics
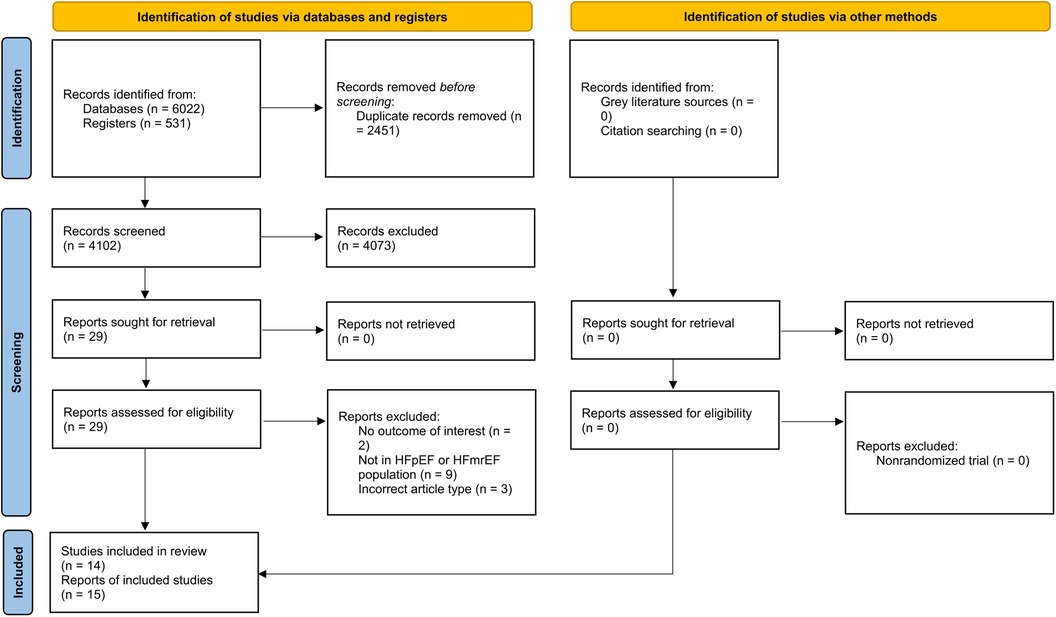
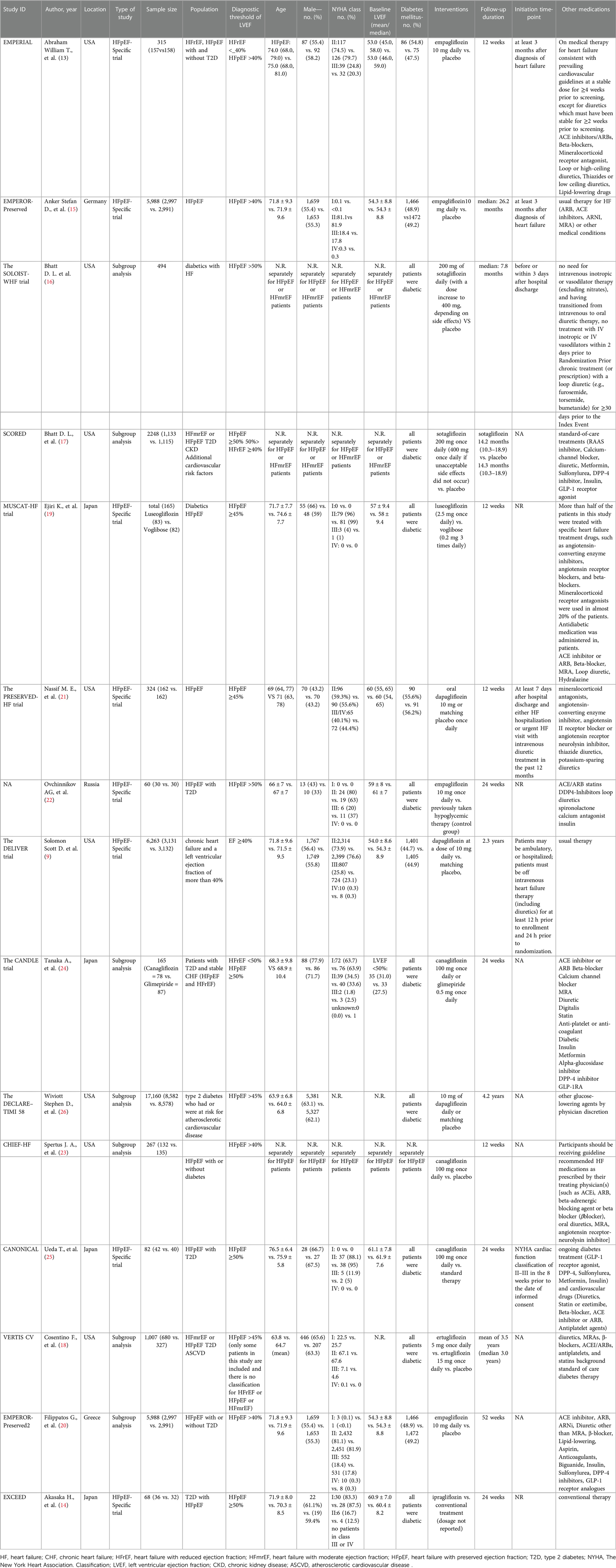
The detailed search result has been provided in Figure 1. Fifteen studies met the eligibility criteria and were included (9, 13, 22–26, 14–21). These reports provided data regarding 14 RCTs. Most of the studies were conducted in the USA. Among the included studies, eight were designed specifically for HFpEF patients (HFpEF-specific trial). For the definition of HFpEF/HFmrEF, five studies designated EF >40%, four designated EF >45%, and six designated EF >50% as the diagnostic thresholds to distinguish from HFrEF. The main population of 9 studies was diabetic patients. The duration of SGLT2i treatment ranged from 12 weeks to 3.5 years. Detailed characteristics of the included studies are available in Table 1.
Risk of bias in included studies
Overall, nine studies had a low risk of bias, six had some concerns (primarily due to issues in the domain of randomization), and none were at high risk (Supplementary Figure 1).
Results of the meta-analysis
Primary composite outcome (cardiovascular death and hospitalization/urgent visit)
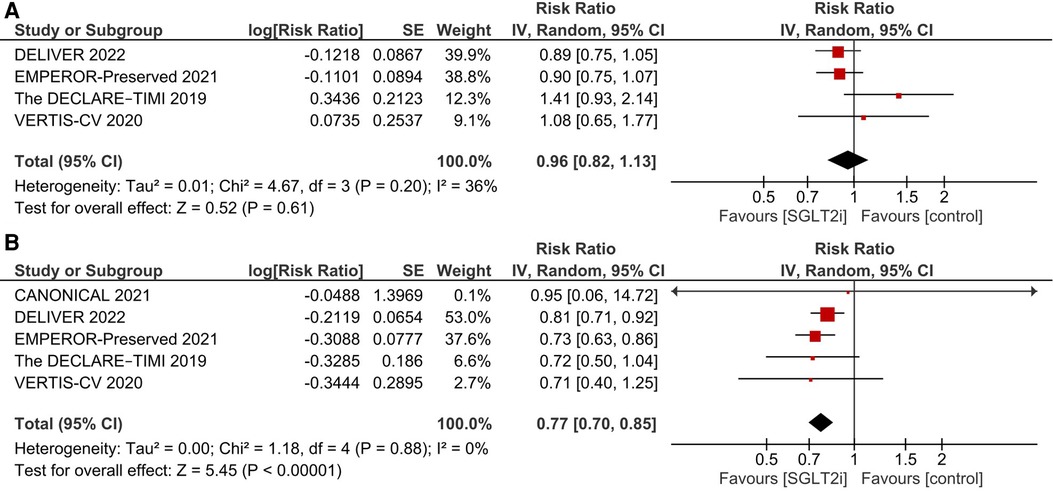
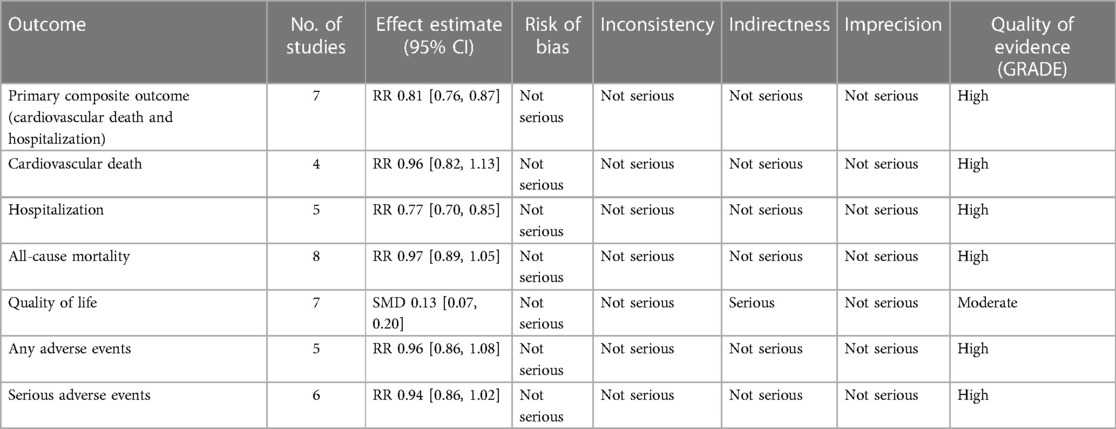
After pooling the results of 7 studies (9, 15–18, 25, 26), a significant reduction in the incidence of primary composite outcome was observed in the SGLT2i group (RR 0.81, 95% CI: 0.76, 0.87; I2 = 0%; Figure 2).
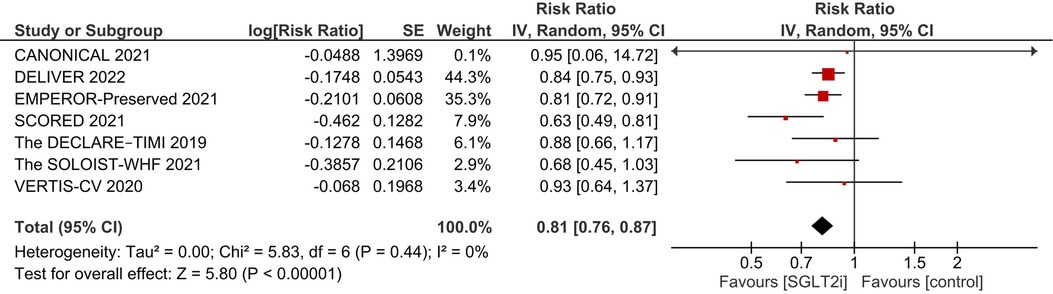
Figure 2. Effect of SGLT2i on the primary composite endpoint of incidence of first HF hospitalization and cardiovascular death.
Cardiovascular death
There was no significant difference between the SGLT2i and the control groups regarding cardiovascular death (RR 0.96, 95% CI: 0.82, 1.13; I2 = 36%; Figure 3).
First HF hospitalization
SGLT2i were associated with a significant reduction in the incidence of first HF hospitalization (RR 0.77, 95% CI: 0.70, 0.85; I2 = 0%; Figure 3).
All-cause mortality
There was no significant difference in all-cause mortality between the two groups (RR 0.97, 95% CI: 0.89, 1.05; I2 = 0%) (Supplementary Figure 2).
Quality of life
Several questionnaires were used to assess the patient's quality of life including the KCCQ and MLHF scales. Overall, there was a significant improvement in the quality of life in the SGLT2i group (SMD 0.13, 95% CI: 0.06, 0.20; I2 = 51%; Supplementary Figure 3).
Safety
There was no significant difference in the incidence of AEs between the two groups (RR 0.96, 95% CI: 0.86, 1.08; I2 = 22%; Supplementary Figure 4). Furthermore, no significant difference was observed in the risk of SAEs (RR 0.94, 95% CI: 0.86, 1.02; I2 = 46%; Supplementary Figure 5). Our analyses regarding specific AEs of interest showed a significant increase in the rate of hypotension and urinary tract infection (Supplementary Table 3).
Subgroup analyses
Diabetes
There was no significant change in the effects of the SGLT2i on the primary composite outcome (cardiovascular death and hospitalization) (Pinteraction = 0.91; Supplementary Figure 6), cardiovascular death (Pinteraction = 0.09; Supplementary Figure 7), and hospitalization (Pinteraction = 0.78; Supplementary Figure 8) due to diabetes.
EF diagnostic threshold
There were no between-group differences (EF >40%, >45% or >50%) in the primary composite outcome (Pinteraction = 0.57; Supplementary Figure 9), and risk of hospitalization (Pinteraction = 0.88; Supplementary Figure 10). Regarding cardiovascular death, there was a trend towards greater benefit with SGLT2i use in studies with EF >40% as the cutoff (RR 0.89, 95% CI: 0.79, 1.01; Supplementary Figure 11) as compared with studies with EF >45% which showed a non-significant increase in cardiovascular mortality (RR 1.26, 95% CI: 0.92, 1.74; Pinteraction = 0.05).
Baseline LVEF
The results of our primary analysis for the primary composite outcome were consistent across the HFmrEF (EF between 40% and 50%) and HFpEF patient cohorts (EF more than 50%) (Pinteraction = 0.48; Supplementary Figure 12).
Type of study
The results of all three primary outcomes were consistent across the type of study (HFpEF-specific trials vs. subgroup/posthoc analysis studies) (Supplementary Figures 13–S15).
Certainty of evidence
The summary of findings and quality of evidence for study outcomes is available in Table 2. The certainty of evidence was high for all outcomes except for the quality of life which was downgraded to moderate due to the issue of indirectness.
Discussion
To the best of our knowledge, this is the most comprehensive systematic review and meta-analysis to date investigating the effect of SGLT2i on the clinical outcomes of patients with HFpEF and HFmrEF. Based on our analyses, SGLT2i significantly decrease the incidence of primary composite outcome (cardiovascular death and first HF hospitalization) mainly driven by the decrease in hospitalization, and substantially improve the quality of life. In addition, our subgroup analyses did not show any significant between-group differences in most of the outcomes assessed.
Overall, the results of our meta-analysis are congruent with the findings of prior meta-analyses, demonstrating a significant benefit of SGLT2i in HFpEF patients (27, 28). We included the DELIVER trial which is the largest RCT specifically conducted for HFpEF and HFmrEF patient population. This enabled us to extend the results of the previous meta-analyses by pooling a significantly greater cumulative sample size. Notably, our finding of no reduction in cardiovascular death is contrary to the results of a recent meta-analysis which found that SGLT2i decreased the risk of cardiovascular death (29). This meta-analysis, however, pooled results only from the EMPEROR-Preserved and DELIVER trials whereas we also included data available from other RCTs. Although this may have introduced some heterogeneity due to the inclusion of non-HF-specific RCTs, it also increases the statistical power required to discern a potential benefit. Nevertheless, further large-scale RCTs are required to resolve this inconsistency and establish the benefit of SGLT2i for reducing cardiovascular mortality with greater confidence. This also indicates that therapies that decrease the risk of mortality in HFpEF/HFmrEF patients are still desperately needed.
A recent meta-analysis reported similar findings to ours but it did not explore the benefit of SGLT2i in improving the quality of life of patients (30). Our study showed that SGLT2i significantly improve the quality of life based on KCCQ and MLHF questionnaires which is an important finding for patients who desire effective treatment options that can not only alleviate symptoms but also improve their overall well-being and day-to-day functioning. Most importantly, since there is a paucity of data regarding the use of SGLT2i in HFmrEF patients, we performed a subgroup analysis based on individual LVEF status and demonstrated that the beneficial effect of SGLT2i is consistent in this population too. Although the overlap of results of trials with HFrEF and HFpEF patients may shed light on the pharmacological treatment of HFmrEF, there is still a lack of specifically designed RCTs for this group of patients (31). More RCTs exclusively designed for this subset of HF patients are required to determine whether or not SGLT2i will reduce the rate of cardiovascular death alone.
The mechanism of action of SGLT2i involves the inhibition of sodium-glucose cotransporter-2 located in the S1 and S2 segments of the proximal convoluted tubule (PCT). Simultaneous prevention of sodium and glucose reabsorption leads to glucosuria and natriuresis (32). However, since the cardiovascular benefits of SGLT2i are demonstrated early after the initiation of therapy, mechanisms of action other than glycemic control seem to be responsible for these effects since improved glycemic control requires years to be effective (33, 34). Several cardioprotective effects including decreased risk of the development and decompensation of HF, reduction in blood pressure, and maintaining proper renal glomerular function are resulted from the diuretic effects along with tissue sodium regulation provided by SGLT2i (35). A recent proteomics study suggests the enhanced autophagy induced by SGLT2i as a potential mechanism underlying the cardioprotective effects (36). Moreover, a metabolomic study stated that alterations in cardiac cell metabolism towards the increased consumption of ketone bodies and free fatty acids may be responsible for these effects (37). Other suggested benefits include prevention of left ventricular hypertrophy, adaptive cellular reprogramming, vascular compliance, reduced blood pressure, reduced systemic inflammation, weight loss, enhanced myocardial energetics, lower uric acid levels, and positive effects on endothelial progenitor cells (38–41).
The use of SGLT2i is associated with several adverse events, including a higher risk of amputations, fractures, bladder cancer, and diabetic ketoacidosis (DKA) (42). When considering specific adverse events, hypotension and urinary tract infection were more frequently seen in the intervention group which aligns with the previously published literature as well-known adverse events of SGLT2i (43, 44).
Our study has several strengths. Our study has the largest cumulative sample size by including comprehensive and up-to-date results. We included only RCTs to review the highest level of clinical evidence. In addition, the GRADE criteria were used for assessing the quality of the evidence. supplementary material from all of the studies was meticulously explored to achieve comprehensive data. Moreover, we performed several subgroup analyses stratified on different EF intervals as well as concurrent diagnoses of DM. Furthermore, the heterogeneity of the studies in our analyses was assessed as very low as demonstrated by the I2 statistic. Our study is also susceptible to certain limitations. First, some of the data were obtained from the post-hoc analyses as the original studies included HFrEF patients as well. Second, the proportion of patients with DM varied among different studies, with some of the studies not including DM patients. Third, studies differed in terms of EF thresholds attributed to each type of heart failure. Fourth, differences exist in both the type and dosages of SGLT2i used as well as the duration of the studies.
Based on our analysis, the use of SGLT2i is associated with a lower risk of the primary composite outcome of hospitalization and cardiovascular death mainly driven by the reduction in hospitalization, and a higher quality of life among HFpEF/HFmrEF patients. Further research involving longer follow-up periods is required to draw a comprehensive conclusion regarding the efficacy and safety of SGLT2i in HFpEF and HFmrEF patients, especially for cardiovascular death.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
HC: Conceptualization, Writing – original draft, Writing – review & editing. AS: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. MA: Writing – review & editing. MR: Data curation, Writing – original draft, Writing – review & editing. AM: Writing – original draft, Writing – review & editing. KJ: Writing – review & editing. AS: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. AA: Writing – review & editing. SI: Data curation, Writing – review & editing. SD: Formal analysis, Writing – review & editing. AM: Data curation, Writing – review & editing. AN: Writing – review & editing. MF: Conceptualization, Writing – review & editing. GF: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
Open Access funding provided by the Qatar National Library.
Conflict of interest
GF reports consulting for Abbott, Amgen, AstraZeneca, Bayer, Cytokinetics, Edwards, Eli Lilly, Janssen, Medtronic, Merck, Novartis, and Pfizer.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor SV declared a past co-authorship with the author SD.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1273781/full#supplementary-material
References
1. Roger VL. Epidemiology of heart failure. Circ Res. (2021) 128:1421–34. doi: 10.1161/CIRCRESAHA.121.318172
2. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. (2017) 14:591–602. doi: 10.1038/nrcardio.2017.65
3. Gladden JD, Linke WA, Redfield MM. Heart failure with preserved ejection fraction. Pflügers Arch - Eur J Physiol. (2014) 466:1037–53. doi: 10.1007/s00424-014-1480-8
4. Chan MMY, Lam CSP. How do patients with heart failure with preserved ejection fraction die? Eur J Heart Fail. (2013) 15:604–13. doi: 10.1093/eurjhf/hft062
5. Komajda M, Lam CSP. Heart failure with preserved ejection fraction: a clinical dilemma. Eur Heart J. (2014) 35:1022–32. doi: 10.1093/eurheartj/ehu067
6. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. (2006) 355:251–9. doi: 10.1056/nejmoa052256
7. Biykem B AHP, David A AAL, M JBJ, Anita D CM, M HDM, R DS, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure. J Am Coll Cardiol. (2022) 79:e263–421. doi: 10.1016/j.jacc.2021.12.012
8. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2023 focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2023):ehad195. doi: 10.1093/eurheartj/ehad195
9. Solomon SD, McMurray JJV, Claggett B, de Boer RA, DeMets D, Hernandez AF, et al. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. (2022) 387:1089–98. doi: 10.1056/NEJMoa2206286
10. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editors. Cochrane handbook for systematic reviews of interventions. 2nd ed. Hoboken, New Jersey: Wiley Blackwell (2019). doi: 10.1002/9781119536604
11. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
12. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Schünemann HJ. GRADE: what is “quality of evidence” and why is it important to clinicians? Br Med J. (2008) 336:995–8. doi: 10.1136/bmj.39490.551019.BE
13. Abraham WT, Lindenfeld J, Ponikowski P, Agostoni P, Butler J, Desai AS, et al. Effect of empagliflozin on exercise ability and symptoms in heart failure patients with reduced and preserved ejection fraction, with and without type 2 diabetes. Eur Heart J. (2021) 42:700–10. doi: 10.1093/eurheartj/ehaa943
14. Akasaka H, Sugimoto K, Shintani A, Taniuchi S, Yamamoto K, Iwakura K, et al. Effects of ipragliflozin on left ventricular diastolic function in patients with type 2 diabetes and heart failure with preserved ejection fraction: the EXCEED randomized controlled multicenter study. Geriatr Gerontol Int. (2022) 22:298–304. doi: 10.1111/ggi.14363
15. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. (2021) 385:1451–61. doi: 10.1056/NEJMoa2107038
16. Bhatt DL, Szarek M, Pitt B, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med. (2021) 384:129–39. doi: 10.1056/NEJMoa2030186
17. Bhatt DL, Szarek M, Steg PG, Cannon CP, Leiter LA, McGuire DK, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. (2021) 384:117–28. doi: 10.1056/NEJMoa2030183
18. Cosentino F, Cannon CP, Cherney DZI, Masiukiewicz U, Pratley R, Dagogo-Jack S, et al. Efficacy of ertugliflozin on heart failure-related events in patients with type 2 diabetes mellitus and established atherosclerotic cardiovascular disease: results of the VERTIS CV trial. Circulation. (2020) 142:2205–15. doi: 10.1161/circulationaha.120.050255
19. Ejiri K, Miyoshi T, Kihara H, Hata Y, Nagano T, Takaishi A, et al. Effect of luseogliflozin on heart failure with preserved ejection fraction in patients with diabetes mellitus. J Am Heart Assoc. (2020) 9:e015103. doi: 10.1161/JAHA.119.015103
20. Filippatos G, Butler J, Farmakis D, Zannad F, Ofstad AP, Ferreira JP, et al. Empagliflozin for heart failure with preserved left ventricular ejection fraction with and without diabetes. Circulation. (2022) 146:676–86. doi: 10.1161/circulationaha.122.059785
21. Nassif ME, Windsor SL, Borlaug BA, Kitzman DW, Shah SJ, Tang F, et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: a multicenter randomized trial. Nat Med. (2021) 27:1954–60. doi: 10.1038/s41591-021-01536-x
22. Ovchinnikov AG, Borisov AA, Zherebchikova KY, Ryabtseva OY, Gvozdeva AD, Masenko VP, et al. Effects of empagliflozin on exercise tolerance and left ventricular diastolic function in patients with heart failure with preserved ejection fraction and type 2 diabetes: a prospective single-center studyAim. To assess the effect of the sodium-glucose. Russ J Cardiol. (2021) 26:4304. doi: 10.15829/1560-4071-2021-4304
23. Spertus JA, Birmingham MC, Nassif M, Damaraju C V, Abbate A, Butler J, et al. The SGLT2 inhibitor canagliflozin in heart failure: the CHIEF-HF remote, patient-centered randomized trial. Nat Med. (2022) 28:809–13. doi: 10.1038/s41591-022-01703-8
24. Tanaka A, Hisauchi I, Taguchi I, Sezai A, Toyoda S, Tomiyama H, et al. Effects of canagliflozin in patients with type 2 diabetes and chronic heart failure: a randomized trial (CANDLE). ESC Hear Fail. (2020) 7:1585–94. doi: 10.1002/ehf2.12707
25. Ueda T, Kasama S, Yamamoto M, Nakano T, Ueshima K, Morikawa Y, et al. Effect of the sodium-glucose cotransporter 2 inhibitor canagliflozin for heart failure with preserved ejection fraction in patients with type 2 diabetes. Circ Reports. (2021) 3:CR-21-0030. doi: 10.1253/circrep.CR-21-0030
26. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. (2019) 380:347–57. doi: 10.1056/NEJMoa1812389
27. Fukuta H, Hagiwara H, Kamiya T. Sodium-glucose cotransporter 2 inhibitors in heart failure with preserved ejection fraction: a meta-analysis of randomized controlled trials. Int J Cardiol Hear Vasc. (2022) 42:101103. doi: 10.1016/j.ijcha.2022.101103
28. Zhou H, Peng W, Li F, Wang Y, Wang B, Ding Y, et al. Effect of sodium-glucose cotransporter 2 inhibitors for heart failure with preserved ejection fraction: a systematic review and meta-analysis of randomized clinical trials. Front Cardiovasc Med. (2022) 9:875327. doi: 10.3389/fcvm.2022.875327
29. Vaduganathan M, Docherty KF, Claggett BL, Jhund PS, de Boer RA, Hernandez AF, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet. (2022) 400:757–67. doi: 10.1016/S0140-6736(22)01429-5
30. Wang Y, Gao T, Meng C, Li S, Bi L, Geng Y, et al. Sodium-glucose co-transporter 2 inhibitors in heart failure with mildly reduced or preserved ejection fraction: an updated systematic review and meta-analysis. Eur J Med Res. (2022) 27:314. doi: 10.1186/s40001-022-00945-z
31. Srivastava PK, Hsu JJ, Ziaeian B, Fonarow GC. Heart failure with mid-range ejection fraction. Curr Heart Fail Rep. (2020) 17:1–8. doi: 10.1007/s11897-019-00451-0
32. Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Intern Med. (2007) 261:32–43. doi: 10.1111/j.1365-2796.2006.01746.x
33. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. (2008) 359:1577–89. doi: 10.1056/NEJMoa0806470
34. Hayward RA, Reaven PD, Wiitala WL, Bahn GD, Reda DJ, Ge L, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. (2015) 372:2197–206. doi: 10.1056/NEJMoa1414266
35. Cowie MR, Fisher M. SGLT2 inhibitors: mechanisms of cardiovascular benefit beyond glycaemic control. Nat Rev Cardiol. (2020) 17:761–72. doi: 10.1038/s41569-020-0406-8
36. Gutmann C, Zelniker TA, Mayr M. SGLT2 inhibitors in heart failure: insights from plasma proteomics. Eur Heart J. (2022) 43(48):5003–5. doi: 10.1093/eurheartj/ehac624
37. Selvaraj S, Fu Z, Jones P, Kwee LC, Windsor SL, Ilkayeva O, et al. Metabolomic profiling of the effects of dapagliflozin in heart failure with reduced ejection fraction: DEFINE-HF. Circulation. (2022) 146:808–18. doi: 10.1161/CIRCULATIONAHA.122.060402
38. Hess DA, Terenzi DC, Trac JZ, Quan A, Mason T, Al-Omran M, et al. SGLT2 inhibition with empagliflozin increases circulating provascular progenitor cells in people with type 2 diabetes mellitus. Cell Metab. (2019) 30:609–13. doi: 10.1016/j.cmet.2019.08.015
39. Sherman SE, Bell GI, Teoh H, Al-Omran M, Connelly KA, Bhatt DL, et al. Canagliflozin improves the recovery of blood flow in an experimental model of severe limb ischemia. JACC Basic to Transl Sci. (2018) 3:327–9. doi: 10.1016/j.jacbts.2018.01.010
40. Packer M. SGLT2 inhibitors produce cardiorenal benefits by promoting adaptive cellular reprogramming to induce a state of fasting mimicry: a paradigm shift in understanding their mechanism of action. Diabetes Care. (2020) 43:508–11. doi: 10.2337/dci19-0074
41. Verma S, Mazer CD, Yan AT, Mason T, Garg V, Teoh H, et al. Effect of empagliflozin on left ventricular mass in patients with type 2 diabetes mellitus and coronary artery disease: the EMPA-HEART CardioLink-6 randomized clinical trial. Circulation. (2019) 140:1693–702. doi: 10.1161/circulationaha.119.042375
42. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. (2017) 377:644–57. doi: 10.1056/nejmoa1611925
43. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. (2015) 373:2117–28. doi: 10.1056/NEJMoa1504720
Keywords: SGLT2I, empagliflozin, dapagliflozin, heart failure, HFPEF
Citation: Cheema HA, Shafiee A, Athar MMT, Rafiei MA, Mehmannavaz A, Jafarabady K, Shahid A, Ahmad A, Ijaz SH, Dani SS, Minhas AMK, Nashwan AJ, Fudim M and Fonarow GC (2023) Efficacy and safety of sodium-glucose cotransporter-2 inhibitors for heart failure with mildly reduced or preserved ejection fraction: a systematic review and meta-analysis of randomized controlled trials. Front. Cardiovasc. Med. 10:1273781. doi: 10.3389/fcvm.2023.1273781
Received: 7 August 2023; Accepted: 29 September 2023;
Published: 12 October 2023.
Edited by:
Saraschandra Vallabhajosyula, Wake Forest University, United StatesReviewed by:
Mridul Bansal, East Carolina University, United StatesAryan Mehta, University of Connecticut, United States
© 2023 Cheema, Shafiee, Athar, Rafiei, Mehmannavaz, Jafarabady, Shahid, Ahmad, Ijaz, Dani, Minhas, Nashwan, Fudim and Fonarow. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huzaifa Ahmad Cheema aHV6YWlmYWFobWFkY2hlZW1hQGdtYWlsLmNvbQ== Abdulqadir J. Nashwan YW5hc2h3YW5AaGFtYWQucWE=
†These authors have contributed equally to this work and share first authorship
 Huzaifa Ahmad Cheema
Huzaifa Ahmad Cheema Arman Shafiee2,3,†
Arman Shafiee2,3,† Sourbha S. Dani
Sourbha S. Dani Abdul Mannan Khan Minhas
Abdul Mannan Khan Minhas Abdulqadir J. Nashwan
Abdulqadir J. Nashwan Gregg C. Fonarow
Gregg C. Fonarow