- 1Department of Endocrinology, The Forth Hospital of Hebei Medical University, Shijiazhuang, China
- 2Department of War and Rescue Medicine Field Internal Medicine Teaching and Research Office, NCO School, Army Medical University, Shijiazhuang, China
- 3Department of Orthopedics, The Affiliated Hospital, NCO School of Army Medical University, Shijiazhuang, China
- 4Department of Endocrinology, The Forth Hospital of Hebei Medical University, Shijiazhuang, China
- 5Department of Orthopedics, The Forth Hospital of Hebei Medical University, Shijiazhuang, China
- 6Second Department of General Surgery, The Forth Hospital of Hebei Medical University, Shijiazhuang, China
- 7Department of Cardiology, Bethune International Peaceful Hospital, Shijiazhuang, China
- 8Department of Gastroenterology, Baoding First Central Hospital, Baoding, China
- 9Department of Endocrinology, The Second Hospital of Hebei Medical University, Shijiazhuang, China
Background: Short sleep duration and poor sleep quality are important risk factors for atherosclerosis. The use of smart bracelets that measure sleep parameters, such as sleep stage, can help determine the effect of sleep quality on lower-extremity atherosclerosis in patients with type 2 diabetes.
Objective: To investigate the correlation between sleep disorders and lower-extremity atherosclerosis in patients with type 2 diabetes.
Methods: After admission, all patients were treated with lower-extremity arterial ultrasound and graded as having diabetic lower-extremity vascular lesions according to the results. A smart bracelet was used to obtain the patient sleep data. The correlation between sleep patterns and diabetic lower-extremity atherosclerosis, diabetic foot, and various metabolic indices was verified.
Results: Between August 2021 and April 2022, we screened 100 patients with type 2 diabetes, with 80 completing sleep monitoring. Univariate ordered logistic regression analysis indicated that patients with a sleep score below 76 (OR = 2.707, 95%CI: 1.127–6.488), shallow sleep duration of 5.3 h or more (OR=3.040, 95 CI: 1.005–9.202), wakefulness at night of 2.6 times or more (OR = 4.112, 95%CI: 1.513–11.174), and a deep sleep continuity score below 70 (OR = 4.141, 95%CI: 2.460–615.674) had greater risk of high-grade lower limb atherosclerosis. Multivariate ordinal logistic regression analysis revealed that the risk of high-grade lower limb atherosclerosis was higher in patients with 2.6 or more instances of nighttime wakefulness (OR = 3.975, 95%CI: 1.297–12.182) compared with those with fewer occurrences. The sleep duration curve of patients with different grades of diabetic lower-extremity atherosclerosis was U-shaped. According to the results of the one-way analysis of variance, the higher the deep sleep continuity score, the lower the Wagner scale score for diabetic foot (P < 0.05).
Conclusions: Sleep disorders (long, shallow sleep duration, frequent wakefulness at night, and poor continuity of deep sleep) can worsen lower limb atherosclerosis in patients with type 2 diabetes. This finding can provide a new method for medical professionals to prevent and treat diabetic lower-extremity vascular lesions.
Introduction
Lower extremity atherosclerosis is a common complication of diabetes that can progress to ischemic foot lesions and endanger the lives of patients. The prevalence of foot ulcers ranges from 4%–10%, with an annual population-based incidence of 1.0%–4.1%, and a lifetime incidence as high as 25%. Diabetic ischemic foot disease is the most common cause of nontraumatic amputation (1). Diabetic foot is characterized by treatment difficulties, high disability and fatality rates, and high treatment costs. Collectively, these factors result in physical and mental distress for patients, contributing to a huge social and economic burden. An early understanding of the risk factors for diabetic lower-extremity atherosclerosis is important for the prevention and treatment of ischemic disease in diabetic foot.
Recently, sleep disorders have received increasing attention in the prevention and treatment of vascular diseases. Sleep deprivation has been shown to increase the risk of atherosclerosis (2, 3). Non-apnea sleep disorders are also risk factors for peripheral artery disease. Sleep fragmentation increases oxidative stress, which leads to vascular dysfunction and atherosclerosis (4). Therefore, sleep disorders are irrefutably associated with atherosclerosis. Combined with our clinical observations, we found that the proportion of patients with both sleep disorders and diabetic lower-extremity vascular lesions increased significantly. However, there are few relevant studies, and there is a lack of evidence. Although sleep is known to have an impact on the body, research on this subject has been difficult.
The traditional polysomnographic sleep monitor has limitations in monitoring patients’ long-term sleep status, and the sleep score is a patient recall index that is subject to various interference factors and lacks reliability. To address these issues, we used smart bracelet monitoring to collect sleep data and explore the correlation between sleep patterns and diabetic lower-extremity atherosclerosis. With the advancement of science and technology, the monitoring of daily life signs is easier to be monitored. Smart bracelets are small and convenient, and are gradually recognized by the public. It can record data and save in real time, which significantly improves the patient's compliance. Therefore, as a record requipment, the bracelet is one of the excellent choices.
Methods
Study design and patients
A smart bracelet was used to monitor sleep indicators and analyze the correlations between sleep index, diabetic lower-extremity atherosclerosis, and diabetic foot Wagner grade. Patients aged 18 years or older with type 2 diabetes who were admitted to the Department of Endocrinology, Fourth Hospital of Hebei Medical University, between August 2021 and April 2022 were invited to participate. All enrolled patients signed an informed consent form, and the study was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University (ethics batch number 2022KS016). The inclusion criteria were as follows: (1) Aged 18 years or older; (2) Compliance with the 2021 version of the Chinese diabetes prevention and treatment guidelines. All enrolled patients were required to meet the following diagnostic criteria: presence of typical diabetes symptoms (excessive thirst, polyuria, overeating, unexplained weight loss) + fasting blood glucose ≥ 7.0 mmol/L, or 2-h post-prandial blood glucose ≥ 11.1 mmol/L, or random blood glucose ≥ 11.1 mmol/L. The following categories of patients were excluded: (1) alcoholic patients; (2) pregnant and lactating women; (3) patients with acute exacerbation of chronic obstructive pulmonary disease; (4) patients with sleep apnea; (5) patients suffering from severe organ failure such as heart failure; (6) patients with mental impairments; (7) patients with painful conditions such as diabetic painful neuropathy; (8) patients with severe hypertension (BP > 180/110 mmHg); (9) patients who had recently taken sleep aids; (10) patients with pain score greater than or equal to 1. The exclusion criteria were used to minimize variations in sleep duration and quality due to other medical conditions.
Procedures
All patients underwent measurements of height, weight, abdominal circumference, hip circumference, blood pressure, and other general indicators. Fasting blood glucose, blood lipid, homocysteine, glycosylated hemoglobin, insulin, and other tests were conducted simultaneously. During hospitalization, lower-extremity arterial ultrasonography or arteriography and ankle-brachial index (ABI) of the lower extremities were performed. Based on the ultrasound results, diabetic lower limb atherosclerosis was divided into four grades. Normal blood vessels without atherosclerotic manifestations were graded as 1. Thickened intima-media thickness without obvious plaque formation was graded as 2. The formation of arterial plaque without significant stenosis (narrowing rate of the artery diameter <20%) was graded as 3. Arterial diameter stenosis ≥20% was graded as 4. The final grade was based on the most severe side. All patients underwent Wagner grading for diabetic foot disease, graded on a six-point scale. The presence of risk factors for foot ulcers without ulcers was graded as 0. A superficial ulcer with no co-infection was graded as 1. A deep ulcer, with the infection limited to the soft tissue, and no bone infection or deep abscess was graded as 2. A deeper infection, which could lead to infection of the bone and deep abscesses, was graded as 3. Grade 4 is localized gangrene, and Grade 5 is gangrene of the entire foot. Professional physicians administered the Pittsburgh Sleep Score (PSQI), Hospital Anxiety and Depression Scale (HADS), and Activity of Daily Living score (ADL) questionnaire.
Smart bracelet monitoring
Twenty-four hours after hospitalization, a qualified physician placed a bracelet 2 cm above the horizontal line on the patient's left wrist, ensuring it remained close to the patient's skin for over 72 h. The bracelet was inspected by a specialist once a day to ensure the patient wore it correctly and that the battery capacity of the bracelet was sufficient. The bracelet automatically collected data for at least 72 h and generated data analysis charts. The sleep monitoring report recorded the sleep onset, end time, wake time, proportion of deep sleep, proportion of shallow sleep, proportion of rapid eye movement, continuity of deep sleep, wakefulness duration, and breathing quality.
Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics for Windows, version 26.0. Statistical significance was set at P < 0.05. First, Spearman correlation analysis was used to screen the risk factors for diabetic lower-extremity vascular lesions and diabetic foot. Subsequently, the correlation between sleep indices, diabetic lower extremity vascular lesions, and the Wagner scale for diabetic foot was further analyzed using one-way analysis of variance, and ordered logistic regression analysis was used to further clarify the causal relationship between sleep indicators, diabetic lower-extremity atherosclerosis, and the Wagner scale for diabetic foot.
Results
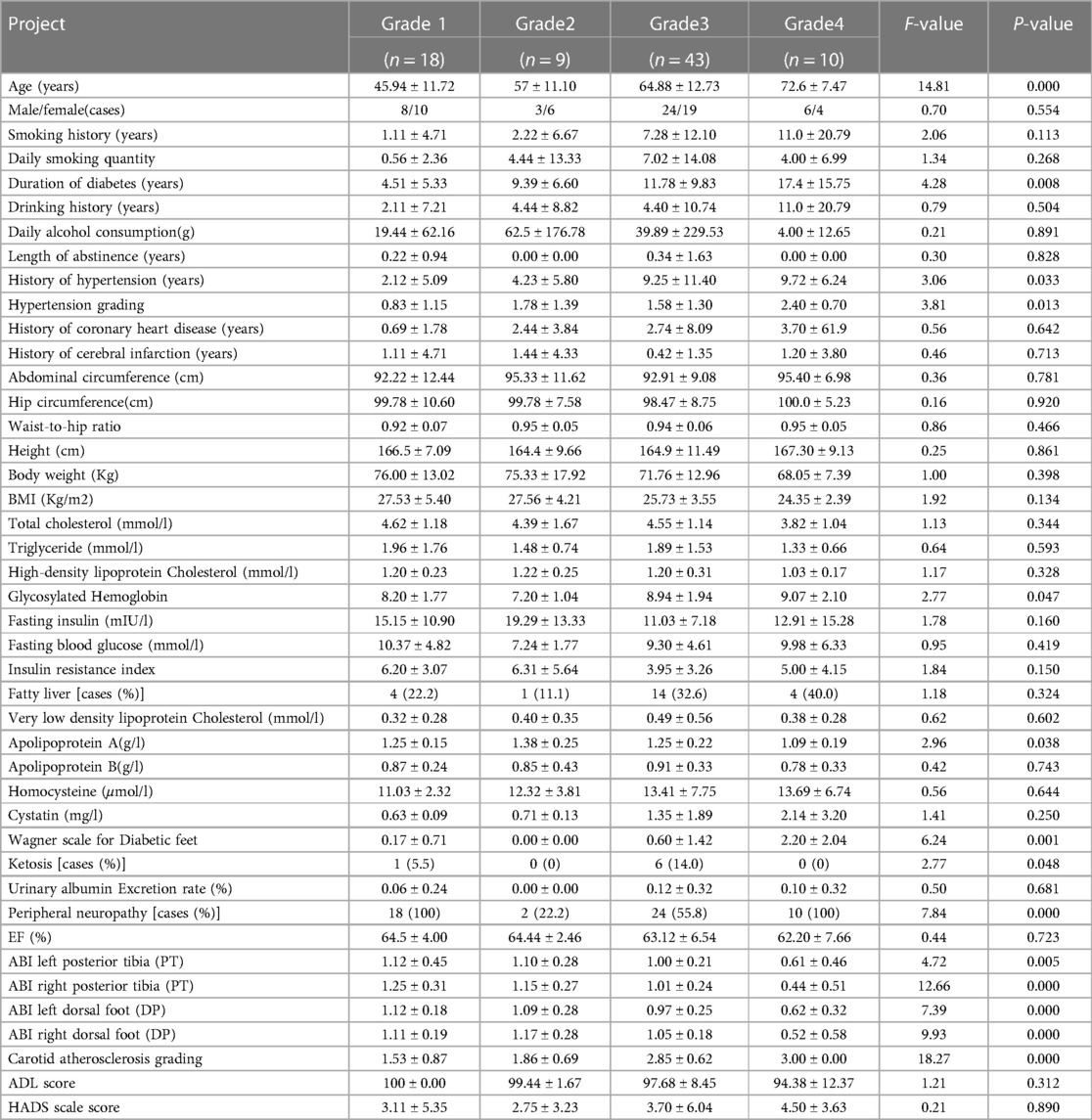
One hundred patients with type 2 diabetes were enrolled after screening, of whom 80 patients had complete sleep data, including 41 men and 39 women. The experimental procedure is illustrated in (Figure 1). The general characteristics of patients in the different diabetic lower-extremity arteriosclerosis grade groups are shown in (Table 1).
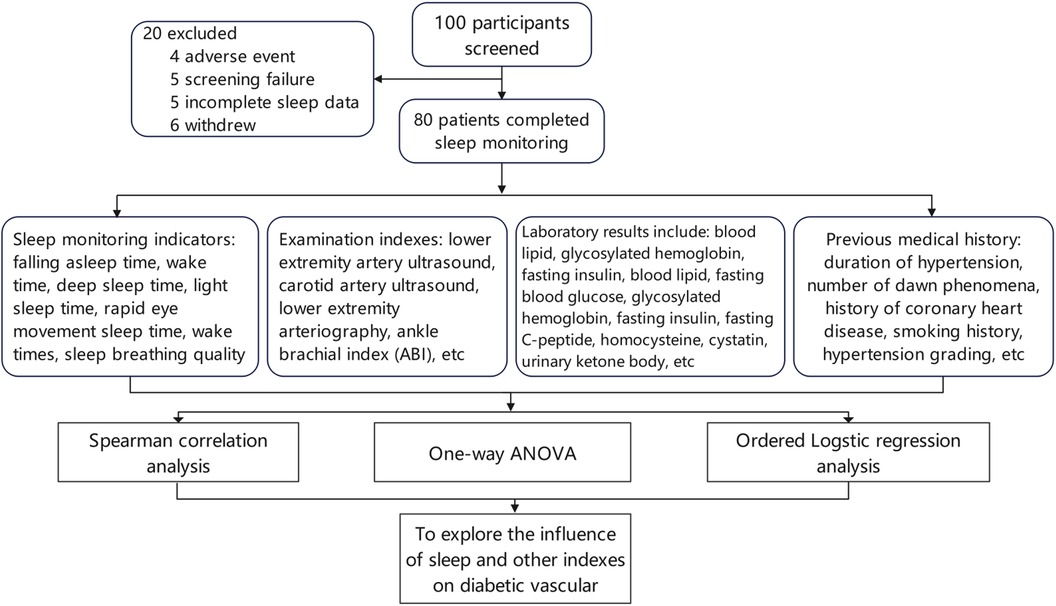
Figure 1. Process technology roadmap. One hundred patients with type 2 diabetes were enrolled after screening, including 41 men and 39 women, of whom 80 patients had complete sleep data. The experimental procedure is shown in the figure.
Screengrab of sleep data monitored by the bracelet
Daily, weekly and monthly sleep statistics were monitored by the bracelet (Figure 2).
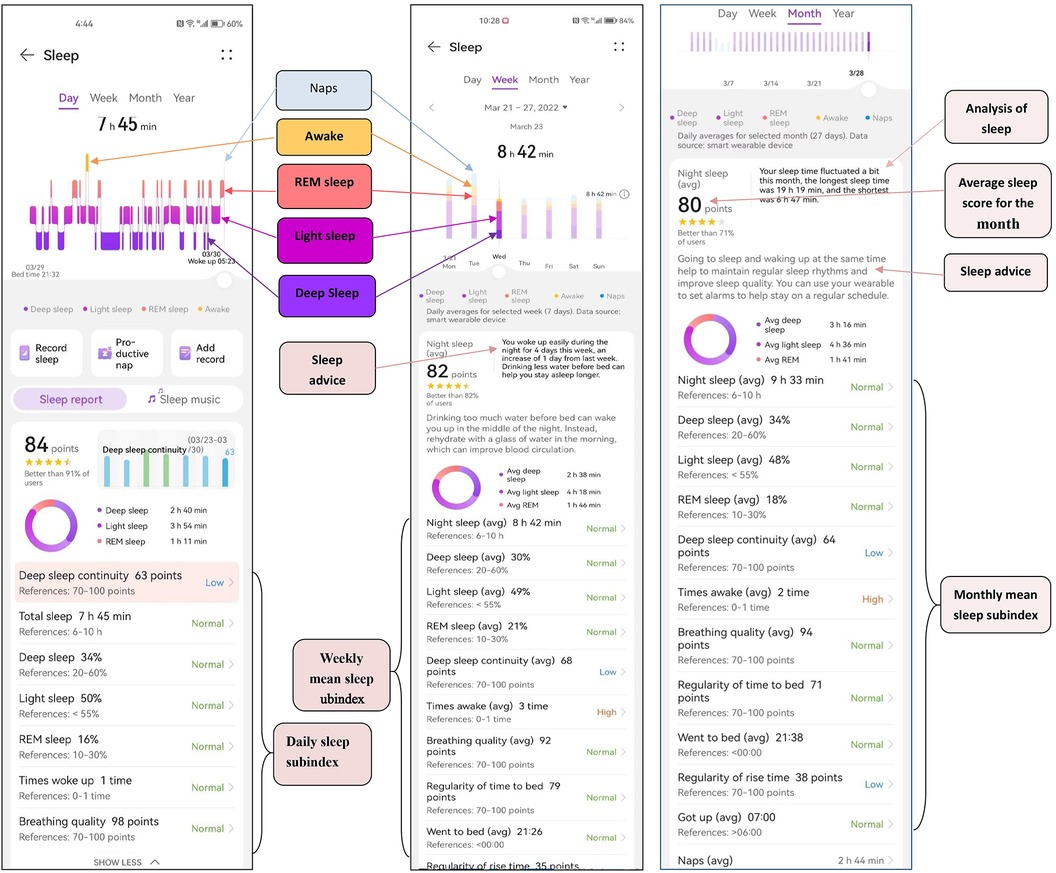
Figure 2. Screengrab of sleep data monitored by the bracelet. Daily, weekly and monthly sleep statistics were monitored by the bracelet.
Analysis of correlative factors of lower extremity vascular atherosclerosis in diabetic patients
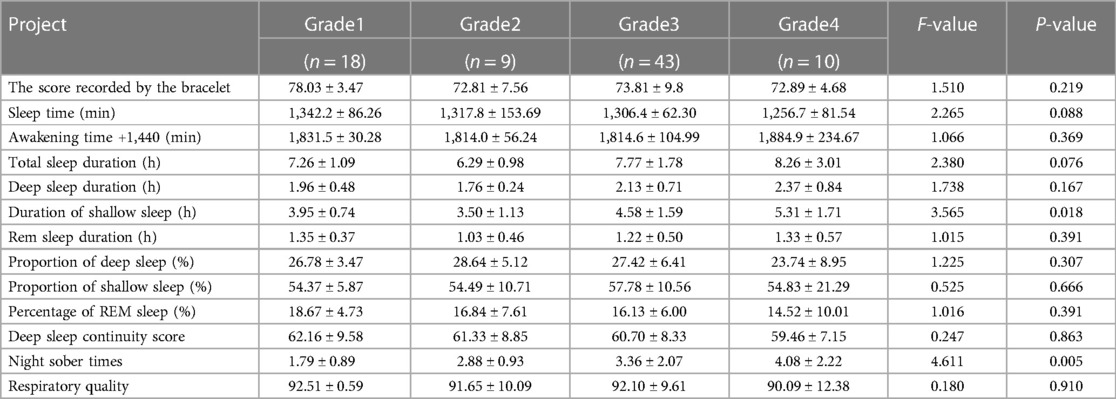
The wake time was recorded as 1,440 min (24 h) + wake time (min). Results from the one-way analysis of variance suggested that age, duration of diabetes, duration of hypertension, grade of hypertension, apolipoprotein A level, glycosylated hemoglobin level, Wagner scale for diabetic feet, and ABI were factors related to diabetic lower-extremity atherosclerosis (P < 0.05, Table 1). The longer the time of awakening at night, the more serious the diabetic lower-extremity atherosclerosis (P < 0.05, Table 2).
Spearman's correlation analysis was used to analyze the correlation between the sleep data monitored by the bracelet, the classification of diabetic lower-extremity vascular lesions, and other general indicators. The correlation coefficients ranked from high to low were age, number of night awakenings recorded by the bracelet, diabetes course, hypertension grade, hypertension course, shallow sleep duration recorded by the bracelet, age of smoking, cystatin level, glycosylated hemoglobin level, average daily smoking amount, homocysteine levels, and very low-density lipoprotein levels (Figure 3).
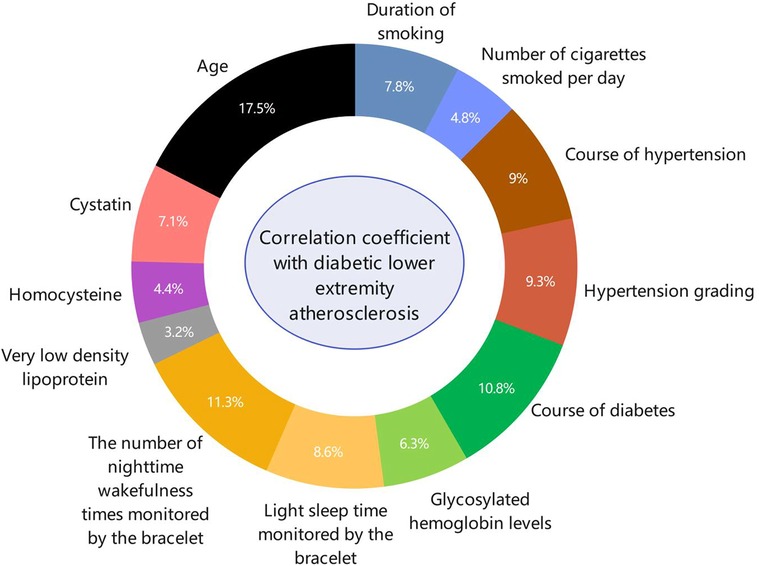
Figure 3. The ring-shaped proportion of risk factors for diabetic lower-extremity vascular disease. Spearman correlation analysis was used to analyze the correlation factors of diabetic lower-limb atherosclerosis. The correlations in descending order were age, number of night awakenings recorded by the bracelet, diabetes course, hypertension grade, hypertension course, light sleep duration recorded by the bracelet, age at smoking, cystatin, glycosylated hemoglobin level, average daily smoking amount, homocysteine level, and very low-density lipoprotein level.
One-way analysis of variance was used to compare the differences in shallow sleep duration among the different diabetic lower limb atherosclerosis groups. The results suggested that the longer the shallow sleep duration, the higher the overall trend in diabetic lower-limb atherosclerosis grade (P < 0.05, Figure 4A). Additionally, the greater the number of night awakenings, the higher the grade of diabetic lower-limb atherosclerosis (P < 0.05, Figure 4B). The sleep duration curve of patients with different grades of diabetic lower extremity atherosclerosis was U-shaped (Figure 4C).
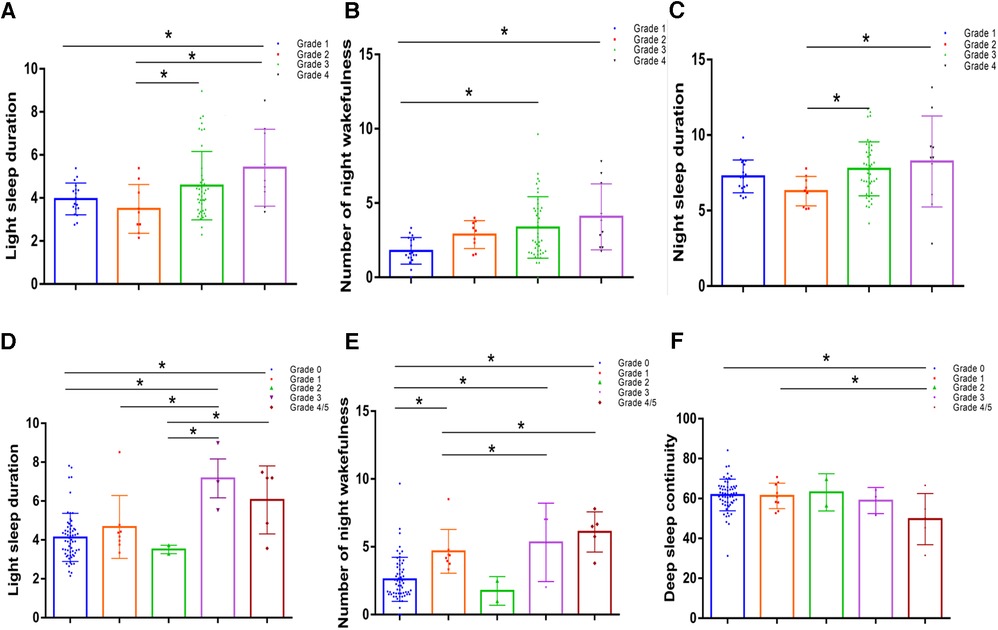
Figure 4. Comparison of sleep indices in patients with different diabetic lower limb atherosclerosis grades and different diabetic foot wagner grades. (A) Comparison of night awakenings in patients with different grades of lower-extremity arteriosclerosis. (B) Comparison of light sleep duration in patients with different grades of lower extremity arteriosclerosis. (C) The sleep duration curve of patients with different grades of diabetic lower-extremity atherosclerosis was U-shaped. (D) Comparison of light sleep duration in patients with different Wagner grades of diabetic feet. (E) Night awakenings in patients with different Wagner grades of diabetic feet. (F) Comparison of deep sleep continuity between patients with different Wagner scores. Values represent the mean and standard deviation (n = 80).
Thirteen Sleep variables were successively included in the univariate ordered logistic regression, and it was found that four variables were correlated with diabetic lower extremity vascular disease, including sleep scores, shallow sleep duration, number of night awakenings, and deep sleep continuity. Patients with a sleep score of below 70 (OR = 2.707, 95% CI: 1.127–6.488), shallow sleep duration exceeding 5.3 h (OR = 3.040, 95% CI: 1.005–9.202), more than 2.6 times occurrences of wakefulness at night (OR = 4.112, 95% CI: 1.513–11.174), and a deep sleep continuity score below 70 (OR = 4.141, 95% CI: 2.460–615.674) had a greater risk of high-grade lower limb atherosclerosis. (P < 0.05, Table 3).
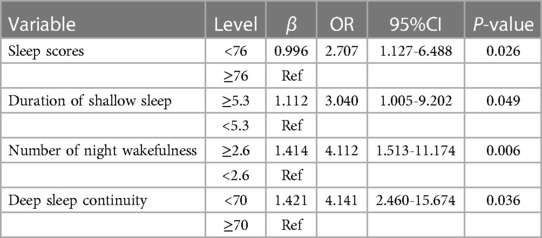
Table 3. Results of univariate ordinal logistic model using 4 levels of diabetic lower extremity vascular disease as response.
Four significant predictors of diabetic lower extremity vasculopathy from the univariate analysis were included in the multiple ordinal logistic models. In the final model, only one variable emerged as a statistically significant independent determinant of the severity of diabetic lower extremity atherosclerosis. Compared with patients with an average of less than 2.6 nighttime awakenings, patients with 2.6 or more awakenings had a higher (OR = 3.975, 95% CI: 1.297–12.182) risk of increased classification of diabetic lower extremity vascular disease (P < 0.05, Table 4).
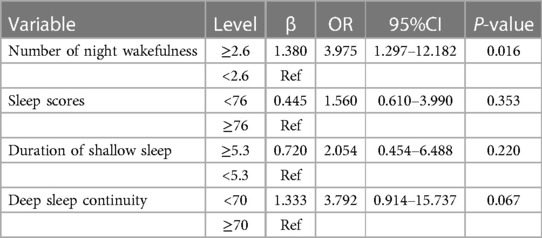
Table 4. Results of multiple ordinal logistic model using 4 levels of diabetic lower extremity vascular disease as response.
Analysis of related factors of diabetic foot
Because the number of patients with diabetic foot grades 4 and 5 was small, the statistical analyses of grades 4 and 5 were combined as grade 4. According to the results of a one-way analysis of variance, the higher the deep sleep continuity score, the lower the Wagner scale score for diabetic foot (P < 0.05, Figure 4F). The shallow sleep and night wake times of patients with different Wagner grades of diabetic foot did not show a significant step-change trend (Figures 4D,E), which may be related to the small number of cases, most of which were grade 0.
Discussion
There is growing evidence that people with peripheral artery disease are at increased risk of other health complications, including cardiovascular death, stroke, heart failure, and myocardial infarction (5). Diabetic lower-extremity atherosclerosis can also progress into diabetic foot, which is one of the most serious complications of diabetes. If diabetic foot is not treated promptly, severe cases can lead to amputation and even death (6). This disease has a high disability rate, mortality rate, and huge treatment costs, causing physical and mental pain in patients and adding to the social and economic burden (7). This has become an urgent issue that needs to be addressed.
Studies have linked sleep disorders with diabetes (8). Research suggests that approximately 42%–71% of patients with type 2 diabetes have varying degrees of sleep disorders (9). Many studies have found that lack of sleep increases the incidence of diabetes and aggravates metabolic disorders (10–12). Meanwhile, studies have found that sleep disorders also play an important role in the occurrence, development, and treatment of atherosclerosis, especially cardiovascular and cerebrovascular diseases (13–15). Sleep disorders cause hyperfunction of the sympathetic nervous system and neuroendocrine abnormalities, and promote the secretion of the adrenal hormones melatonin, angiotensin, and catechin, thus leading to vasoconstriction, and increased blood pressure (16), and increased platelet viscosity, thus promoting the progression of vascular lesions (17).
Smart wearable devices are increasingly being used in clinical settings. Data generated by intelligent wearable devices are often referred to as “big data,” and various machine algorithms used to predict health outcomes have become an area of common interest in industry, academia, and healthcare research (18). Smart wearable devices have great potential in personal health management and clinical care. It can continuously monitor health data, provide clues for disease diagnosis, discover disease symptoms promptly, and help doctors make comprehensive and accurate judgments. With progress in science and technology, various brands of smart bracelets or watches can monitor the general vital signs of human beings in a noninvasive, continuous, and convenient manner, such as heart rate, oxygen saturation, amount of exercise, blood pressure, and other indicators. However, sleep monitoring remains in its infancy. To address this, we selected a smart bracelet that primarily utilizes cardiopulmonary coupling (CPC) technology through the heart rate and acceleration sensors built into the watch. Chen et al. demonstrated that an accelerometer provided good results in predicting sleep duration, while CPC technology is useful for obtaining data on sleep apnea and assessing sleep conditions for insomnia (19–21). According to the Center for Dynamic Biomarkers, Beth Israel Deaconess Medical Center/Harvard Medical School, the results of sleep state analysis based on TruSleep™ sleep detection technology were highly consistent with the results of cardiopulmonary coupling analysis based on electrocardiography (ECG). The accuracy of comprehensive sleep duration was 93.8%, deep sleep duration was 88.8%, shallow sleep duration was 90.7%, and rapid eye movement (REM) sleep duration was 85.6%. According to verification by the University of Bern in Switzerland, the accuracy of TruSleep™ sleep detection technology for the recognition of sleep state was 96.3% for standard polysomnography monitoring, and the certification results were included in the well-known academic conference of the European Academy of Neurology (EAN) in 2018. In our study, a one-way analysis of variance was used to compare the differences in sleep indicators recorded by the bracelet in different diabetic lower limb atherosclerosis groups. The results showed that the more times of awakening at night, the more severe the diabetic lower-extremity atherosclerosis (P < 0.05).
Although studies on diabetic lower-extremity atherosclerosis are relatively comprehensive, studies on the effect of sleep on diabetic lower-extremity atherosclerosis are still insufficient. Suzuki et al. studied the association between sleep fragmentation and human atherosclerosis and found that sleep fragmentation was independently associated with atherosclerosis (2, 22). Nair and Zhang reported that long-term sleep fragmentation leads to vascular dysfunction and atherosclerosis (23). Increased wakefulness is the main manifestation of sleep fragmentation. In our study, the results showed that sleep disorders in patients with diabetes positively correlated with the progression of lower limb atherosclerosis. Univariate ordered logistic regression analysis results suggested that patients with a sleep score below 70, shallow sleep duration exceeding 5.3 h, more than 2.6 times of wakefulness at night, and a deep sleep continuity score below 70 have a greater risk of high-grade lower limb atherosclerosis. Our study showed that long, shallow sleep durations, frequent wakefulness at night, and poor continuity of deep sleep could aggravate atherosclerosis of the lower limbs in patients with diabetes. Meanwhile, our results suggested that the sleep duration curve of patients with different grades of diabetic lower limb atherosclerosis was U-shaped, which is consistent with the results of Beaman et al. (24, 25).
Studies have shown that after Cognitive Behavioral Therapy for insomnia (CBT-I), patients experience significant improvements in HbA1c levels, diabetes self-care behaviors, and fatigue (26). In our study, the results of multivariate ordinal logistic regression analysis showed that compared with patients with an average of less than 2.6 waking times at night, patients with 2.6 or more waking times at night had a 3.975 times higher risk of advanced diabetic lower extremity vascular disease. We found that increased nocturnal wakefulness was an independent risk factor for lower limb atherosclerosis in patients with diabetes. If our findings are applied in the clinical settings, and CBT-I treatment is guided through education for diabetic patients to reduce their nocturnal waking times, this approach may be a new method for preventing diabetic lower limb atherosclerosis.
Limitation
Our study has some limitations, including insufficient types of bracelets, a relatively small sample size, and a short follow-up period. Additionally, our study was cross-sectional; therefore, the results need to be confirmed in further longitudinal studies.
Conclusion
We explored the relationship between sleep and diabetic lower-extremity atherosclerosis via a smart bracelet, a modern technological product, using a simple, effective, and rapid method. These results can guide in further understanding of the relationship between sleep and diabetic lower-extremity atherosclerosis. These findings partly demonstrate that sleep disorders (long, shallow sleep, frequent wakefulness at night, and poor continuity of deep sleep) aggravate lower-extremity atherosclerosis in patients with type 2 diabetes. Moreover, this study can provide a new method for medical professionals to prevent and treat diabetic lower-extremity vascular lesions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University (Ethics batch number 2022KS016. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
BF: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. TT: Writing – review & editing. XZ: Writing – review & editing. HD: Writing – review & editing. PG: Writing – review & editing, Conceptualization. HM: Writing – review & editing. YC: Writing – review & editing, Investigation. YY: Writing – review & editing. LZ: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank all physicians, nurses, and other staff who contributed to the study. This study was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1267539/full#supplementary-material
References
1. Chemello G, Salvatori B, Morettini M, Tura A. Artificial intelligence methodologies applied to technologies for screening, diagnosis and care of the diabetic foot: a narrative review. Biosensors (Basel). (2022) 12(11):2–3. doi: 10.3390/bios12110985
2. Domínguez F, Fuster V, Fernández-Alvira JM, Fernández-Friera L, López-Melgar B, Blanco-Rojo R, et al. Association of sleep duration and quality with subclinical atherosclerosis. J Am Coll Cardiol. (2019) 73(2):134–44. doi: 10.1016/j.jacc.2018.10.060
3. Full KM, Huang T, Shah NA, Allison MA, Michos ED, Duprez DA, et al. Sleep irregularity and subclinical markers of cardiovascular disease: the multi-ethnic study of atherosclerosis. J Am Heart Assoc. (2023) 12(4):e027361. doi: 10.1161/JAHA.122.027361
4. Bhagavan SM, Sahota PK. Sleep fragmentation and atherosclerosis: is there a relationship. Mo Med. (2021) 118(3):272–6. PMID: 34149089.34149089
5. Vitalis A, Shantsila A, Proietti M, Vohra RK, Kay M, Olshansky B, et al. Peripheral arterial disease in patients with atrial fibrillation: the AFFIRM study. Am J Med. (2021) 134(4):514–8. doi: 10.1016/j.amjmed.2020.08.026
7. Rodrigues BT, Vangaveti VN, Urkude R, Biros E, Malabu UH. Prevalence and risk factors of lower limb amputations in patients with diabetic foot ulcers: a systematic review and meta-analysis. Diabetes Metab Syndr. (2022) 16(2):102397. doi: 10.1016/j.dsx.2022.102397
8. Schipper S, Van Veen MM, Elders P, Van Straten A, Van Der W, Ysbrand D, et al. Sleep disorders in people with type 2 diabetes and associated health outcomes: a review of the literature. Diabetologia. (2021) 64(11):2367–77. doi: 10.1007/s00125-021-05541-0
9. Zhu B, Quinn L, Fritschi C. Relationship and variation of diabetes related symptoms, sleep disturbance and sleep-related impairment in adults with type 2 diabetes. J Adv Nurs. (2018) 74(3):689–97. doi: 10.1111/jan.13482
10. Fan Y, Wang R, Ding L, Meng Z, Zhang Q, Shen Y, et al. Waist circumference and its changes are more strongly associated with the risk of type 2 diabetes than body mass Index and changes in body weight in Chinese adults. J Nutr. (2020) 150(5):1259–65. doi: 10.1093/jn/nxaa014
11. Huang T, Redline S. Cross-sectional and prospective associations of actigraphy-assessed sleep regularity with metabolic abnormalities: the multi-ethnic study of atherosclerosis. Diabetes Care. (2019) 42(8):1422–9. doi: 10.2337/dc19-0596
12. Huang Y, Xie T, Cao Y, Wu M, Yu L, Lu S, et al. Comparison of two classification systems in predicting the outcome of diabetic foot ulcers: the wagner grade and the saint elian wound score systems. Wound Repair Regen. (2015) 23(3):379–85. doi: 10.1111/wrr.12289
13. Cherubini JM, Cheng JL, Williams JS, MacDonald MJ. Sleep deprivation and endothelial function: reconciling seminal evidence with recent perspectives. Am J Physiol Heart Circ Physiol. (2021) 320(1):H29–35. doi: 10.1152/ajpheart.00607.2020
14. Kadoya M, Koyama H. Sleep, autonomic nervous function and atherosclerosis. Int J Mol Sci. (2019) 20(4):1–4. doi: 10.3390/ijms20040794
15. Li X, Cao Y, Xu X, Wang C, Ni Q, Lv X, et al. Sleep deprivation promotes endothelial inflammation and atherogenesis by reducing exosomal miR-182-5p. Arterioscler Thromb Vasc Biol. (2023) 43(6):995–1014. doi: 10.1161/ATVBAHA.123.319026
16. Sá Gomes E Farias AV, de Lima Cavalcanti MP, de Passos Junior MA, Vechio Koike BD. The association between sleep deprivation and arterial pressure variations: a systematic literature review. Sleep Med X. (2022) 4:100042. doi: 10.1016/j.sleepx.2022.100042
17. Tan X, Chapman CD, Cedernaes J, Benedict C. Association between long sleep duration and increased risk of obesity and type 2 diabetes: a review of possible mechanisms. Sleep Med Rev. (2018) 40:127–34. doi: 10.1016/j.smrv.2017.11.001
18. Witt D, Kellogg R, Snyder M, Dunn J. Windows into human health through wearables data analytics. Curr Opin Biomed Eng. (2019) 9:28–46. doi: 10.1016/j.cobme.2019.01.001
19. Magnusdottir S, Hilmisson H. Ambulatory screening tool for sleep apnea: analyzing a single-lead electrocardiogram signal (ECG). Sleep Breath. (2018) 22(2):421–9. doi: 10.1007/s11325-017-1566-6
20. Lu M, Fang F, Sanderson JE, Ma C, Wang Q, Zhan X, et al. Validation of a portable monitoring device for the diagnosis of obstructive sleep apnea: electrocardiogram-based cardiopulmonary coupling. Sleep Breath. (2019) 23(4):1371–8. doi: 10.1007/s11325-019-01922-3
21. Song X, Ge YJ, Kong XY, Li XY, Zhang P, Hu T, et al. Cardiopulmonary coupling analysis in diagnosis of sleep disorders. Zhonghua Yi Xue Za Zhi. (2020) 100(11):817–22. doi: 10.3760/cma.j.cn112137-20190911-02008
22. Suzuki M, Shimamoto K, Sekiguchi H, Harada T, Satoya N, Inoue Y, et al. Arousal index as a marker of carotid artery atherosclerosis in patients with obstructive sleep apnea syndrome. Sleep Breath. (2019) 23(1):87–94. doi: 10.1007/s11325-018-1664-0
23. Fortis S, O’Shea A, Beck BF, Comellas A, Vaughan Sarrazin M, Kaboli PJ. Association between rural residence and in-hospital and 30-day mortality among veterans hospitalized with COPD exacerbations. Int J Chron Obstruct Pulmon Dis. (2021) 16:191–202. doi: 10.2147/COPD.S281162
24. Beaman A, Bhide MC, McHill AW, Thosar SS. Biological pathways underlying the association between habitual long-sleep and elevated cardiovascular risk in adults. Sleep Med. (2021) 78:135–40. doi: 10.1016/j.sleep.2020.12.011
25. Ahmad A, Didia SC. Effects of sleep duration on cardiovascular events. Curr Cardiol Rep. (2020) 22(4):18. doi: 10.1007/s11886-020-1271-0
Keywords: sleep disorders, type 2 diabetes mellitus, atherosclerosis, peripheral arterial, smart wristband
Citation: Fan B, Tang T, Zheng X, Ding H, Guo P, Ma H, Chen Y, Yang Y and Zhang L (2023) Sleep disturbance exacerbates atherosclerosis in type 2 diabetes mellitus. Front. Cardiovasc. Med. 10:1267539. doi: 10.3389/fcvm.2023.1267539
Received: 26 July 2023; Accepted: 10 November 2023;
Published: 1 December 2023.
Edited by:
Ling-bing Meng, Tsinghua University, ChinaReviewed by:
David John Vigerust, Vanderbilt University, United StatesZhigang Guo, Tianjin Chest Hospital, China
© 2023 Fan, Tang, Zheng, Ding, Guo, Ma, Chen, Yang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lihui Zhang 15803210962@hebmu.edu.cn
Abbreviations ABI, ankle-brachial index; PSQI, pittsburgh sleep score; HADS, hospital anxiety and depression scale; ADL, activity of daily living score; CPC, cardiopulmonary coupling; ECG, electrocardiogram, electrocardiogram; REM, rapid eye movement; EAN, European Academy of Neurology; CBT-I, cognitive behavioral therapy for insomnia; EF, ejection fraction; PT, posterior tibia; DP, dorsal foot.
 Bingge Fan1
Bingge Fan1 Peng Guo
Peng Guo Hongqing Ma
Hongqing Ma Lihui Zhang
Lihui Zhang