- 1Department of Epidemiology, Human Genetics and Environmental Sciences, School of Public Health, The University of Texas Health Science Center at Houston, Houston, TX, United States
- 2Department of Biostatistics and Data Science, Coordinating Center for Clinical Trials, School of Public Health, The University of Texas Health Science Center at Houston, Houston, TX, United States
Purpose: This study aimed to determine the 18-year risk of cancer, angioedema, insomnia, depression, and erectile dysfunction in association with antihypertensive drug use.
Methods: This is a post-trial passive follow-up study of Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) participants between 1994 and 1998 that was conducted by linking their follow-up data with Medicare claims data until 2017 of subjects who were free of outcomes at baseline on 1 January 1999. The main outcomes were the occurrence of cancer (among n = 17,332), angioedema (among n = 17,340), insomnia (among n = 17,340), depression (among n = 17,330), and erectile dysfunction (among n = 7,444 men) over 18 years of follow-up.
Results: The 18-year cumulative incidence rate of cancer other than non-melanoma skin cancer from Medicare inpatient claims was 23.9% for chlorthalidone, 23.4% for amlodipine, and 25.3% for lisinopril. There were no statistically significant differences in the 18-year risk of cancer, depression, and erectile dysfunction among the three drugs based on the adjusted hazard ratios. The adjusted 18-year risk of angioedema was elevated in those receiving lisinopril than in those receiving amlodipine (hazard ratio: 1.63, 95% CI: 1.14–2.33) or in those receiving chlorthalidone (1.33, 1.00–1.79), whereas the adjusted 18-year risk of insomnia was statistically significantly decreased in those receiving lisinopril than in those receiving amlodipine (0.90, 0.81–1.00).
Conclusions: The 18-year risk of angioedema was significantly higher in patients receiving lisinopril than in those receiving amlodipine or chlorthalidone; the risk of insomnia was significantly lower in patients receiving lisinopril than in those receiving amlodipine; and the risk of cancer, depression, and erectile dysfunction (in men) was not statistically significantly different among the three drug groups.
Introduction
The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), which was a multicenter, randomized, double-blind, active-controlled trial in 623 North American centers and was completed with in-trial follow-up in 2002, produced some major findings (1, 2) that have translated into clinical practice guidance (3–8). For example, this study found that amlodipine (calcium channel blocker), lisinopril [angiotensin-converting enzyme (ACE) inhibitor], and doxazosin were no superior to chlorthalidone (thiazide diuretic) in preventing most types of cardiovascular disease (CVD) and chlorthalidone was superior in preventing heart failure (1, 2). This study also found that the secondary outcomes, such as cancer and hospitalized gastrointestinal (GI) bleeding, had no significantly different 6-year incidence rates and adjusted hazard ratios between these three treatment groups (9). The cumulative incidence rates of non-hospitalized GI bleeding were also similar across the three groups (12.0%, 12.2%, and 12.0% for amlodipine, lisinopril, and chlorthalidone, respectively) (10). According to previous studies, the use of antihypertensive drugs might be associated with an increased risk of angioedema, insomnia, depression, and erectile dysfunction, in addition to cancer, GI bleeding, and dementia (1, 2, 9–17). Some of these side effects were reported from clinical observations, cross-sectional surveys, or retrospective cohort studies, which are extremely vulnerable to selection bias and confounding. The causations have not been well established through the confirmation of long-term follow-up studies of well-conducted randomized clinical trials or cohort studies. Now, data of ALLHAT participants were linked with their Medicare data until December 2017, which enabled us to examine the long-term outcomes associated with different antihypertensive drugs used among the trial participants. Therefore, this study aimed to determine the risk of cancer, angioedema, insomnia, depression, and erectile dysfunction in association with three antihypertensive drugs and other factors among ALLHAT participants with up to 23-year follow-up from 1994 to 2017 and 18-year Medicare-linked data from 1999 to 2017.
Methods
Study design, population, and data sources
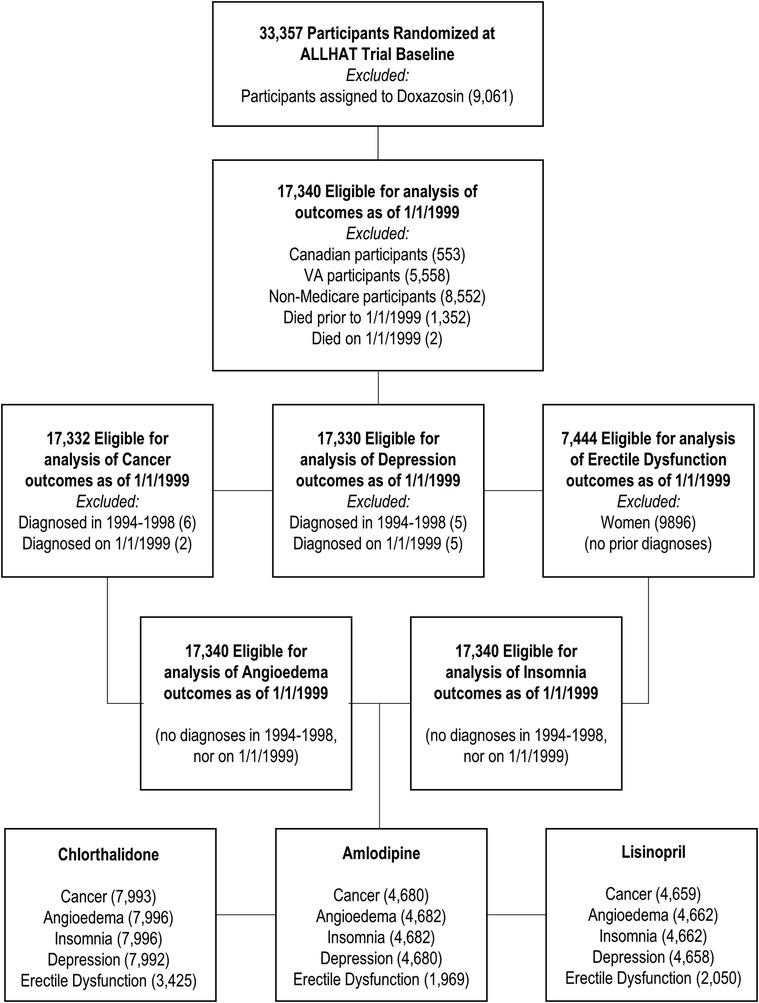
The detailed methods of the ALLHAT have been reported previously (1, 2). In brief, the ALLHAT was a multicenter, randomized, double-blind, active-controlled trial conducted on 42,418 participants aged ≥55 years with hypertension and at least one other coronary heart disease (CHD) risk factor in 623 North American centers. Those patients who were eligible and agreed to participate were randomly assigned to four treatment groups: an ACE inhibitor (lisinopril) (n = 9,054), a calcium channel blocker (CCB, amlodipine) (n = 9,048), an α-blocker (doxazosin) (n = 9,061), or a thiazide-type diuretic (chlorthalidone) (n = 15,255). This study did not include patients on doxazosin because this group was terminated earlier. Data of ALLHAT participants aged ≥65 years (because the nationwide Medicare health insurance program covers those aged 65 years or older) were linked with their Medicare inpatient, outpatient, and physician carrier claims data available from 1 January 1999 to 31 December 2017. Of the 33,357 participants (n = 9,054 for lisinopril, n = 9,048 for amlodipine, and n = 15,255 for chlorthalidone), the study excluded the following subjects: Canadian participants (553), VA participants (5,558), non-Medicare participants (8,552), and those who died on or prior to 1 January 1999 (1,354), leaving 17,340 participants for this study. Because we examined five different study outcomes and considered the baseline as 1 January 1999, we excluded a varying number of subjects based on whether Medicare inpatient claims data from 1 January 1994 to 31 December 1998 had each respective outcome. These individuals were considered to have the condition at baseline and, therefore, were excluded, leaving between 17,330 and 17,340 total subjects and 7,444 men in the final analysis (see Figure 1 for the flowchart, Table 1, and Supplementary Tables S1A–D).
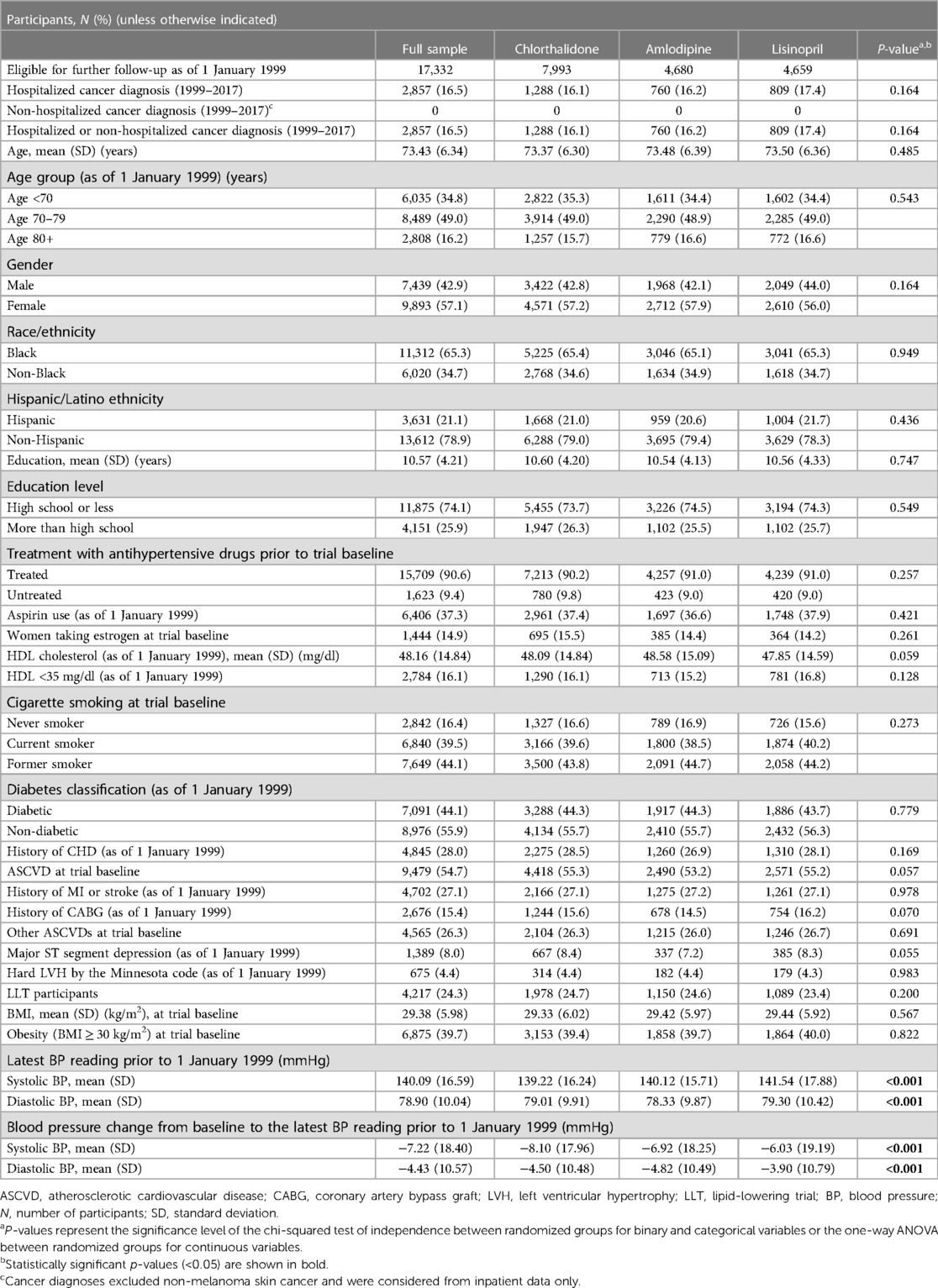
Table 1. Baseline characteristics of the cancer outcome cohort: full sample and by the randomized group.
Study variables
Main exposures
The main exposures were three antihypertensive drugs (lisinopril, amlodipine, and chlorthalidone) that were prescribed to participants through initial trial randomization and continued until 1 January 1999 as the baseline for this passive follow-up because complete Medicare data (inpatient, outpatient, and physician carrier data files) were available from this time to 31 December 2017. After the ALLHAT ended in March 2002, participants were not followed for their post-study antihypertensive treatment.
Main outcomes
The main outcomes were the occurrence of cancer (any cancer other than non-melanoma skin cancer), angioedema, insomnia, depression, and erectile dysfunction (in men). These outcomes were defined if there were ICD-9 or ICD-10 diagnosis codes (Supplementary Tables S2A–C) in Medicare claims data (inpatient, outpatient, and physician carrier claims) that occurred on one or the first of more occasions after the baseline (1 January 1999) to the date of last follow-up (31 December 2017). For sensitivity analyses, the findings were presented when the outcomes were defined from any diagnosis codes and primary diagnosis codes only (i.e., the first diagnosis code out of 12 diagnosis codes for carrier data and 25 diagnosis codes for inpatient and outpatient data) that occurred on at least two separate occasions 30 days apart in Medicare claims data from 1999 to 2017 (Supplementary Tables S3A–D and S4).
Covariates
ALLHAT baseline (at the time of randomization) demographic and clinical data including age, gender, race/ethnicity, education, prior receipt of antihypertensive drug therapy, estrogen use (for women), smoking, history of atherosclerotic cardiovascular disease, other atherosclerotic cardiovascular disease, and obesity (body mass index, BMI ≥30 kg/m2), were incorporated into analyses. Wherever possible, covariate data were gathered from extension trial follow-up visits that were most proximal to but did not extend beyond 1 January 1999. Extension trial data up to 1 January 1999 were available for aspirin use, high-density lipoprotein (HDL) cholesterol level <35 mg/dl, diabetes, history of coronary heart disease, coronary artery bypass graft, major ST segment depression, left ventricular hypertrophy by the Minnesota code, and systolic and diastolic blood pressure. In the absence of targeted follow-up during the extension phase on the status of myocardial infarction (MI) or stroke, data on the history of MI or stroke were supplemented with relevant diagnosis codes garnered from Medicare inpatient claims data from 1 January 1994 to 31 December 1998.
Statistical analysis
Baseline characteristics among the study comparison groups were compared using chi-squared statistics for categorical variables and one-way analysis of variance (ANOVA) for continuous variables. The 6-year and 18-year cumulative incidence rates of cancer (any cancer other than non-melanoma skin cancer), angioedema, insomnia, depression, and erectile dysfunction (in men) were calculated from the baseline on 1 January 1999 to 1 January 2005 for the 6-year rate and to the date of last follow-up (31 December 2017) for the 18-year rate using the Kaplan–Meier method. Subjects who died or were lost to follow-up were censored. The 6-year incidence rates and hazard ratios of outcomes from Medicare claims data were presented to be compared with the findings of original ALLHAT reports that used the 6-year rates and criteria (1).
The population at risk were those who were free of the respective outcomes (cancer, angioedema, insomnia, depression, or erectile dysfunction) at baseline in 1999, as determined from the data available in Medicare inpatient, outpatient, and carrier data sets. In addition, Cox regression models were used to perform time-to-event analyses to determine the risk of developing the above outcomes by the three study drugs while adjusting for all measured confounding factors listed in the tables. The proportionality assumption for multivariable models was assessed by the Schoenfeld residuals test and by visually inspecting whether the log–log Kaplan–Meier curves were parallel and did not intersect. There was no adjustment for multiple comparisons. A p < 0.05 was considered statistically significant. Analyses were conducted using SAS version 9.4 and R version 4.0.2 (R Foundation for Statistical Computing).
Results
Comparisons of baseline characteristics among study participants taking three antihypertensive drugs (chlorthalidone, amlodipine, and lisinopril) on 1 January 1999 were presented for cancer (Table 1) and other outcomes (Supplementary Table S1A–D). Of those subjects who were free of outcomes (cancer, angioedema, insomnia, depression, and erectile dysfunction), the baseline characteristics such as age, gender, race/ethnicity, history of vascular diseases, diabetes, and obesity were similar without statistically significant differences among the three drug groups.
Table 2 presents the cumulative incidence of outcomes (cancer, angioedema, insomnia, depression, and erectile dysfunction) that occurred over the next 6 and 18 years of follow-up from 1 January 1999 to 31 December 2017 and were identified from Medicare inpatient hospitalization data only. For example, the 6-year cumulative incidence rate of cancer other than non-melanoma skin cancer was 9.5% for chlorthalidone, 9.8% for amlodipine, and 10.5% for lisinopril, which was identical to what was reported (9.7%, 10.0%, and 9.9%, respectively, for the above three drug groups) in an ALLHAT main report (1). The 18-year cumulative incidence rate of cancer other than non-melanoma skin cancer from Medicare inpatient hospitalization data was 23.9% for chlorthalidone, 23.4% for amlodipine, and 25.3% for lisinopril (Table 2). After adjusting for socio-demographics, comorbidities, and other potential confounding factors in the time-to-event Cox models (Table 3), the 6-year risk of cancer was statistically significantly higher for lisinopril vs. chlorthalidone (adjusted hazard ratio: 1.19, 95% CI: 1.03–1.36) but was not statistically significantly different among two other drug groups for chlorthalidone vs. amlodipine (1.03, 0.90–1.19) and for lisinopril vs. amlodipine (1.15, 0.98–1.34). The 18-year risk of cancer was not statistically significantly different among the three drug groups [0.98 (95% CI: 0.89–1.09) for chlorthalidone vs. amlodipine, 1.06 (0.95–1.19) for lisinopril vs. amlodipine, and 1.05 (0.95–1.16) for lisinopril vs. chlorthalidone, all ps > 0.05)]. Including cases with outcomes that were identified from Medicare inpatient, outpatient, and physician carrier claims data, the cumulative incidence rate of cancer was much higher. For example, the 6-year cumulative incidence rate of cancer other than non-melanoma skin cancer from Medicare inpatient, outpatient, and physician carrier claims data was 38.5% for chlorthalidone, 38.5% for amlodipine, and 39.3% for lisinopril, whereas the 18-year cumulative incidence rate of cancer other than non-melanoma skin cancer from Medicare inpatient, outpatient, and physician carrier claims data was 61.1% for chlorthalidone, 60.4% for amlodipine, and 62.7% for lisinopril (Supplementary Table S3A). The adjusted hazard ratios of cancer were not statistically significantly different among these three drug groups (Supplementary Table S4).
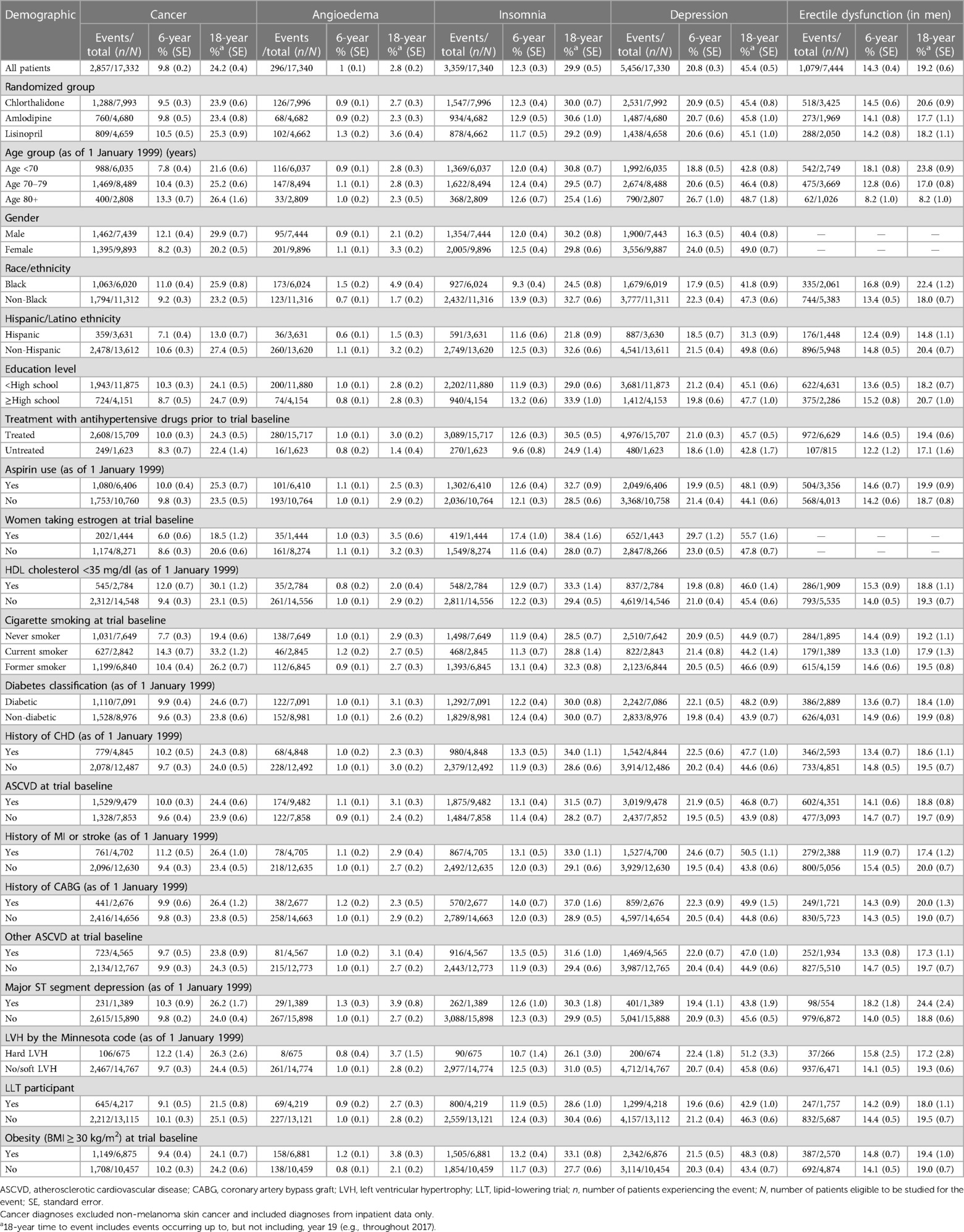
Table 2. Cumulative incidence (%) of five outcomes from any diagnosis in Medicare inpatient data on ≥1 time by the three study drugs and other factors (1999–2017).
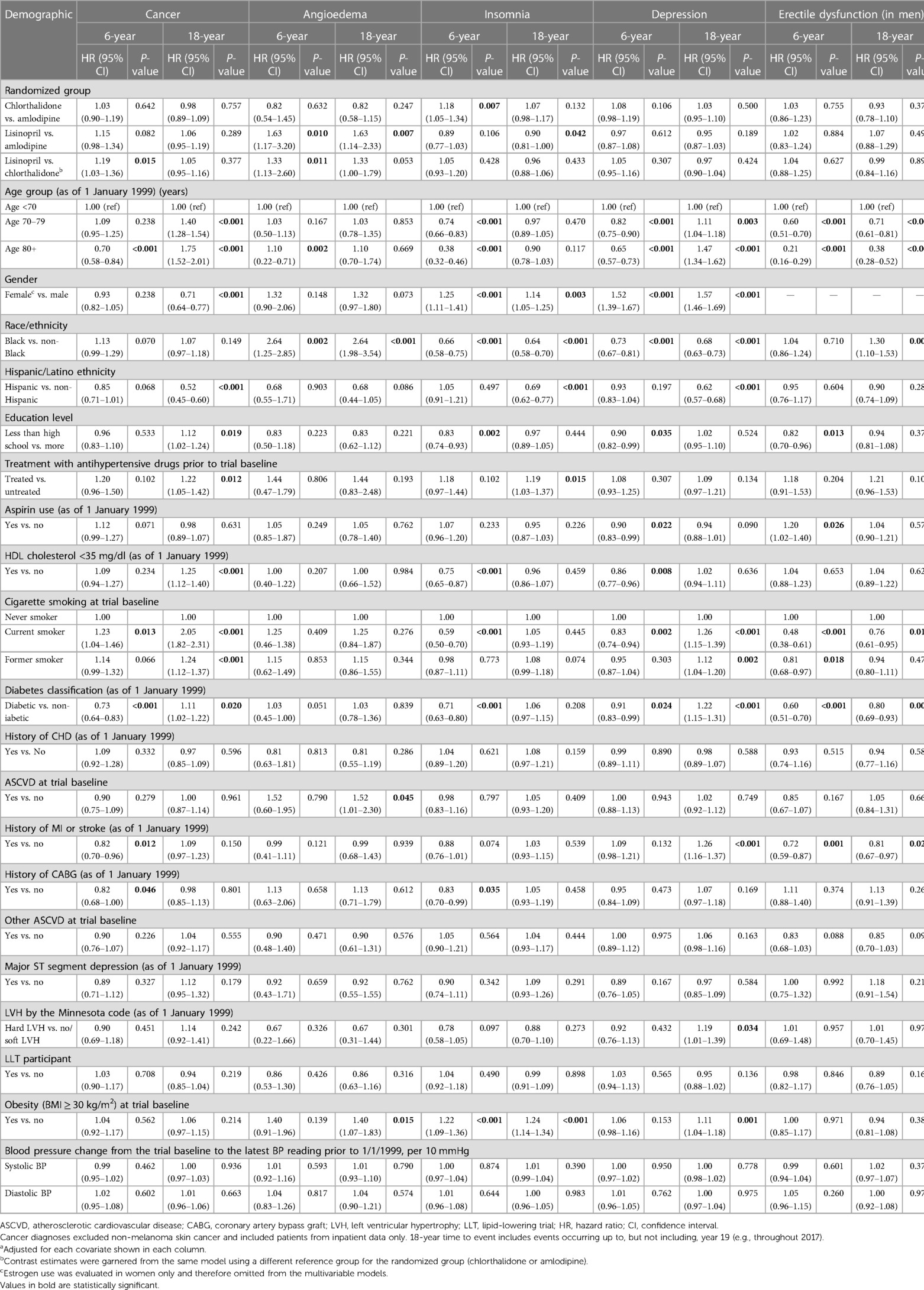
Table 3. Adjusteda HRs of having five outcomes from any diagnosis in Medicare inpatient data on ≥1 time by the three study drugs and other factors (1999–2017).
There were some significant differences in the risk of angioedema and insomnia among these antihypertensive drug groups. For example, the adjusted hazard ratio of angioedema was statistically significantly elevated in those receiving lisinopril than in those receiving amlodipine (1.63, 1.17–3.20, for 6-year risk and 1.63, 1.14–2.33, for 18-year risk) and in those receiving chlorthalidone (1.33, 1.13–2.60, for 6-year risk and 1.33, 1.00–1.79, for 18-year risk). The adjusted hazard ratio of insomnia was statistically significantly higher in patients receiving chlorthalidone (1.18, 1.05–1.34, for 6-year risk) than in those receiving amlodipine and significantly decreased in those receiving lisinopril than in those receiving amlodipine (0.90, 0.81–1.00, for 18-year risk). There were no significant differences in the 6-year and 18-year risk of depression and erectile dysfunction (in men) among the three antihypertensive drug groups.
Figure 2 presents the Kaplan–Meier cumulative incidence rate curves of cancer, angioedema, insomnia, depression, and erectile dysfunction (Figures 2A–E) by the three study drugs from 1999 to 2017 using Medicare inpatient hospitalization data. The log-rank test was statistically significant only (p = 0.01) for the cumulative incidence rate curves of angioedema by the three drug groups but was not statistically significant in the other four cumulative incidence rate curves of insomnia, depression, cancer, and erectile dysfunction by the three study drug groups. In the Kaplan–Meier cumulative incidence rate curve of erectile dysfunction in men (Figure 2E), the plateau was observed between 6 and 9 years. In that time, there were 987 deaths and only two erectile dysfunction diagnoses from inpatient hospitalization data.
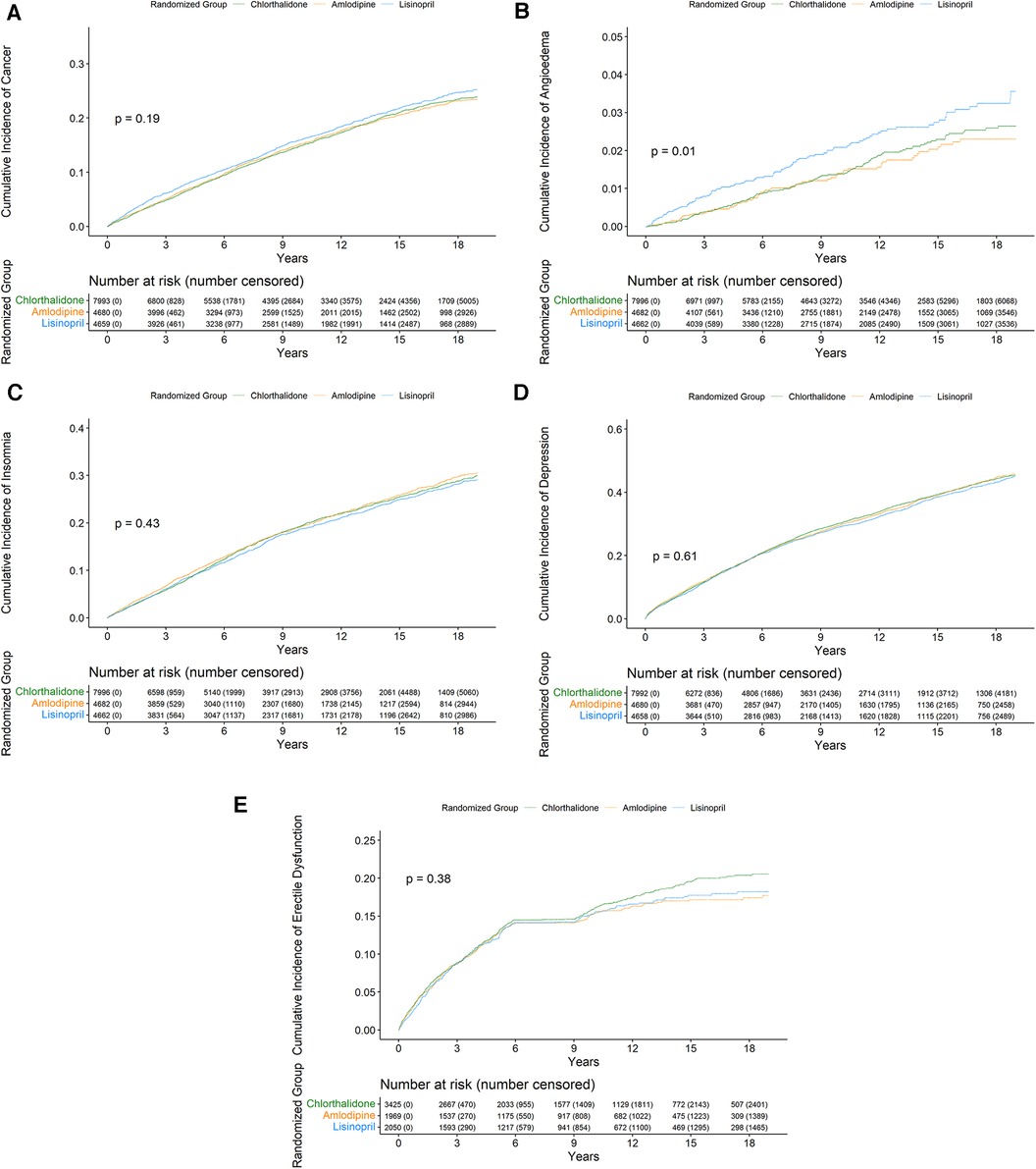
Figure 2. Cumulative incidence of cancer (A), angioedema (B), insomnia (C), depression (D), and erectile dysfunction (E) from 1999 to 2017 by the three study groups.
We also performed several sensitivity analyses by defining the study outcomes differently using Medicare inpatient, outpatient, and physician carrier claims data that occurred at least once (Supplementary Table S3A), using diagnosis codes that occurred at least two times 30 days apart from any diagnosis codes from Medicare inpatient claims only (Supplementary Table S3B), using any diagnosis codes from Medicare inpatient, outpatient, and physician carrier claims data (Supplementary Table S3C), using primary diagnosis only from Medicare inpatient claims alone (Supplementary Table S3D), or using primary diagnosis only from Medicare inpatient, outpatient, and physician carrier claims data (Supplementary Table S3E). The cumulative incidence rates of cancer, angioedema, insomnia, depression, and erectile dysfunction were higher when the diagnosis codes for outcomes were identified from Medicare inpatient, outpatient, and physician carrier claims and lower when the diagnosis codes for outcomes that required at least two claims 30 days apart or when the codes were restricted to primary diagnosis only in Medicare inpatient claims. However, regardless of the criteria for the five outcomes, findings from the three study drug groups were similar to those presented in Tables 2 and 3. Overall, there were no statistically significant differences in the adjusted hazard ratio of cancer, depression, and erectile dysfunction. The adjusted hazard ratio of angioedema was significantly higher in patients receiving lisinopril vs. amlodipine and marginally higher in those receiving lisinopril vs. chlorthalidone, whereas the adjusted hazard ratio of insomnia was significantly lower in patients receiving lisinopril vs. amlodipine (Supplementary Table S4).
Tables 2 and 3 also present the cumulative incidence rates and hazard ratios of cancer, angioedema, insomnia, depression, and erectile dysfunction by other sociodemographic and comorbidity factors. For example, the cumulative incidence rates of cancer and depression increased with advanced age, whereas the cumulative incidence rate of angioedema did not differ significantly with age and the cumulative incidence rates of insomnia and erectile dysfunction (in men) decreased with age (Table 2). The 6-year cumulative incidence rate of cancer was 7.8% in patients aged <70 years, 10.4% in patients aged 70–79 years, and 13.3% in patients aged ≥80 years, whereas the 18-year cumulative incidence rate of cancer was 21.6% in patients aged <70 years, 25.2% in patients aged 70–79 years, and 26.4% in patients aged ≥80 years. The adjusted 18-year risk of cancer was significantly higher in patients aged 70–79 years (hazard ratio: 1.40, 95% CI: 1.28–1.54) and ≥80 years (1.75, 1.52–2.01) (Table 3). There was no significant difference in the 18-year risk of angioedema with age, but the 18-year risk of depression was significantly higher in patients aged 70–79 years (hazard ratio: 1.11, 95% CI 1.04–1.18) and ≥80 years (1.47, 1.34–1.62), whereas the 18-year risk of insomnia and erectile dysfunction in men was lower in older patients. Women had a significantly lower risk of cancer but a significantly higher risk of insomnia and depression than men. Black patients were significantly more likely to have angioedema (2.64, 1.98–3.54) and erectile dysfunction (1.30, 1.10–1.53) and significantly less likely to have insomnia (0.64, 0.58–0.70) and depression (0.68, 0.63–0.73) than non-Black patients, but they did not have significantly different risk of cancer. Hispanics were significantly less likely to develop cancer (0.52, 0.45–0.60), insomnia (0.69, 0.62–0.77), and depression (0.62, 0.57–0.68), but they did not have a significantly different risk of angioedema and erectile dysfunction compared to non-Hispanics. Smoking (current or former), diabetes, myocardial infarction or stroke, left ventricular hypertrophy by the Minnesota code, and obesity were associated with a significantly higher risk of depression. Less than high school education, prior antihypertensive treatment, low HDL cholesterol, smoking (current or former), and diabetes were associated with a significantly higher risk of cancer. Diabetes, smoking (current), and a history of myocardial infarction or stroke were associated with a significantly lower risk of erectile dysfunction in men.
Discussion
This study examined the 6-year and 18-year risk of cancer, angioedema, insomnia, depression, and erectile dysfunction in association with antihypertensive drugs by the three study groups and other factors among a large number of ALLHAT participants using the passive follow-up data of ALLHAT and Medicare-linked data from 1999 to 2017. We found that the risk of angioedema was significantly higher in patients receiving lisinopril than in those receiving amlodipine or chlorthalidone; the risk of insomnia was significantly lower in patients receiving lisinopril than in those receiving amlodipine; and the risk of cancer, depression, and erectile dysfunction (in men) was not statistically significantly different among the three drug groups. The risk of cancer and depression increased with advanced age, while the risk of insomnia and erectile dysfunction (in men) decreased with age; however, the risk of angioedema did not vary significantly with age. The risk of some of these outcomes was also associated with gender, race/ethnicity, education, smoking, and comorbidities.
Hypertension is a prevalent medical condition, accounting for 47% of adults in the United States in 2017–2018 and 33% of adults aged 30–79 years globally in 2019 (18–20). Many patients with hypertension need pharmaceutical treatment to have their blood pressure under control. Thankfully, there are multiple classes of antihypertensive drugs available, which are essential to treat hypertension and prevent more serious complications related to hypertension. However, all medications have a certain degree of side effects, and antihypertensive drugs are no exceptions (21–43). The important consideration in choosing various classes of antihypertensive drugs includes a well-accepted tolerance and a well-maintained quality of life (21–24). Some common side effects of antihypertensive drugs include dizziness, GI bleeding, hypotension, angioedema, insomnia, depression, and erectile dysfunction, depending on different antihypertensive drugs and duration of therapies (1, 2, 9–17, 25–43). Clinical trials often routinely report the side effects (adverse events or toxicities) of intervention drugs detected during the in-trial periods. Indeed, ALLHAT (1, 2) and other clinical trials such as the Systolic Blood Pressure Intervention Trial (SPRINT) (11, 12) reported some common serious side effects associated with antihypertensive drugs, including GI bleeding, angioedema, hypotension, syncope, and acute kidney injury or acute renal failure. However, due to short follow-ups, clinical trials are typically not well suited for measuring rare side effects and/or late (long-term) side effects. The literature that examined the potential links between antihypertensive drugs and the increased risks of side effects suggested that the use of antihypertensive drugs may also be associated with an increased risk of cancer, insomnia, depression, and erectile dysfunction.
An ALLHAT in-trial report did not find a significant association between amlodipine or lisinopril vs. chlorthalidone regarding the risk of GI bleeding (1, 2). A more recent ALLHAT study, which specifically focused on the risk of hospitalized GI bleeding using Medicare inpatient data concluded that hypertensive patients on amlodipine did not have an increased risk of GI bleeding compared to those on chlorthalidone or lisinopril (9). We later used the ALLHAT–Medicare-linked data to examine the risk of both hospitalized and non-hospitalized GI bleeding in association with the use of three study antihypertensive drugs (lisinopril, amlodipine, and chlorthalidone) (10). We found that the cumulative incidence rate of hospitalized GI bleeding until 31 March 2002 (the end of the ALLHAT in-trial) was 5.4%, 5.8%, and 5.4% for amlodipine, lisinopril, and chlorthalidone groups, and the cumulative incidence rate of non-hospitalized GI bleeding was 12.0%, 12.2%, and 12.0% for amlodipine, lisinopril, and chlorthalidone, respectively, which were not statistically significant among the three groups after adjusting for confounders in Cox regression models (10).
The link between the use of antihypertensive drugs and an increased risk of cancer was proposed but is still inconsistent (37, 38). Bangalore et al. summarized 70 clinical trials and concluded that an increased risk of cancer with the combination of ACE inhibitors and angiotensin-receptor blockers (ARBs) cannot be ruled out (37). Later, Copland et al. summarized 33 clinical trials and found no consistent evidence that antihypertensive medication use had any effect on cancer risk (38). The ALLHAT study also reported the 6-year rate of cancer (other than non-melanoma skin cancer) at 9.7% for chlorthalidone, 10.0% for amlodipine, and 9.9% for lisinopril. Our study using ALLHAT–Medicare-linked data had similar findings on the 6-year cumulative incidence rate of cancer (other than non-melanoma skin cancer) at 9.5%, 9.8%, and 10.5%, respectively, for the above drugs from Medicare inpatient hospitalization data. In addition, our study examined that by adding cases from outpatient and physician claims data, the 6-year rate of cancer (other than non-melanoma skin cancer) was much higher than that of using inpatient hospitalization data alone at 38.5% for chlorthalidone, 38.5% for amlodipine, and 39.3% for lisinopril, although the adjusted hazard ratio of cancer was not statistically significantly different between each pair of the three drugs (Supplementary Table S4).
Several studies suggested an association between antihypertensive drugs and depression (15, 16, 34–36) and sexual dysfunction (13, 14, 39–43). This study also found that there were no statistically significant differences in the 6-year and 18-year risk of depression and erectile dysfunction between the three drugs. However, our study did find that the risk of angioedema was statistically significantly increased in those receiving lisinopril than in those receiving amlodipine or chlorthalidone and the risk of insomnia was statistically significantly lower in those receiving lisinopril than in those receiving amlodipine. Several previous studies reported that ACE inhibitors, such as lisinopril, were related to an increased risk of angioedema mainly due to inhibition of the angiotensin-converting enzyme and subsequent blockade of bradykinin degradation (25–29). The weighted incidence rate of angioedema polled from over 40 trials was 0.3%, which was remarkably identical to 0.3% for lisinopril in our study (Table 2). Our study also found that those receiving lisinopril were significantly more likely to develop angioedema than those receiving calcium channel blockers (amlodipine) or a thiazide-type diuretic (chlorthalidone). Although some studies showed that there was a significant association between hypertension and insomnia (30–33) and that insomnia was considered a side-effect of both lisinopril and amlodipine (30–33, 44, 45), little information was available to compare the risk of insomnia between these antihypertensive drugs. Our study that found a lower risk in those receiving lisinopril than those receiving amlodipine may stimulate more research on this comparison. If it is confirmed, it will be of clinical importance and implications to patients and providers. Furthermore, previous studies demonstrated that the incidence and prevalence of erectile dysfunction increase with age (46, 47). However, our study showed that the risk of insomnia and erectile dysfunction decreased with age. This finding may require some caution for its interpretation because patients may not volunteer this information or significantly under-report their private health conditions to the providers.
Our study has several limitations. First, we only studied the trial participants who were still alive in 1999 and enrolled in Medicare insurance program, due to which the trial randomization was no longer intact and any analyses done off-randomization may be subject to unmeasured or unknown confounders. Although the distribution of all baseline characteristics among the three study drug groups by 1999 was not significantly different, unmeasured or unknown confounders cannot be ruled out. Also, because there was no information on actual blood pressure measurements after the trial ended in 2002, it was unknown whether the study outcomes were affected by changes in blood pressure. Second, subjects who were free of outcomes at baseline were determined from Medicare inpatient data only in January 1994–December 1998, which might have missed some cases. The underestimates were particularly possible on the outcomes such as insomnia, depression, and erectile dysfunction, which mostly relied on self-reporting during the medical encounters or more objective measures. Therefore, caution should be needed in determining the incidence rates of these outcomes, especially insomnia and erectile dysfunction, for which the risk expectedly decreased with age, although it may not be different among the three study groups. Third, although the 6-year cumulative incidence rate of outcomes such as cancer from Medicare inpatient data was identical to that of an original ALLHAT report (1), the incidence of outcomes from a complete Medicare data set (inpatient, outpatient, and physician carrier data) in this study could not be compared with another source because of the lack of such analysis from original ALLHAT reports. On the other hand, this study demonstrated that the incidence of these outcomes could be underestimated or missed if only Medicare inpatient data were used to ascertain the outcomes, even though those outcomes might not vary significantly by the three study drugs. Fourth, this study only compared the risk of five outcomes among the three study drug groups, but it is unknown about the gap in the risk of these outcomes between antihypertensive drug users and nonusers or what was the attributable risk of these outcomes due to antihypertensive drug use. Fifth, the study excluded trial participants from Canada and Veteran Affairs (VA) because they were ineligible for Medicare or had incomplete Medicare claims; the findings may just be generalizable to Medicare beneficiaries from the United States.
In conclusion, the 6-year and 18-year risk of angioedema was significantly higher in patients receiving lisinopril than in those receiving amlodipine or chlorthalidone; the risk of insomnia was significantly lower in patients receiving lisinopril than in those receiving amlodipine; and the risk of cancer, depression, and erectile dysfunction (in men) was not statistically significantly different among the three drug groups. The risk of cancer and depression increased with advanced age, while the risk of insomnia and erectile dysfunction (in men) decreased with age; however, the risk of angioedema did not vary significantly with age. The findings on the unique and long-term incidence of cancer, angioedema, insomnia, depression, and erectile dysfunction associated with various antihypertensive drugs would be helpful to care providers and patients with hypertension in their choice and management of antihypertensive medications.
Data availability statement
The data sets presented in this article are not readily available because the ALLHAT data and Medicare claims data are not public-use data sets. However, researchers may request the ALLHAT data with the approval from the ALLHAT Coordinating Center in Houston and the Medicare claims data with the approval from the Center for Medicare and Medicaid Services (CMS). The authors plan to share the statistical models and statistical programs that they used to analyze these data upon request. Requests to access the data sets should be directed to the ALLHAT Coordinating Center in Houston, School of Public Health, The University of Texas Health Science Center at Houston, 1200 Pressler Street, Houston, TX 77030, USA.
Ethics statement
The studies involving humans were approved by the Committee for Protection of Human Subjects (CPHS) at the University of Texas Health Science Center in Houston (HSC-SPH-17-1035). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
XD: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. JM: Data curation, Formal Analysis, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. JY: Conceptualization, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft. LS: Data curation, Funding acquisition, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. BD: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The authors declare that financial support was received for the research, authorship, and/or publication of this article.
This study was supported by the grants (numbers R01AG058971 and R01AG067498) from the National Institute on Aging (NIA) of the National Institutes of Health (NIH), USA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH/NIA.
Acknowledgments
The authors thank other original ALLHAT investigators and trial participants for their contributions in making the rich data sets available to be linked with Medicare claims data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1272385/full#supplementary-material
References
1. The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA. (2002) 288:2981–97. doi: 10.1001/jama.288.23.2981
2. The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT-LLT). JAMA. (2002) 288:2998–3007. doi: 10.1001/jama.288.23.2998
3. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. National heart, lung, and blood institute joint national committee on prevention, detection, evaluation, and treatment of high blood pressure, national high blood pressure education program coordinating committee. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC-7 report. JAMA. (2003) 289: 2560–72. doi: 10.1001/jama.289.19.2560
4. Heidenreich PA, Davis BR, Cutler JA, Furberg CD, Lairson DR, Shlipak MG, et al. Cost-effectiveness of chlorthalidone, amlodipine, and lisinopril as first-step treatment for patients with hypertension: an analysis of the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). J Gen Intern Med. (2008) 23:509–16. doi: 10.1007/s11606-008-0515-2
5. Muntner P, Krousel-Wood M, Hyre AD, Stanley E, Cushman WC, Cutler JA, et al. Antihypertensive prescriptions for newly treated patients before and after the main antihypertensive and lipid-lowering treatment to prevent heart attack trial results and seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure guidelines. Hypertension. (2009) 53:617–23. doi: 10.1161/HYPERTENSIONAHA.108.120154
6. Stafford R, Batholomew LK, Cushman WC, Cutler JA, Davis BR, Dawson G, et al. Impact of the ALLHAT/JNC7 dissemination project on thiazide-type diuretic use. Arch Int Med. (2010) 170:851–8. doi: 10.1001/archinternmed.2010.130
7. Krousel-Wood M, Muntner P, Carson A, Anderson AH, Delaune E, Cushman WC, et al. Hypertension control among newly treated patients before and after publication of the main ALLHAT results and JNC 7 guidelines. J Clin Hypertens (Greenwich). (2012) 14:277–83. doi: 10.1111/j.1751-7176.2012.00609.x
8. Ovbiagele B, Goldstein LB, Higashida RT, Howard VJ, Johnston SC, Khavjou OA, et al. Forecasting the future of stroke in the United States: a policy statement from the American Heart Association and American Stroke Association. Stroke. (2013) 44:2361–75. doi: 10.1161/STR.0b013e31829734f2
9. Phillips W, Piller LB, Williamson JD, Whittle J, Jafri SZ, Ford CE, et al. Risk of hospitalized gastrointestinal bleeding in persons randomized to diuretic, ACE-inhibitor, or calcium-channel blocker in ALLHAT. J Clin Hypertens (Greenwich). (2013) 15(11):825–32. doi: 10.1111/jch.12180
10. Du XL, Simpson LM, Tandy BC, Bettencourt JL, Davis BR. Risk of hospitalized and non-hospitalized gastrointestinal bleeding in ALLHAT trial participants receiving diuretic, ACE-inhibitor, or calcium-channel blocker. PLoS One. (2021) 16(11):e0260107. doi: 10.1371/journal.pone.0260107
11. SPRINT Research Group, Wright JT, Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. (2015) 373(22):2103–16. doi: 10.1056/NEJMoa1511939
12. SPRINT Research Group, Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged ≥75 years: a randomized clinical trial. JAMA. (2016) 315(24):2673–82. doi: 10.1001/jama.2016.7050
13. Cordero A, Bertomeu-Martínez V, Mazón P, Fácila L, Bertomeu-González V, Conthe P, et al. Erectile dysfunction in high-risk hypertensive patients treated with beta-blockade agents. Cardiovasc Ther. (2010) 28(1):15–22. doi: 10.1111/j.1755-5922.2009.00123.x
14. Selvin E, Burnett AL, Platz EA. Prevalence and risk factors for erectile dysfunction in the US. Am J Med. (2007) 120(2):151–7. doi: 10.1016/j.amjmed.2006.06.010
15. Prisant LM, Spruill WJ, Fincham JE, Wade WE, Carr AA, Adams MA. Depression associated with antihypertensive drugs. J Fam Pract. (1991) 33(5):481–5.1682414
16. Ringoir L, Pedersen SS, Widdershoven JW, Pouwer F, Keyzer JM, Romeijnders AC, et al. Beta-blockers and depression in elderly hypertension patients in primary care. Fam Med. (2014) 46(6):447–53.24911300
17. Chang CH, Yang YH, Lin SJ, Su JJ, Cheng CL, Lin LJ. Risk of insomnia attributable to β-blockers in elderly patients with newly diagnosed hypertension. Drug Metab Pharmacokinet. (2013) 28(1):53–8. doi: 10.2133/dmpk.DMPK-12-RG-004
18. Chobufo MD, Gayam V, Soluny J, Rahman EU, Enoru S, Foryoung JB, et al. Prevalence and control rates of hypertension in the USA: 2017-2018. Int J Cardiol Hypertens. (2020) 6:100044. doi: 10.1016/j.ijchy.2020.100044
19. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. (2020) 16(4):223–37. doi: 10.1038/s41581-019-0244-2
20. NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. (2021) 398(10304):957–80. doi: 10.1016/S0140-6736(21)01330-1
21. Handler J. Quality of life and antihypertensive drug therapy. J Clin Hypertens (Greenwich). (2005) 7(5):274–85. doi: 10.1111/j.1524-6175.2005.04470.x
22. Moser M. Relative efficacy of, and some adverse reactions to, different antihypertensive regimens. Am J Cardiol. (1989) 63(4):2B–7B. doi: 10.1016/0002-9149(89)90931-4
23. Gebreyohannes EA, Bhagavathula AS, Abebe TB, Tefera YG, Abegaz TM. Adverse effects and non-adherence to antihypertensive medications in University of Gondar Comprehensive Specialized Hospital. Clin Hypertens. (2019) 25:1. doi: 10.1186/s40885-018-0104-6
24. Dimmitt SB, Stampfer HG, Martin JH, Ferner RE. Efficacy and toxicity of antihypertensive pharmacotherapy relative to effective dose 50. Br J Clin Pharmacol. (2019) 85(10):2218–27. doi: 10.1111/bcp.14033
25. Brown T, Gonzalez J, Monteleone C. Angiotensin-converting enzyme inhibitor-induced angioedema: a review of the literature. J Clin Hypertens (Greenwich). (2017) 19(12):1377–82. doi: 10.1111/jch.13097
26. Melendez M, Grosel JM. ACE inhibitor-induced angioedema causing small bowel obstruction. JAAPA. (2020) 33(8):28–31. doi: 10.1097/01.JAA.0000668864.61980.c0
27. Montinaro V, Cicardi M. ACE inhibitor-mediated angioedema. Int Immunopharmacol. (2020) 78:106081. doi: 10.1016/j.intimp.2019.106081
28. Gillion V, Dragean CA, Dahlqvist G, Jadoul M. Intestinal angioedema from angiotensin converting enzyme inhibitor. Kidney Int. (2019) 96(3):798. doi: 10.1016/j.kint.2019.02.031
29. Rasmussen ER, Pottegård A, Bygum A, von Buchwald C, Homøe P, Hallas J. Angiotensin II receptor blockers are safe in patients with prior angioedema related to angiotensin-converting enzyme inhibitors—a nationwide registry-based cohort study. J Intern Med. (2019) 285(5):553–61. doi: 10.1111/joim.12867
30. Prejbisz A, Kabat M, Januszewicz A, Szelenberger W, Piotrowska AJ, Piotrowski W, et al. Characterization of insomnia in patients with essential hypertension. Blood Press. (2006) 15(4):213–9. doi: 10.1080/08037050600963040
31. Calhoun DA, Harding SM. Sleep and hypertension. Chest. (2010) 138(2):434–43. doi: 10.1378/chest.09-2954
32. Hernández-Aceituno A, Guallar-Castillón P, García-Esquinas E, Rodríguez-Artalejo F, Banegas JR. Association between sleep characteristics and antihypertensive treatment in older adults. Geriatr Gerontol Int. (2019) 19(6):537–40. doi: 10.1111/ggi.13660
33. Jarrin DC, Alvaro PK, Bouchard MA, Jarrin SD, Drake CL, Morin CM. Insomnia and hypertension: a systematic review. Sleep Med Rev. (2018) 41:3–38. doi: 10.1016/j.smrv.2018.02.003
34. van Sloten TT, Souverein PC, Stehouwer CD, Driessen JH. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers and risk of depression among older people with hypertension. J Psychopharmacol. (2022) 36(5):594–603. doi: 10.1177/02698811221082470
35. Muldoon MF, Waldstein SR, Ryan CM, Jennings JR, Polefrone JM, Shapiro AP, et al. Effects of six anti-hypertensive medications on cognitive performance. J Hypertens. (2002) 20(8):1643–52. doi: 10.1097/00004872-200208000-00028
36. Louis WJ, Mander AG, Dawson M, O'Callaghan C, Conway EL. Use of computerized neuropsychological tests (CANTAB) to assess cognitive effects of antihypertensive drugs in the elderly. Cambridge neuropsychological test automated battery. J Hypertens. (1999) 17(12 Pt 2):1813–9. doi: 10.1097/00004872-199917121-00005
37. Bangalore S, Kumar S, Kjeldsen SE, Makani H, Grossman E, Wetterslev J, et al. Antihypertensive drugs and risk of cancer: network meta-analyses and trial sequential analyses of 324,168 participants from randomised trials. Lancet Oncol. (2011) 12(1):65–82. doi: 10.1016/S1470-2045(10)70260-6
38. Copland E, Canoy D, Nazarzadeh M, Bidel Z, Ramakrishnan R, Woodward M, et al. Antihypertensive treatment and risk of cancer: an individual participant data meta-analysis. Lancet Oncol. (2021) 22(4):558–70. doi: 10.1016/S1470-2045(21)00033-4
39. Spatz ES, Canavan ME, Desai MM, Krumholz HM, Lindau ST. Sexual activity and function among middle-aged and older men and women with hypertension. J Hypertens. (2013) 31(6):1096–105. doi: 10.1097/HJH.0b013e32835fdefa
40. Moulik PK, Hardy KJ. Hypertension, anti-hypertensive drug therapy and erectile dysfunction in diabetes. Diabet Med. (2003) 20(4):290–3. doi: 10.1046/j.1464-5491.2003.00911.x
41. Grimm RH Jr, Grandits GA, Prineas RJ, McDonald RH, Lewis CE, Flack JM, et al. Long-term effects on sexual function of five antihypertensive drugs and nutritional hygienic treatment in hypertensive men and women. Treatment of mild hypertension study (TOMHS). Hypertension. (1997) 29(1 Pt 1):8–14. doi: 10.1161/01.HYP.29.1.8
42. Ferrario CM, Levy P. Sexual dysfunction in patients with hypertension: implications for therapy. J Clin Hypertens (Greenwich). (2002) 4(6):424–32. doi: 10.1111/j.1524-6175.2002.00862.x
43. Akinyede AA, Nwaiwu O, Fasipe OJ, Olusanya A, Olayemi SO, Akande B. A prospective study of the effect of antihypertensive medications on the sexual functions of hypertensive adult male patients. Future Sci OA. (2020) 6(6):FSO479. doi: 10.2144/fsoa-2020-0030
44. Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. (1995) 18(6):425–32. doi: 10.1093/sleep/18.6.425
45. Spencer PZ. Gender, age, and the risk of insomnia. CNS Spectr. (2008) 13(12 Suppl 17):7–9. doi: 10.1017/s1092852900003357
46. Laumann EO, West S, Glasser D, Carson C, Rosen R, Kang JH. Prevalence and correlates of erectile dysfunction by race and ethnicity among men aged 40 or older in the United States: from the male attitudes regarding sexual health survey. J Sex Med. (2007) 4(1):57–65. doi: 10.1111/j.1743-6109.2006.00340.x
Keywords: cancer, angioedema, antihypertensive drugs, ALLHAT, Medicare claims data
Citation: Du XL, Martinez J, Yamal J-M, Simpson LM and Davis BR (2023) The 18-year risk of cancer, angioedema, insomnia, depression, and erectile dysfunction in association with antihypertensive drugs: post-trial analyses from ALLHAT–Medicare linked data. Front. Cardiovasc. Med. 10:1272385. doi: 10.3389/fcvm.2023.1272385
Received: 3 August 2023; Accepted: 31 October 2023;
Published: 17 November 2023.
Edited by:
Tlili Barhoumi, King Abdullah International Medical Research Center (KAIMRC), Saudi ArabiaReviewed by:
Dimitris Konstantinidis, Hippokration General Hospital, GreeceNicolas Renna, Universidad Nacional de Cuyo, Argentina
© 2023 Du, Martinez, Yamal, Simpson and Davis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianglin L. Du WGlhbmdsaW4uTC5EdUB1dGgudG1jLmVkdQ==
 Xianglin L. Du
Xianglin L. Du Journey Martinez2
Journey Martinez2 Jose-Miguel Yamal
Jose-Miguel Yamal