
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 18 December 2023
Sec. Cardiac Rhythmology
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1270422
 Eleonora Angelini1,†
Eleonora Angelini1,† Jan-Thorben Sieweke1,†
Jan-Thorben Sieweke1,† Dominik Berliner1
Dominik Berliner1 Saskia Biber1
Saskia Biber1 Stephan Hohmann1
Stephan Hohmann1 Maximiliane Oldhafer1
Maximiliane Oldhafer1 Sven Schallhorn1
Sven Schallhorn1 David Duncker1
David Duncker1 Christian Veltmann1,2
Christian Veltmann1,2 Johann Bauersachs1
Johann Bauersachs1 Udo Bavendiek1*
Udo Bavendiek1*
Background: The echocardiographic parameters total atrial conduction time (PA-TDI duration), left atrial (LA) volume index (LAVI), and LA strain reflect adverse atrial remodeling and predict atrial fibrillation (AF).
Objectives: The aim of this study was to investigate echocardiographic parameters indicating reverse LA remodeling and potential associations with AF recurrence after pulmonary vein isolation (PVI).
Methods: This prospective observational study consecutively enrolled patients scheduled for PVI for symptomatic AF. Electrocardiogram (ECG) test and transthoracic echocardiography were performed the day before and after PVI and again 3 months later. AF recurrence was determined by Holter ECG at 3 months, and telephone follow-up at 12 months, after PVI. The parameters of LA remodeling [PA-TDI, LAVI, and LA strain analysis: reservoir strain (LASr), conduit strain (LAScd), contraction strain (LASct)] were determined by transthoracic echocardiography.
Results: A total of 48 patients were included in the study (mean age: 61.4 ± 12.2 years). PA-TDI significantly decreased the day after PVI compared with the baseline (septal PA-TDI 103 ± 13 vs. 82 ± 14.9 ms, p ≤ 0.001; lateral PA-TDI 122.4 ± 14.8 vs. 106.9 ± 14.4 ms, p ≤ 0.001) and at the 3-month follow-up (septal PA-TDI: 77.8 ± 14.5, p ≤ 0.001; lateral PA-TDI 105.2 ± 16.1, p ≤ 0.001). LAVI showed a significant reduction at the 3-month follow-up compared with the baseline (47.7 ± 14.4 vs. 40.5 ± 9.7, p < 0.05). LASr, LAScd, and LASct did not change after PVI compared with the baseline. AF recurred in 10 patients after PVI (21%). Septal PA-TDI, septal a', and LAVI/a' determined the day after PVI were associated with AF recurrence.
Conclusion: Changes in echocardiographic parameters of LA remodeling and function indicate that functional electromechanical recovery preceded morphological reverse remodeling of the left atrium after PVI. Furthermore, these changes in echocardiographic parameters indicating LA reverse remodeling after PVI may identify patients at high risk of AF recurrence.
Catheter ablation has become a standard therapy for patients with symptomatic non-permanent atrial fibrillation (AF) (1). Catheter ablation of AF by pulmonary vein isolation (PVI) has rapidly developed in recent years after the first description of the role of pulmonary veins in triggering AF (2, 3). However, despite technical advances, successful maintenance of sinus rhythm (SR) in the first year after PVI is approximately 70% in paroxysmal AF and 50% in persistent AF (4–6).
AF causes electrical and structural left atrial (LA) remodeling, which contributes to the susceptibility to AF perpetuation and its progression (7, 8). Echocardiography is the most widely used modality to evaluate LA dimension and function before scheduling AF ablation. Echocardiographic parameters of LA remodeling comprise total atrial conduction time (PA-TDI interval), the ratio of LA volume indexed to tissue Doppler a' (LAVI/a'), and left atrial strain (9). PA-TDI indicates left atrial electromechanical coupling (10). PA-TDI and LAVI/a' have been shown to predict incident AF in different patient populations in SR (8–12). A reduced LA strain is associated with an increased risk of AF occurrence and recurrence of cerebral infarction (13).
The mechanisms explaining a reduction in LA dimensions after PVI have been described in basic science studies, showing reverse atrial remodeling following restoration of sinus rhythm as occurs following PVI (14). However, there are no data available about a potential change and time-course of the echocardiographic parameters PA-TDI, LAVI/a', and LA strain after PVI. Therefore, the present study investigated whether PVI affects echocardiographic parameters of LA remodeling and function indicating reverse LA remodeling after restoration of SR.
The present study is a prospective, longitudinal, single-center study, which was approved by the local ethics committee of the Hannover Medical School (# 7910_BO_S_2018). The study complies with the Declaration of Helsinki. All participants gave their written informed consent. We consecutively screened patients for eligibility, selecting those who had undergone PVI due to symptomatic AF in the Department of Cardiology and Angiology between June 2018 and July 2019. Of importance, patients were only included in this study (T0) if they presented in SR during the echocardiographic examination because parameters for LA remodeling cannot be determined during AF. The exclusion criteria were defined as follows: age less than 18 years, severe mitral valve stenosis or regurgitation, history of aortic or mitral valve replacement, AF during echocardiography, hyperthyroidism, previously diagnosed channelopathies, alcohol abuse, contraindications against PVI, active devices (left ventricular assist device, pacemaker, implantable cardioverter-defibrillator, cardiac resynchronization therapy), patients lost to follow-up, radio-frequency (RF) catheter ablation, and patients who could not provide informed consent. In particular, RF ablation was an exclusion criterion because all included patients underwent AF ablation for the first time, and to strengthen the primary endpoint in a homogeneous patient cohort. Inclusion of the participants and workflow are presented in Figure 1.
Standard transthoracic echocardiography was performed according to the American Society of Echocardiography guidelines at inclusion visit (T0) and in the follow-up (T1, T2) as described in Figure 1 (15). In addition, specific echocardiographic parameters reflecting LA function and remodeling were determined (7, 15). Left atrial volume was determined by biplane area length method in apical four- and two-chamber views at the ventricular end-systole from the apical approach in brief breathhold (15). Subsequently, left atrial volume was indexed (LAVI) to body surface area (BSA). Using tissue Doppler imaging, septal and lateral late diastolic peak tissue Doppler velocity (a') was determined. The average of the septal and lateral a' was used to assess LAVI/a'. In tissue Doppler imaging, the interval between the onset of P wave in lead II of the ECG and the peak A'-wave of the septal or lateral mitral valve (MV) annulus was defined as septal or lateral PA-TDI. Two-dimensional (2D) speckle tracking analyses of the left atrium were conducted offline with customized computer software (TOMTEC Autostrain for LA; TOMTEC Imaging Systems GmbH, Unterschleissheim, Germany) using zoom mode images of the LA in four- and two-chamber views to obtain LA reservoir, conduit, and contraction strains (LASr, LAScd, LASct). Investigators analyzing the echo images were not involved in PVI and were unaware of patients' ECG analyses. To evaluate the accuracy of the echocardiographic parameters and to exclude a change of the echocardiographic parameters over time, we compared echocardiographic examinations of the inclusion visit (T0) with echocardiographic examinations conducted before study inclusion in the outpatient clinic (T1) if available in a subpopulation of patients.
All participants received 12-lead ECG at inclusion and follow-up (T1, T2). In addition, 24 h Holter was performed at 3 months after PVI (T2). All ECG recordings were analyzed during clinical routine by blinded professionals applying current guidelines on AF (1).
Pre-procedure transesophageal echocardiography was performed to assess the left atrium, left atrium appendage, and pulmonary vein anatomy. Cryoballoon ablation was performed using a 28-mm cryoballoon (Artic Front Advance; Medtronic) using standard protocols to achieve persistent bidirectional conduction block in all pulmonary veins. During the procedure, esophageal temperature was monitored (CIRCA S-Cath, Circa Scientific Inc.). After circumferential antral vein isolation, a 30-min waiting period was conducted to assess spontaneous conduction recovery. Pericardial effusion was ruled out after PVI by echocardiography.
Follow-ups were performed after PVI during in-hospital stay (T1), in the outpatient clinic after 3 months (T2), and by telephone call 12 months (T3) after catheter ablation. Transthoracic echocardiography and 12-lead ECG were scheduled for T1 and T2. In addition, Holter ECG was performed at the follow-up 3 months after PVI (T2). The follow-up visit after 12 months (T3) was performed by telephone call. AF recurrence was defined by AF detection in Holter ECG at the 3-month follow-up and telephone follow-up 12 months after PVI (Figure 1).
The primary endpoint of the analysis was the change in the total atrial conduction time (PA-TDI interval) assessed by transthoracic echocardiography, indicating LA remodeling after PVI. Key secondary endpoints included change in the ratio of the indexed left atrial volume and mitral annulus velocity during atrial contraction a' (LAVI/a'), change in LA dimension, and changes in 2D speckle tracking parameters of the left atrium (LA strain), which also indicate LA remodeling.
Statistical analysis and graphical presentation were performed using SPSS Statistics 27 (IBM SPSS Statistics 27) and GraphPad Prism 7.04 (GraphPad Software, San Diego, CA, USA). Categorical variables are presented as n (%). Normally distributed continuous variables are given as mean ± standard deviation (SD), or median and interquartile ranges (IQR) for non-normally distributed variables. Normality and variance homogeneity were checked by the Shapiro–Wilk test. Comparison between the groups was performed using the Student's t-test for Gaussian distributed data and the Mann–Whitney U test for non-normally distributed data. ANOVA was performed followed by Bonferroni test or Dunn's test for multiple comparisons, respectively. The categorical variables were evaluated by the chi-square test. A two-sided p-value of <0.05 was considered statistically significant. In a sample size analysis before the start of the study, based on the publication by Fukushima et al. (16), a number of 44 patients was calculated under the condition of a power of 80% in order to achieve a two-sided significance level of 5% (p = 0.05). The discriminative ability of septal PA-TDI at T1 was assessed by the area under the receiver operating characteristic (ROC) curve. The prediction values were defined as the cut-off point having the highest Youden index (Yi = sensitivity + specificity − 1). The sensitivity, specificity, and accuracy for determining cut-offs were calculated.
Between June 2018 and July 2019, a total of 58 of the 110 eligible patients were included in the study after applying the inclusion and exclusion criteria. Of these patients, 48 had a transthoracic echocardiography and an ECG taken one day after PVI. At the second visit three months after PVI, all 48 patients participated. Furthermore, all 48 patients were available at a telephone visit after 12 months. The study flow is presented in Figure 1.
Baseline characteristics of the study patients are summarized in Table 1. The patients with symptomatic paroxysmal AF (EHRA 2a-3) had a mean age of 61.4 ± 12.2 years, and 66% were male. Hypertension was apparent in 33 patients (69%). All study participants underwent first-time PVI using a cryoballoon. Primary successful isolation of all pulmonary veins was performed in all patients. No complications occurred. The medical treatment is presented in Supplementary Table S3.
The parameters of echocardiography are summarized in Table 2. Patients had a normal left ventricular ejection fraction with a mean of 60.9 ± 4.9%. After PVI, septal PA-TDI and lateral PA-TDI were significantly reduced in the follow-up visits T1 and T2 compared with T0 (Figures 2A,B). By contrast, LAVI did not change at in-hospital visit T1, although it was significantly reduced compared with the baseline at follow-up visit T2 at 3 months after PVI (Figure 2C). The LA strain parameter did not significantly change over the course of the entire follow-up period after PVI (Figures 2D–F). Of note, the internal control group showed that echocardiographic parameters of LA remodeling and function were not significantly altered over time without PVI intervention (T0 vs. T-1) as presented in Supplementary Table S1.
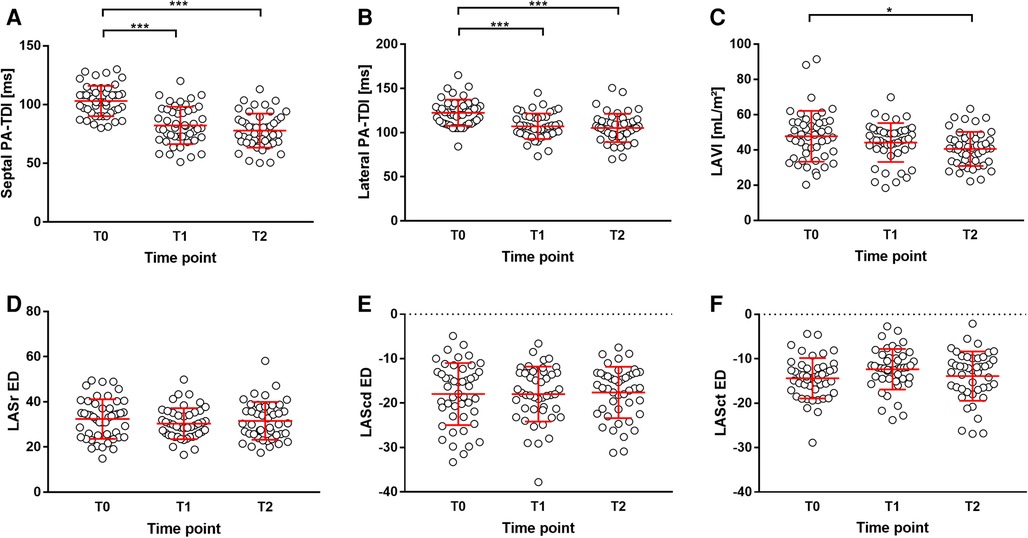
Figure 2. Parameters of LA remodeling during study. (A) septal PA-TDI, (B) lateral PA-TDI, (C) LAVI, (D) LASr ED, (E) LAScd ED, and (F) LASct ED. *p < 0.05; ***p < 0.001. LAScd ED, left atrial conduit strain at end-diastole; LASct ED, left atrial contractile strain at end-diastole; LASr ED, left atrial reservoir strain at end-diastole.
Overall, 10 patients had a recurrence of AF after PVI (21%) within the 12-month follow-up. After 3 months (T2), nine patients showed AF in a 12-lead ECG or in a Holter ECG. At the telephone follow-up visit (T3) 12 months after ablation, one patient reported symptomatic documented recurrence of AF.
There were no differences in echocardiographic baseline characteristics between patients with and without recurrence of AF (Table 2). Septal PA-TDI, LAVI/a', and A' septal—echocardiographic parameters of LA remodeling, dimension, and function, respectively—differed significantly in patients with AF recurrence after PVI (T1) (Figure 3). The ROC analysis and subsequent analysis of discriminators identified a septal PA-TDI at T1 of 87 ms as optimal cut-off for the prediction of AF recurrence with a specificity of 74% and sensitivity of 80% (Supplementary Figure S1).
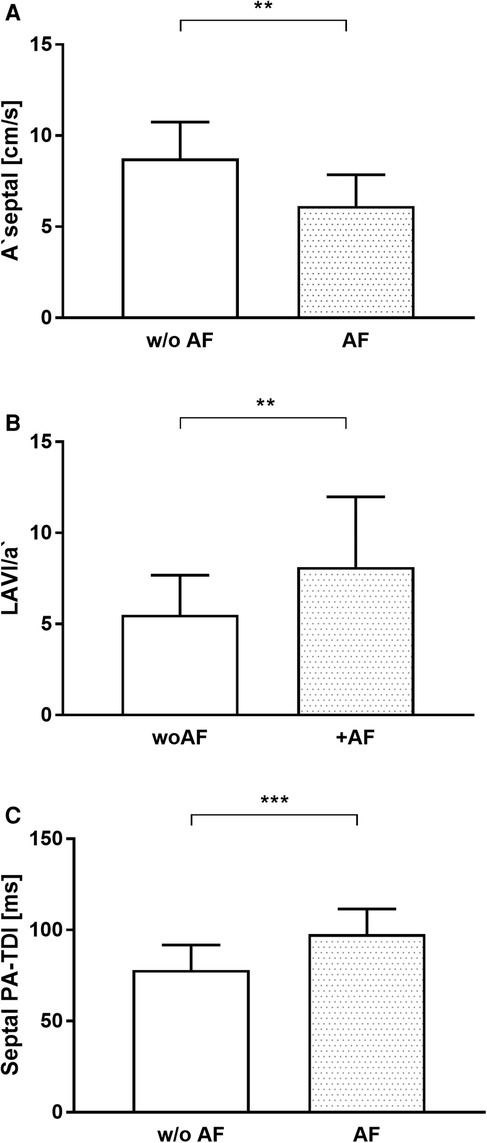
Figure 3. Echocardiographic parameters of LA remodeling and function associated with the recurrence of AF. (A) A ‘septal, (B) LAVI/a’, (C) Septal PA-TDI. AF, atrial fibrillation; w/o AF, without atrial fibrillation. **p < 0.01; ***p < 0.001.
In the present study, for the first time the change of echocardiographic parameters indicating reverse LA remodeling after PVI over time was described. Immediately after PVI, there was a change in electromechanical coupling as indicated by PA-TDI. Delayed reverse remodeling led to a significant reduction in LA dimensions illustrated by LAVI. This study indicated that functional electromechanical recovery precedes morphological reverse remodeling of the LA after PVI. However, there was no statistically significant improvement in LA function assessed by LAVI/a' and LA strain during the 3 months follow-up period.
LA remodeling is crucial for the development and persistence of AF and primarily consists of electrical, contractile and structural remodeling processes (17). Furthermore, to increase the success rate of PVI and to enable enhanced patient selection, it is important to understand LA remodeling in AF more precisely and thereby achieve optimal treatment planning and risk stratification. Transthoracic echocardiography, a widely available non-invasive and accurate imaging approach, allows determination of parameters associated with structural processes in LA remodeling. Structural remodeling is associated with dilatation and progressive fibrosis of the left atrium, and the electrical remodeling process is manifested by a shortening of the action potential and atrial refractory period (18).
Of note, in the present study septal PA-TDI after PVI was longer in the AF recurrence group than in the group without AF recurrence. The significant reduction in PA-TDI duration occurred immediately after successful PVI and persisted 3 months later indicating early functional electromechanical recovery. den Uijl et al. (19) described that PA-TDI duration before PVI is an independent predictor of AF recurrence after PVI. Due to the lower sample size calculated for the primary hypothesis in the present study compared with den Uijl et al., the number of patients may not be sufficient to calculate predictions for AF recurrence. However, the septal PA-TDI, collected after PVI at time T1, showed a high degree of discriminatory ability between patients with recurrence of AF and patients without recurrence [area under the curve (AUC): 0.853]. The ROC analysis followed by determination of Youden’s index calculated a separation value of 87 ms (sensitivity 80%, specificity 74%) for septal PA-TDI after PVI (T1) associated with recurrence of AF (Supplementary Figure S1). Although our current study pursued a different primary objective, the power analysis based on our results generate the hypothesis that septal PA-TDI after PVI could be used as a parameter for risk stratification of AF recurrence.
LA enlargement is known to be characteristic in patients with AF as well as a predictor for AF occurrence (20, 21). Current guidelines recommend the measurement of LA volume indexed to BSA when assessing the LA size, since indexing accounts for the gender differences in LA size and hence provides comparable values (13). The ratio of LAVI to LAVI/a' has been demonstrated to allow patients with arterial hypertension and AF to be distinguished from those without AF more precisely than LA size alone (8). In addition, LAVI/a’ was demonstrated to be an independent predictor of AF in patients with cryptogenic cerebral infarction (9). In our analysis, LAVI was significantly reduced 3 months after PVI. Furthermore, LAVI/a' was significantly greater in patients with AF recurrence after PVI compared with patients without AF recurrence. LA size, assessed by LAV and/or LAVI, is an independent predictor of maintenance of SR after catheter ablation of AF (17, 21). In our analysis, PVI was followed by a decrease in LA size, which correlated with persistence of SR.
LA strain correlates with the progress of LA fibrosis and hence impaired function in AF (7, 22). Alterations in strain rate analysis are predictive for successful ablation of AF during follow-up (23). This evidence is supported by Tops et al. describing a significant increase in LA strain, measured by TDI suggesting reverse remodeling 12 months after PVI (24). In our speckle tracking analysis, LA strain parameters did not change significantly after PVI within the 3-month follow-up presumably because reversal of LA fibrosis reflected by improvement of LA strain parameters takes longer as indicated by Tops et al. (24).
In our study, the PVI success rate of 79% is within the range known from the literature (62%–86% during a follow-up of 6–12 months) (25). PVI was considered successful if patients’ symptoms improved and a Holter ECG and 12-lead ECG showed SR 3 months after PVI. In the present study, echocardiography did not demonstrate maladaptive consequences of PVI in patients who had AF recurrence. Our results corroborate the work of Verma et al. (26), who also excluded any impairment of LA function after PVI.
Previous studies (27, 28) reported that LA remodeling is partially reversible: in the context of electrophysiological studies during PVI, the electrical changes might completely regress after termination of atrial tachycardia, whereas structural remodeling reverts slower or not at all depending on AF burden. Our findings support this assumption and consolidate the value of echocardiographic assessment of LA remodeling, especially with regard to septal PA-TDI. In addition, parameters used to assess LA remodeling and LA function, particularly septal PA-TDI and LAVI/a', showed little variance over time. This finding reinforces the importance of using these parameters to optimize the risk stratification of patients with AF.
The following aspects may limit our findings: (1) The sample size was tailored for the primary endpoint (change in the total atrial conduction time) and required a low number of patients enrolled. The analysis was not aimed to accurately investigate the change of other echocardiographic parameters, which also reflect adverse atrial remodeling. (2) We adjudicated PVI to be successful if patients’ symptoms improved and a Holter ECG and 12-lead ECG showed SR 3 months after PVI. However, we did not assess AF burden after ablation. (3) After pulmonary vein isolation, there was significantly more frequent use of non-vitamin K antagonists, especially apixaban (Supplementary Table S3). In animal models, a pleiotropic effect of another anti-Xa antagonist, edoxaban, was recognized with less progression of fibrotic progression of the atrium during tachypacing (29). However, it is puzzling whether this is a class effect that also occurs in humans and influences reverse remodeling. (4) In the follow-up at the time point of echocardiographic examination, an electrophysiological study to assess atrial reconduction would have been most accurate. However, like other authors, we are convinced that assessment of recurrent atrial fibrillation by long-term ECG is sufficient for this question. This is especially justifiable under consideration of ethics and patient safety. (5) Furthermore, the underlying mechanisms causal for the reduction of PA-TDI duration immediately after PVI remains open. Future studies should address this issue.
To increase the success rate of PVI and to enable enhanced patient selection, it is important to understand LA remodeling in AF more precisely and thereby achieve optimal treatment planning and risk stratification. Transthoracic echocardiography, a widely available, non-invasive, and accurate imaging approach, allows the determination of parameters associated with structural processes in LA remodeling. Based on our data, future studies should investigate potential associations of echocardiographic parameters indicating LA reverse remodeling with AF recurrence after PVI.
Changes of echocardiographic parameters after PVI indicate that functional electromechanical recovery precedes morphological reverse remodeling of the left atrium. Our data provide the basis for further studies that investigate potential associations of echocardiographic parameters indicating LA reverse remodeling with AF recurrence after PVI to optimize the selection of PVI candidates using echocardiographic parameters and to identify patients at high risk of AF recurrence after PVI.
This manuscript is part of the doctoral thesis of Eleonora Angelini supervised by Jan-Thorben Sieweke.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by local ethics committee of the Hannover Medical School (# 7910_BO_S_2018). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
EA: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Writing – original draft, Writing – review & editing. JS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. DB: Formal analysis, Writing – review & editing. SB: Data curation, Writing – review & editing. SH: Writing – review & editing. MO: Data curation, Writing – review & editing. SS: Data curation, Writing – review & editing. DD: Writing – review & editing. CV: Conceptualization, Data curation, Writing – review & editing. JB: Data curation, Supervision, Writing – review & editing. UB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
We thank Julia Afanasjew for her technical assistance and Lukas Aguirre Dávila for the sample size calculation. We also thank the staff of the echocardiography and EP lab for supporting this analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1270422/full#supplementary-material
AF, atrial fibrillation; BSA, body surface area; ECG, electrocardiogram; EHRA, European Heart Rhythm Association; LA, left atrium; LAScd, left atrial conduit strain; LASct, left atrial contractile strain; LASr, left atrial reservoir strain; LAVI, left atrial volume indexed; LAVI/a', ratio of LA volume index to tissue Doppler a'; MV, mitral valve; PA-TDI, total atrial conduction time; PVI, pulmonary vein isolation; TDI, tissue Doppler imaging.
1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. (2021) 42(5):373–498. doi: 10.1093/eurheartj/ehaa612
2. Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. (1998) 339(10):659–66. doi: 10.1056/NEJM199809033391003
3. Chen S, Pürerfellner H, Ouyang F, Kiuchi MG, Meyer C, Martinek M, et al. Catheter ablation vs. antiarrhythmic drugs as ‘first-line’ initial therapy for atrial fibrillation: a pooled analysis of randomized data. Europace. (2021) 23(12):1950–60. doi: 10.1093/europace/euab185
4. Ganesan AN, Shipp NJ, Brooks AG, Kuklik P, Lau DH, Lim HS, et al. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc. (2013) 2(2):e004549. doi: 10.1161/JAHA.112.004549
5. Wazni OM, Dandamudi G, Sood N, Hoyt R, Tyler J, Durrani S, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. (2021) 384(4):316–24. doi: 10.1056/NEJMoa2029554
6. Andrade JG, Wells GA, Deyell MW, Bennett M, Essebag V, Champagne J, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. (2021) 384(4):305–15. doi: 10.1056/NEJMoa2029980
7. Donal E, Lip GY, Galderisi M, Goette A, Shah D, Marwan M, et al. EACVI/EHRA expert consensus document on the role of multi-modality imaging for the evaluation of patients with atrial fibrillation. Eur Heart J Cardiovasc Imaging. (2016) 17(4):355–83. doi: 10.1093/ehjci/jev354
8. Toh N, Kanzaki H, Nakatani S, Ohara T, Kim J, Kusano KF, et al. Left atrial volume combined with atrial pump function identifies hypertensive patients with a history of paroxysmal atrial fibrillation. Hypertension. (2010) 55(5):1150–6. doi: 10.1161/HYPERTENSIONAHA.109.137760
9. Sieweke JT, Biber S, Weissenborn K, Heuschmann PU, Akin M, Zauner F, et al. Septal total atrial conduction time for prediction of atrial fibrillation in embolic stroke of unknown source: a pilot study. Clin Res Cardiol. (2019) 109(2):205–14. doi: 10.1007/s00392-019-01501-2
10. Müller P, Hars C, Schiedat F, Bösche LI, Gotzmann M, Strauch J, et al. Correlation between total atrial conduction time estimated via tissue Doppler imaging (PA-TDI interval), structural atrial remodeling and new-onset of atrial fibrillation after cardiac surgery. J Cardiovasc Electrophysiol. (2013) 24(6):626–31. doi: 10.1111/jce.12084
11. Stahrenberg R, Edelmann F, Haase B, Lahno R, Seegers J, Weber-Krüger M, et al. Transthoracic echocardiography to rule out paroxysmal atrial fibrillation as a cause of stroke or transient ischemic attack. Stroke. (2011) 42(12):3643–5. doi: 10.1161/STROKEAHA.111.632836
12. Tjahjadi C, Hiemstra YL, van der Bijl P, Pio SM, Bootsma M, Ajmone Marsan N, et al. Assessment of left atrial electro-mechanical delay to predict atrial fibrillation in hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging. (2021) 22(5):589–96. doi: 10.1093/ehjci/jeaa174
13. Leong DP, Joyce E, Debonnaire P, Katsanos S, Holman ER, Schalij MJ, et al. Left atrial dysfunction in the pathogenesis of cryptogenic stroke: novel insights from speckle-tracking echocardiography. J Am Soc Echocardiogr. (2017) 30(1):71–9.e1. doi: 10.1016/j.echo.2016.09.013
14. Ausma J, van der Velden HM, Lenders MH, van Ankeren EP, Jongsma HJ, Ramaekers FC, et al. Reverse structural and gap-junctional remodeling after prolonged atrial fibrillation in the goat. Circulation. (2003) 107(15):2051–8. doi: 10.1161/01
15. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. (2015) 28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003
16. Fukushima K, Fukushima N, Ejima K, Kato K, Sato Y, Uematsu S, et al. Left atrial appendage flow velocity and time from P-wave onset to tissue Doppler-derived A’ predict atrial fibrillation recurrence after radiofrequency catheter ablation. Echocardiography. (2015) 32(7):1101–8. doi: 10.1111/echo.12823
17. Njoku A, Kannabhiran M, Arora R, Reddy P, Gopinathannair R, Lakkireddy D, et al. Left atrial volume predicts atrial fibrillation recurrence after radiofrequency ablation: a meta-analysis. Europace. (2018) 20(1):33–42. doi: 10.1093/europace/eux013
18. Nattel S, Harada M. Atrial remodeling and atrial fibrillation: recent advances and translational perspectives. J Am Coll Cardiol. (2014) 63(22):2335–45. doi: 10.1016/j.jacc.2014.02.555
19. den Uijl DW, Gawrysiak M, Tops LF, Trines SA, Zeppenfeld K, Schalij MJ, et al. Prognostic value of total atrial conduction time estimated with tissue Doppler imaging to predict the recurrence of atrial fibrillation after radiofrequency catheter ablation. Europace. (2011) 13(11):1533–40. doi: 10.1093/europace/eur186
20. Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, et al. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. (2006) 47(12):2357–63. doi: 10.1016/j.jacc.2006.02.048
21. Albano AJ, Bush J, Parker JL, Corner K, Lim HW, Brunner MP, et al. Left atrial volume index predicts arrhythmia-free survival in patients with persistent atrial fibrillation undergoing cryoballoon ablation. J Atr Fibrillation. (2019) 12(2):2192. doi: 10.4022/jafib.2192
22. Kuppahally SS, Akoum N, Burgon NS, Badger TJ, Kholmovski EG, Vijayakumar S, et al. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging. (2010) 3(3):231–9. doi: 10.1161/CIRCIMAGING.109.865683
23. Schneider C, Malisius R, Krause K, Lampe F, Bahlmann E, Boczor S, et al. Strain rate imaging for functional quantification of the left atrium: atrial deformation predicts the maintenance of sinus rhythm after catheter ablation of atrial fibrillation. Eur Heart J. (2008) 29(11):1397–409. doi: 10.1093/eurheartj/ehn168
24. Tops LF, Delgado V, Bertini M, Marsan NA, Den Uijl DW, Trines SA, et al. Left atrial strain predicts reverse remodeling after catheter ablation for atrial fibrillation. J Am Coll Cardiol. (2011) 57(3):324–31. doi: 10.1016/j.jacc.2010.05.063
25. Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. (2006) 27(8):949–53. doi: 10.1093/eurheartj/ehi825
26. Verma A, Kilicaslan F, Adams JR, Hao S, Beheiry S, Minor S, et al. Extensive ablation during pulmonary vein antrum isolation has no adverse impact on left atrial function: an echocardiography and cine computed tomography analysis. J Cardiovasc Electrophysiol. (2006) 17(7):741–6. doi: 10.1111/j.1540-8167.2006.00488.x
27. Manning WJ, Silverman DI, Katz SE, Riley MF, Come PC, Doherty RM, et al. Impaired left atrial mechanical function after cardioversion: relation to the duration of atrial fibrillation. J Am Coll Cardiol. (1994) 23(7):1535–40. doi: 10.1016/0735-1097(94)90652-1
28. Pappone C, Oreto G, Rosanio S, Vicedomini G, Tocchi M, Gugliotta F, et al. Atrial electroanatomic remodeling after circumferential radiofrequency pulmonary vein ablation: efficacy of an anatomic approach in a large cohort of patients with atrial fibrillation. Circulation. (2001) 104(21):2539–44. doi: 10.1161/hc4601.098517
Keywords: atrial fibrillation, 2D speckle tracking, atrial remodeling, atrial conduction time, pulmonary vein isolation, catheter ablation
Citation: Angelini E, Sieweke J-T, Berliner D, Biber S, Hohmann S, Oldhafer M, Schallhorn S, Duncker D, Veltmann C, Bauersachs J and Bavendiek U (2023) Echocardiographic parameters indicating left atrial reverse remodeling after catheter ablation for atrial fibrillation. Front. Cardiovasc. Med. 10:1270422. doi: 10.3389/fcvm.2023.1270422
Received: 20 August 2023; Accepted: 29 November 2023;
Published: 18 December 2023.
Edited by:
Young Keun On, Sungkyunkwan University, Republic of KoreaReviewed by:
Antoine Da Costa, Université Jean Monnet, France© 2023 Angelini, Sieweke, Berliner, Biber, Hohmann, Oldhafer, Schallhorn, Duncker, Veltmann, Bauersachs and Bavendiek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Udo Bavendiek YmF2ZW5kaWVrLnVkb0BtaC1oYW5ub3Zlci5kZQ==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.