- 1Cardio-Thoracic Surgery Department, Heart & Vascular Centre, Maastricht University Medical Centre, Maastricht (MUMC), Maastricht, Netherlands
- 2Division of Cardiology, Lausanne University Hospital, Lausanne, Switzerland
- 3Division of Cardiology, Università Degli Studi di Milano, Milan, Italy
- 4Division of Cardiology, Istituto Clinico Città Studi, Milan, Italy
- 5Cardiovascular Research Institute Maastricht (CARIM), Maastricht University Medical Centre (MUMC+), Maastricht, Netherlands
Background: Loop diuretics are essential for managing congestion in acute heart failure (AHF) patients, but concerns exist about their dosing and administration. This study aims to explore the relationship between aggressive diuretic treatment and clinical outcomes in AHF patients.
Methods: We randomly selected 370 AHF patients from admissions at Maastricht University Medical Center between January 2011 and March 2017. Patients were divided into four quartiles based on diuretic doses administrated during index hospitalization. The primary endpoint was a composite of cardiovascular (CV) rehospitalization or death at 1 year.
Results: 42.4% of patients experimented the primary outcome The composite endpoint rates were 35.4%, 41.6%, 38.5%, and 54.9%, respectively, from lowest to highest dose quartiles (p = 0.033). In univariate analysis, the outcome was significantly lower in the first three quartiles as compared to the fourth quartile. One-year CV mortality was 9.1%, 10.1%, 20.9% and 27.2%, respectively (p = 0.002). After adjusting for confounders, the association between loop diuretic dosage disappeared for both the primary outcome and one-year CV mortality. Most secondary outcomes and endpoints at 3 months, including worsening renal function, showed no significant differences between groups, while hypokaliemia occurrence, length of hospital stay and weight loss at index admission were higher in the fourth quartile compared to the first one.
Conclusions: High loop diuretic doses are associated with poor outcomes in AHF patients, reflecting disease severity rather than harm from aggressive diuretic use. Furthermore, high diuretic doses do not seem to negatively affect kidney function.
Introduction
Acute heart failure (AHF) is one of the most important causes of hospitalization in Western countries. High readmission and mortality rates continue to be a burden on healthcare systems worldwide (1, 2). The main reason for AHF is worsening congestion, defined as signs and symptoms of extracellular fluid accumulation that result in increased cardiac filling pressures (3).
The goal of therapy in these patients is the relief of congestion achieving a state of euvolemia, mainly through diuretic therapy. Loop diuretics, used in over 90% of patients, are the cornerstone in the treatment of congestion to reduce left ventricular filling, avoid pulmonary oedema and alleviate peripheral fluid retention (1). Despite their importance in the treatment of AHF and the ubiquity of their administration, significant concerns have been raised regarding the balance of risks and benefits, especially regarding the dosage and the administration regimen of loop diuretics.
The analysis by ADHERE Registry showed that patients receiving lower doses have a lower risk of in-hospital mortality, ICU stay, prolonged hospitalization or adverse renal effects (4). However, the DOSE trial, the largest prospective randomized double-blind controlled study assessing loop diuretics in HF, demonstrated that high dose in comparison to low dose (equal to home dose) resulted in no effect on the co-primary endpoints of global assessment of symptoms or change in serum creatinine (s-Cr) over 72 h and on 60-day death or rehospitalization. Still, it demonstrated a favourable effect on secondary endpoints of dyspnoea relief, change in weight and net fluid loss (5). In addition, the high-dose group was associated with better 60-day outcomes when adjusted for cumulative loop diuretics dose received (6).
The association between high doses of loop diuretic and worsening renal function (WRF) is of particular interest since the latter is correlated in observational studies with worse outcomes (including longer length of stay, hospital readmission and increased long term CV mortality) in the setting of AHF (7). However, in the DOSE Trial, WRF occurred more often in the high-dose group, but a post hoc analysis illustrated that this increase in creatinine did not portend a worse outcome (8). In this regard, transient WRF might mirror a more aggressive administration of loop diuretics with effective decongestion and be acceptable in acute decongestion, while persistent WRF is more likely due to more advanced hemodynamic impairment, kidney damage, and more intense neurohormonal activation (9). However, the relationship between decongestion, transient or persistent WRF and diuretic management is still incompletely understood, particularly in unselected AHF populations.
The primary goal of the present study is therefore to investigate the relationship between more aggressive diuretic treatment and clinical and renal outcomes in a cohort of patients with AHF of various aetiologies.
Methods
Subjects
In total, 3,206 patients admitted to the Cardiology department or Intensive Care Unit of the Maastricht University Medical Center (MUMC) between January 2011 and March 2017 were screened, and patients were selected using the following inclusion criteria: (1) age >18years; (2) primary diagnosis with AHF or co-primary diagnosis with newly developed HF during the index admission; (3) length of hospital stay of at least 3 days. In patients with repeated admission, only the first admission was considered. Patients with left and/or right ventricular assistant device and those were undergoing heart or heart-lung transplantation during the index hospitalisation and during follow-up were excluded from the analysis. Patients were also excluded if their medical record could not be accessed for administrative reasons. Of the remaining 2,144 patients, 370 admitted with a diagnosis of acute decompensation HF, hospitalized for at least 3 days, were randomly selected, and enrolled in this retrospective study. The number of included patients was based on a power calculation for an internal quality assessment to investigate the impact of a change in treatment regimens in ADHF (data on file), which was also the reason why the inclusion period ranged over 7 years. The clinical evaluation and AHF evaluation were performed by trained physicians when the patients were admitted at our Institution (details in Supplementary Data).
During hospitalization, every patient underwent standard clinical evaluation and received recommended heart failure treatment. The choice of diuretic regimen was left at a physician's discretion.
We collected patients' characteristics, including their age, gender, comorbidities, previous therapies, the presence of de novo or recurrent HF, the aetiology of HF, the risk factors for atherosclerosis, vital signs, admission symptoms and physical findings, the left ventricular ejection fraction (LVEF) on echocardiography, laboratory data, chest x-ray findings (signs of lung congestion), medications administered during the first week of hospitalization and the length of hospital stay. Obesity was defined as a BMI >30 kg/m2, anaemia was defined as Hb <7.5 mmol/L in women and <8.1 mmol/L in men. Heart valve disease (HVD) was considered significant in case of at least moderate degree severity.
This study complies with the Declaration of Helsinki (10) and the research protocol was approved by the local appointed ethics committee.
Assessment of diuretics doses and quartiles definition
As loop diuretics, either furosemide or bumetanide was used. The doses of loop diuretics (oral or intravenous) during the first week of hospitalization were assessed day by day expressed as mg/24 h in furosemide equivalents. For intravenous diuretics, we considered either boluses or continuous infusion. Bumetanide doses were converted to furosemide equivalents using previously published conversions where 1 mg of bumetanide was equivalent to 40 and 80 mg furosemide for intravenous and oral administration, respectively (11). We then divided the population in 4 groups according to quartiles of daily mean dose of furosemide equivalents.
Assessment of renal function
Serum creatinine levels, collected daily during the hospitalization from admission to day 7, were analysed. Glomerular filtration was estimated with the Cockcroft-Gault equation, and chronic kidney disease (CKD) was defined as estimated glomerular filtration rate (eGRF) lower than 60 ml/min/1.73 m2.
We set the definition of WRF during the acute phase of HF in the present study based on the evidence that almost all WRF occurs within seven days (12). WRF was defined as the occurrence during the first week of hospitalization of a ≥0.3 mg/dl (26.5 µmol/L) increase in s-Cr from admission.
Outcomes
The primary outcome was the combined endpoint of cardiovascular (CV) mortality or CV rehospitalization over a one-year follow-up period. Secondary outcomes were three-month CV mortality or CV rehospitalization, three-month and one-year CV mortality, one-year CV rehospitalization, in-hospital CV mortality, WRF, dyspnoea relief, hypokalaemia (serum potassium <3.5 mmol/L), hyperkalaemia (serum potassium >5 mmol/L), weight loss and length of hospital stay.
Statistical analysis
Results are presented as mean ± standard deviation (SD) for continuous variables or as median (interquartile range) for continuous variables with a skewed distribution and as percentages of the total for categorical variables. Continuous variables were compared by two-tailed paired t-test and analysis of variance in case of normal distribution, and using the Mann–Whitney U-test and Kruskal–Wallis H-test in case of abnormal distribution. Categorical variables were tested using Chi-squared tests. A p-value < 0.05 was considered to be statistically significant.
The treatment groups defined by dose were compared with logistic regression for binary end points. We divided our sample into quartiles according to the mean dose of furosemide equivalents. The highest quartile was used as the reference when calculating odds ratios (ORs), hazard ratios (HRs) and confidence intervals.
The association between diuretic dose with primary and secondary outcomes were tested using univariate logistic regression models. A multivariate logistic regression model was also tested for primary endpoint and 1 year CV mortality. The variables included in the multivariate model were: age, gender, obesity, diabetes mellitus, smoking, hypertension, arrhythmia, chronic heart failure (CHF), admission systolic blood pressure (SBP) ≤90 mmHg, admission creatinine, urea, sodium and N-terminal proB-type natriuretic peptide (NT-proBNP).
In order to test the association between the treatment groups and continuous variables with a linear regression model, every diuretic dose standard deviation parameter was associated with dummy variables for quartile 1, 2 and 3, hence, the unstandardized B coefficient (with confidence intervals at 95%) represented the difference from the highest quartile.
Kaplan–Meier method was used to compare those groups for time-to-event endpoints and differences between the curves were evaluated with the log-rank statistic. In addition, univariable and multivariable Cox proportional hazard models regressed time to primary endpoint on quartiles of diuretic dose, age, gender, obesity, admission systolic blood pressure (SBP) ≤90 mmHg, presence of anemia, admission sodium, creatinine, urea and NT-proBNP. BM SPSS Statistics for Windows, Version 26.0 (Armonk, NY, IBM Corp) was used for all analyses.
Results
Characteristics of the cohort at admission
At admission, characteristics of the study population are presented in Table 1. The age of the cohort ranged from 22 to 98 years (mean 76.4 ± 12 years). Approximately 53% of the study participants were men.
The study population had several high-risk features including obesity, hypertension, dyslipidaemia, diabetes mellitus, smoking, coronary artery disease (CAD), peripheral artery disease, CKD and CHF. The most common triggers of AHF were fluid overload, arrhythmias, acute coronary syndromes, mechanical causes, hypertension and respiratory infection. At index admission, the majority of patients had signs and symptoms of congestion. The mean LVEF was 43.7 ± 16.1%. Patients treated with loop diuretics prior to index admission were 190 (51.4%) with the mean dosage of 120 [80–175] mg/day.
Diuretic dose and patient characteristics
There were 99 patients (26.7%) in the first quartile, with mean daily furosemide dose equivalence of 0–80 mg/day; 89 patients (24.1%) in the second quartile, with dose equivalence of 81–120 mg/day; 91 patients (24.6%) in the third quartile, with dose equivalence of 121–175 mg/day; and 91 patients (24.6%) in the fourth quartile, with dose equivalence >175 mg/day. Differences in patients’ characteristics among the loop diuretic quartiles are depicted in Table 1. Patients receiving higher doses of loop diuretics more often had diabetes mellitus, previous heart failure, arrhythmias, primary cause of admission was more often fluid overload, had more prior history of CABG, experienced more weight gain, and had more signs of right ventricular fluid overload, higher BMI and lower blood pressure and heart rate, higher admission s-Cr, urea and NT-proBNP. They were treated prior to admission more often with loop diuretic at higher doses and mineralcorticoid receptor antagonist (MRA). They also received more often thiazides during hospitalisation and nitrates. In addition, there was a more important prevalence of smokers in the second quartile, and chest pain at presentation and nitrates were prevalent in the third quartile (Supplementary Table S1).
Relation between diuretic dose and primary outcome
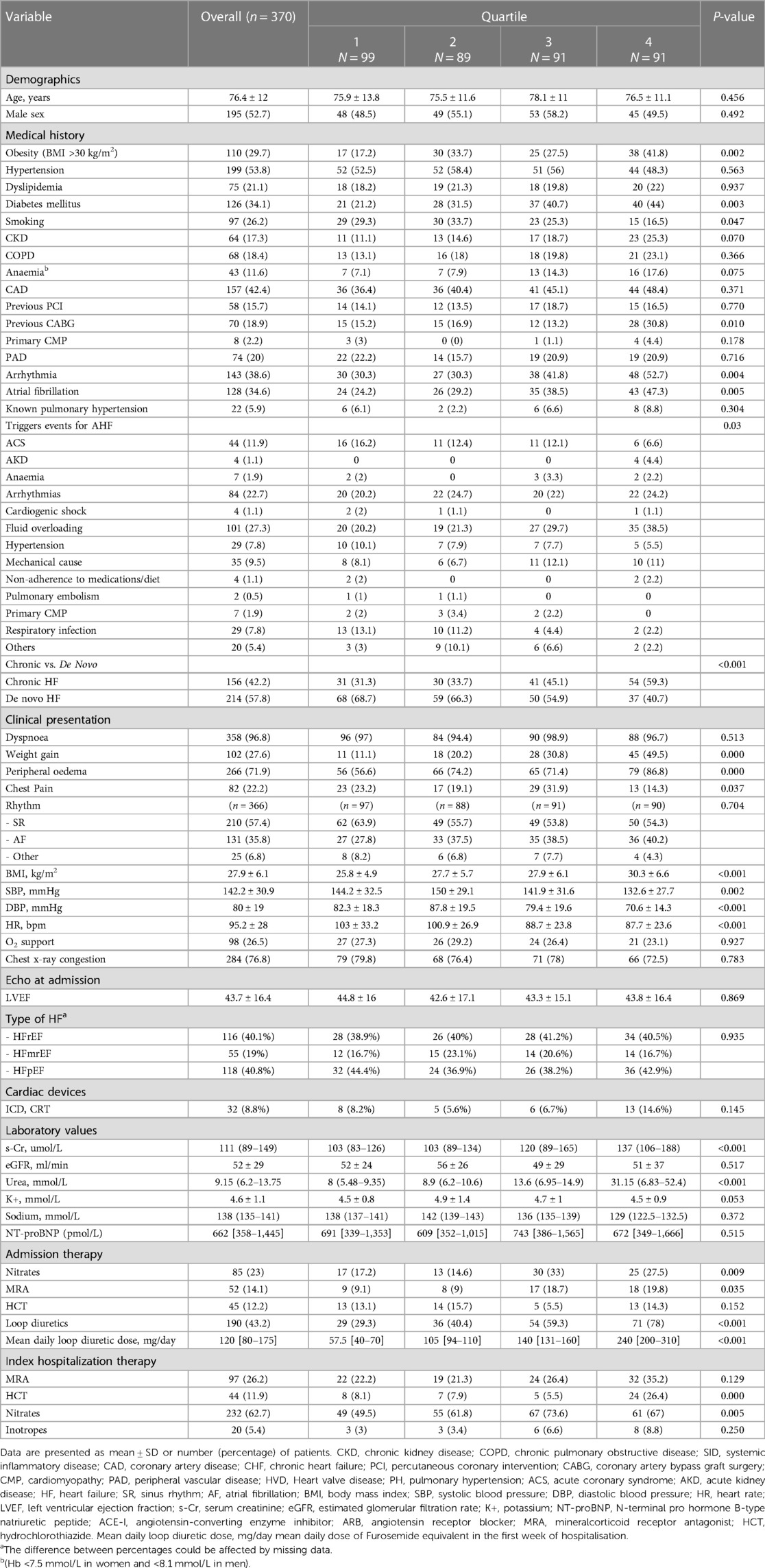
A total of 157 patients (42.4%) had a CV rehospitalization or died with CV causes within the one-year follow-up period. CV rehospitalization or CV mortality estimates at 1 year were 35.4%, 41.6%, 38.5%, and 54.9% for quartiles 1, 2, 3, and 4, respectively (p = 0.033) (Figure 1). In univariate analysis, this composite endpoint was significantly lower in the first three quartiles as compared to the quartile with the highest loop diuretic dose [first quartile, OR 0.45, 95% confidence interval (CI) 0.25–0.8; second quartile, OR 0.58, 95% CI 0.32–1.1 and third quartile, OR 0.51, 95% CI 0.–0.92; p = 0.040], whereas between the first three quartiles, there was no statistically significant difference. In univariable Cox proportional hazard model, highest diuretic dose quartile was associated with an increased risk of primary outcome (HR 1.62, 95% CI 1.05–2.27; p-value = 0.005) compared to the other quartiles (Figure 2).
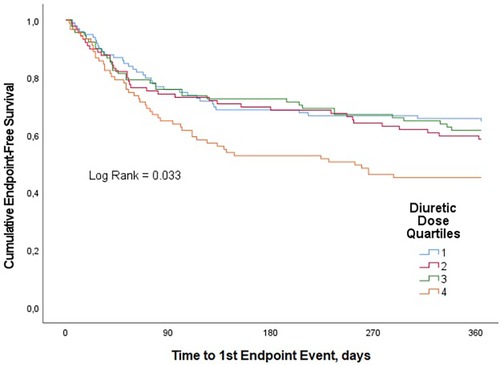
Figure 1. Kaplan–Meier curves for the clinical composite end point of CV mortality or CV rehospitalization for loop diuretic dose quartiles in patients with AHF over one-year follow-up.

Figure 2. Cox proportional hazard model (univariate): risk of composite end point of CV mortality or CV rehospitalization for loop diuretic dose quartiles in patients with AHF over one-year follow-up.
After adjustment for age, gender, diabetes mellitus, smoking, obesity, hypertension, arrhythmia, previous CHF, admission SBP ≤90 mmHg, admission creatinine, admission urea, admission sodium and admission NT-proBNP, the association between loop diuretic dosage and the primary end point disappeared (Table 2).
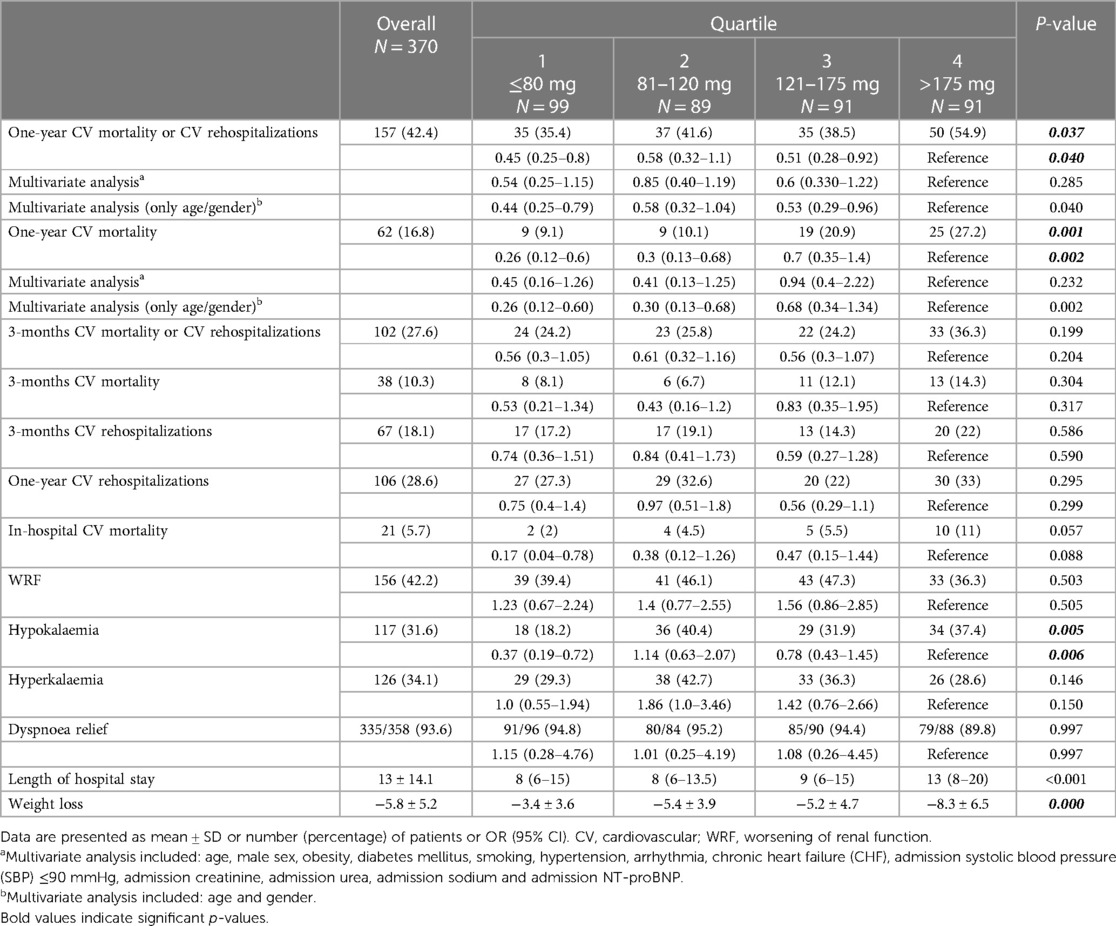
Table 2. Association between quartiles of daily mean dose of furosemide equivalents and clinical end points.
Supplementary Table S2 lists the characteristics for patients with the primary end point compared with patients without it.
Relation between diuretic dose and secondary outcomes
We observed 62 CV deaths (16.8% of study participants) during the one-year follow-up period. In-hospital CV death occurred in 21 (5.7%) patients. Patients in the highest diuretic dose quartile were found to have significantly worse survival compared with patients in the lowest quartile. CV mortality estimates at 1 year were 9.1%, 10.1%, 20.9% and 27.2% for quartiles 1, 2, 3, and 4, respectively (p = 0.002) (Figure 3). In univariate analysis, compared with the fourth quartile, increasing loop diuretic dose quartiles were associated with lower CV mortality in the first two quartiles (first quartile, OR 0.26, 95% CI 0.12–0.6; second quartile, OR 0.3, 95% CI 0.13–0.68; and third quartile, OR 0.7, 95% CI 0.35–1.4; p = 0.002).
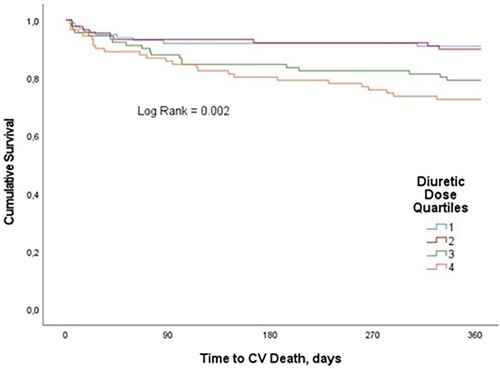
Figure 3. Kaplan–Meier curves for the clinical end point of CV mortality for loop diuretic dose quartiles in patients with AHF over one-year follow-up.
After adjustment for age, male sex, obesity, diabetes mellitus, smoking, hypertension, arrhythmia, CHF, admission SBP ≤90 mmHg, admission creatinine, admission urea, admission sodium and admission NT-proBNP, no statistically significant association was found between loop diuretic dosage and one-year CV mortality (Table 2).
Non-survivors for CV causes had more often CKD, CAD, previous HF, pulmonary hypertension, and previous CABG, and experienced lower blood pressure, heart rate, eGFR and sodium, higher s-Cr and urea. Furthermore, they were more often treated prior to admission with loop diuretic, beta-blocker and MRA, and during the index hospitalisation they received more often MRA and inotropes and higher doses of loop diuretic (Supplementary Table S3).
Subjects in the highest diuretic dose quartile had significantly more often hypokalaemia (serum potassium <3.5 mmol/L) compared with patients in the lowest quartile, whereas there were no statistically significant differences between groups in terms of hyperkalaemia (serum potassium >5 mmol/L).
Moreover, there were no significant between-groups differences for a variety of secondary endpoints (Table 2): CV rehospitalization during the one-year follow-up period, CV mortality or rehospitalizations and CV mortality during the three-month follow-up period, in-hospital CV mortality, WRF and dyspnoea relief at discharge. Patients in the highest diuretic dose quartile were found to have significantly longer hospitalizations and greater weight loss during the index hospitalization compared with patients in the lowest one.
Discussion
Despite the widespread loop diuretics strategy, their appropriate use remains challenging given the limited evidence supporting their efficacy, safety (especially WRF), the optimal dosage and the relative prognostic impact (13). The current study demonstrates that patients requiring higher loop diuretic doses had more comorbidities, more often a history of CHF and impaired clinical conditions including more congestion and lower blood pressure. The potentially negative impact on outcome of higher doses of loop diuretics to recompensate can only be seen long-term and disappears completely in multivariable analysis. This suggests that the need of more intensified diuretic therapy is an indicator of severity of AHF to completely decongest and of co-morbidities but is not harmful per se. Thus, the report of this study supports the findings of the DOSE study in a real-world population that was older and had more co-morbidities.
Other observational studies have found that higher doses may have harmful effects, possibly due to activation of the renin-angiotensin and sympathetic nervous system, or electrolyte disturbance (14, 15). Still, it must be underlined that such observations are confounded by the fact that more severely ill patients require higher doses of diuretic, particularly when the urine output of sodium and water is insufficient. Indeed, our data supports the idea that higher doses of loop diuretics are a consequence of more advanced HF rather than a cause of poor outcome. Therefore, these should be considered as a marker instead of a mechanism of poor outcomes (15, 16). Patients with higher loop diuretic dose also more often required the addition of thiazide-type diuretics. These drugs are prescribed in less responsive patients to address loop diuretic resistance because they might overcome the escape phenomenon due to activation of the renin-angiotensin system (RAS) and sympathetic system and sodium reabsorption by more distal sodium transporters (17). Administration of high loop diuretics doses, particularly in combination with thiazides, may lead to electrolyte imbalances (such hypokalaemia, hyponatremia, and hypomagnesemia), which might exacerbate cardiac arrhythmias and increase the risk of sudden cardiac death (14). It may be speculated that the daily monitoring of electrolytes during decongestion or the occurrence of loop diuretic resistance that limited the excessive loss of fluid and electrolyte in our study population could have contributed to the lack of increased in-hospital CV mortality in the patients receiving higher doses.
WRF during an AHF hospitalization is common ranging from 20 to 40% (18), but not always clinically relevant, especially when it is associated with appropriate decongestion, diuresis and haemoconcentration (1). CKD is a well-recognized risk factor of adverse outcomes in AHF (7). However, the latter was not confirmed for acute renal dysfunction. On one hand, several studies reported that the association between the decline in kidney function and AHF treatment is not related with adverse outcomes (8, 19); on the other hand, the association with poor prognosis (20) through several mechanisms such as inflammation, oxidative stress, impaired hydrosaline homeostasis, and diuretic resistance (21) was demonstrated in other studies. WRF occurred in approximately 40% of our study population, but it was not related to loop diuretic dose. This finding is extremely relevant since it overcomes the prejudice regarding the fear of decreasing renal plasma flow and deterioration of kidney function. WRF is frequently interpreted in clinical practice as a decrease in effective circulating volume, prompting physicians to reduce loop diuretic therapy based on the often-false assumption that further decongestion might result in renal tubular damage (22).
Furthermore, the association between WRF and outcomes might be influenced by other factors. Firstly, patients who experience WRF have often a higher disease severity. Secondly, they are less responsive to AHF therapies and, finally, they are intrinsically at greater risk of adverse events independent of the renal dysfunction (8). WRF alone is not an independent determinant of outcomes in patients with AHF, but it has an additive prognostic value when it occurs in patients with persistent signs of congestion (19). This implies that the renal function impairment is more dependent on venous congestion rather than on impairment of cardiac output (23). The limited impact of aggressive decongestion with higher doses of loop diuretics on renal function is supported by the findings of this study, which is in line with very recent data on the addition of acetazolamide (24) and thiazide (25) to rapidly decongest patients with ADHF. The main goal should therefore be rapid and effective decongestion followed by establishment of standard therapy of chronic HF (22, 26). Our data supports this recommendation also in an elderly all-comers population of ADHF, in which renal function is generally poorer and treating physicians more reluctant to use higher diuretic doses. In fact, despite the use of diuretics, 40% of patients are discharged with unresolved congestion in clinical practice, leading to increased rehospitalization and higher mortality rates (27).
Study limitations
The present study has the following limitations. First, the retrospective nature of this study does not allow to certainly define the causal relationship of the investigated variables.
Second, WRF definition depends on s-Cr, which is primarily a marker of glomerular filtration with a slow kinetics and does not recognize renal tubular injury in the absence of a significant reduction in eGFR (28).
Third, weight loss, as indicator of decongestion and diuretic response in the absence of diuresis data, is influenced by factors other than fluid balance. This may be inaccurate even in the best of circumstances, may be a poor predictor of euvolemia and has a weak correlation with net urine output in AHF clinical trials (29–31). Furthermore, data on predischarge NT-proBNP and delta changes during hospitalisation were available for a very small number of patients, and therefore could not be analysed as a marker of decongestion and clinical improvement. Moreover, a comprehensive haemodynamic phenotyping to proof complete decongestion at discharge (central venous pressure and pulmonary artery wedge pressure) was not collected in the present study. Finally, the limited number of patients included might have contributed to the lack of prognostic impact of diuretic therapy during first months, which is in some contrast to earlier studies.
Conclusion
The highest diuretic dose was associated with a significant increase in CV mortality and in the composite endpoint of CV mortality or CV rehospitalization over the one-year follow-up period, while there was no association between higher doses and outcomes at 3 months. In addition, poor long-term outcome was no longer seen in multivariable analysis. Furthermore, higher diuretic dosage was not associated with a significant worsening in renal function. These findings suggest that higher doses of loop diuretics are not harmful but necessary to manage congestion in patients with advanced HF and worse admission clinical conditions, explaining the poor outcome at 1 year. Our data are in line with recent finding that aggressive decongestion may result in better outcome (24, 25) and suggests that such a treatment regimen is also applicable in an elderly all-comer population with ADHF.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Maastricht Medisch Ethische Toetsings Commissie—METC. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
PM: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Validation, Writing – original draft, Writing – review & editing. MP: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. GM: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. SF: Data curation, Methodology, Writing – original draft, Writing – review & editing. HB: Conceptualization, Investigation, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1267042/full#supplementary-material
References
1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42(36):3599–726. doi: 10.1093/eurheartj/ehab368
2. Writing Committee M, Members AAJC. 2022 AHA/ACC/HFSA guideline for the management of heart failure. J Card Fail. (2022) 28(5):e1–167. doi: 10.1016/j.cardfail.2022.02.010
3. Chioncel O, Mebazaa A, Harjola VP, Coats AJ, Piepoli MF, Crespo-Leiro MG, et al. Clinical phenotypes and outcome of patients hospitalized for acute heart failure: the ESC heart failure long-term registry. Eur J Heart Fail. (2017) 19(10):1242–54. doi: 10.1002/ejhf.890
4. Peacock WF, Costanzo MR, De Marco T, Lopatin M, Wynne J, Mills RM, et al. Impact of intravenous loop diuretics on outcomes of patients hospitalized with acute decompensated heart failure: insights from the ADHERE registry. Cardiology. (2009) 113(1):12–9. doi: 10.1159/000164149
5. Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. (2011) 364(9):797–805. doi: 10.1056/NEJMoa1005419
6. Hanberg JS, Tang WHW, Wilson FP, Coca SG, Ahmad T, Brisco MA, et al. An exploratory analysis of the competing effects of aggressive decongestion and high-dose loop diuretic therapy in the DOSE trial. Int J Cardiol. (2017) 241:277–82. doi: 10.1016/j.ijcard.2017.03.114
7. Damman K, Solomon SD, Pfeffer MA, Swedberg K, Yusuf S, Young JB, et al. Worsening renal function and outcome in heart failure patients with reduced and preserved ejection fraction and the impact of angiotensin receptor blocker treatment: data from the CHARM-study programme. Eur J Heart Fail. (2016) 18(12):1508–17. doi: 10.1002/ejhf.609
8. Brisco MA, Zile MR, Hanberg JS, Wilson FP, Parikh CR, Coca SG, et al. Relevance of changes in serum creatinine during a heart failure trial of decongestive strategies: insights from the DOSE trial. J Card Fail. (2016) 22(10):753–60. doi: 10.1016/j.cardfail.2016.06.423
9. Palazzuoli A, Ruocco G, Ronco C, McCullough PA. Loop diuretics in acute heart failure: beyond the decongestive relief for the kidney. Crit Care. (2015) 19(1):296. doi: 10.1186/s13054-015-1017-3
10. Rickham PP. Human experimentation. Code of ethics of the world medical association. Declaration of Helsinki. Br Med J. (1964) 2(5402):177. doi: 10.1136/bmj.2.5402.177
11. Testani JM, Brisco MA, Turner JM, Spatz ES, Bellumkonda L, Parikh CR, et al. Loop diuretic efficiency: a metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ Heart Fail. (2014) 7(2):261–70. doi: 10.1161/CIRCHEARTFAILURE.113.000895
12. Forman DE, Butler J, Wang Y, Abraham WT, O'Connor CM, Gottlieb SS, et al. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. (2004) 43(1):61–7. doi: 10.1016/j.jacc.2003.07.031
13. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. (2022) 43(7):561–632. doi: 10.1093/eurheartj/ehab395
14. Cooper HA, Dries DL, Davis CE, Shen YL, Domanski MJ. Diuretics and risk of arrhythmic death in patients with left ventricular dysfunction. Circulation. (1999) 100(12):1311–5. doi: 10.1161/01.CIR.100.12.1311
15. Hasselblad V, Gattis Stough W, Shah MR, Lokhnygina Y, O'Connor CM, Califf RM, et al. Relation between dose of loop diuretics and outcomes in a heart failure population: results of the ESCAPE trial. Eur J Heart Fail. (2007) 9(10):1064–9. doi: 10.1016/j.ejheart.2007.07.011
16. Mentz RJ, Kjeldsen K, Rossi GP, Voors AA, Cleland JG, Anker SD, et al. Decongestion in acute heart failure. Eur J Heart Fail. (2014) 16(5):471–82. doi: 10.1002/ejhf.74
17. Michael Felker G. Diuretic management in heart failure. Congest Heart Fail. (2010) 16(Suppl 1):S68–72. doi: 10.1111/j.1751-7133.2010.00172.x
18. Cruz DN, Gheorghiade M, Palazzuoli A, Ronco C, Bagshaw SM. Epidemiology and outcome of the cardio-renal syndrome. Heart Fail Rev. (2011) 16(6):531–42. doi: 10.1007/s10741-010-9223-1
19. Metra M, Davison B, Bettari L, Sun H, Edwards C, Lazzarini V, et al. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ Heart Fail. (2012) 5(1):54–62. doi: 10.1161/CIRCHEARTFAILURE.111.963413
20. Heywood JT, Fonarow GC, Costanzo MR, Mathur VS, Wigneswaran JR, Wynne J, et al. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail. (2007) 13(6):422–30. doi: 10.1016/j.cardfail.2007.03.011
21. Ruocco G, Palazzuoli A, Ter Maaten JM. The role of the kidney in acute and chronic heart failure. Heart Fail Rev. (2020) 25(1):107–18. doi: 10.1007/s10741-019-09870-6
22. Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner-La Rocca HP, Martens P, et al. The use of diuretics in heart failure with congestion—a position statement from the heart failure association of the European society of cardiology. Eur J Heart Fail. (2019) 21(2):137–55. doi: 10.1002/ejhf.1369
23. Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. (2009) 53(7):589–96. doi: 10.1016/j.jacc.2008.05.068
24. Mullens W, Dauw J, Martens P, Verbrugge FH, Nijst P, Meekers E, et al. Acetazolamide In acute decompensated heart failure with volume overload. N Engl J Med. (2022) 387(13):1185–95. doi: 10.1056/NEJMoa2203094
25. Trulls JC, Morales-Rull JL, Casado J, Carrera-Izquierdo M, Snchez-Marteles M, Conde-Martel A, et al. Combining loop with thiazide diuretics for decompensated heart failure: the CLOROTIC trial. Eur Heart J. (2023) 44(5):411–21. doi: 10.1093/eurheartj/ehac689
26. Boulos J, Darawsha W, Abassi ZA, Azzam ZS, Aronson D. Treatment patterns of patients with acute heart failure who develop acute kidney injury. ESC Heart Fail. (2019) 6(1):45–52. doi: 10.1002/ehf2.12364
27. Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. (2013) 34(11):835–43. doi: 10.1093/eurheartj/ehs444
28. Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol. (2009) 20(3):672–9. doi: 10.1681/ASN.2008070669
29. Kociol RD, McNulty SE, Hernandez AF, Lee KL, Redfield MM, Tracy RP, et al. Markers of decongestion, dyspnea relief, and clinical outcomes among patients hospitalized with acute heart failure. Circ Heart Fail. (2013) 6(2):240–5. doi: 10.1161/CIRCHEARTFAILURE.112.969246
30. Ambrosy AP, Cerbin LP, Armstrong PW, Butler J, Coles A, DeVore AD, et al. Body weight change during and after hospitalization for acute heart failure: patient characteristics, markers of congestion, and outcomes: findings from the ASCEND-HF trial. JACC Heart Fail. (2017) 5(1):1–13. doi: 10.1016/j.jchf.2016.09.012
Keywords: loop diuretics, acute heart failure, acute heart failure treatment, acute heart failure outcomes, worsening renal function
Citation: Meani P, Pagnoni M, Mondellini GM, Fiorenza S and Brunner-La Rocca HP (2023) Impact of loop diuretic dosage in a population of patients with acute heart failure: a retrospective analysis. Front. Cardiovasc. Med. 10:1267042. doi: 10.3389/fcvm.2023.1267042
Received: 25 July 2023; Accepted: 31 October 2023;
Published: 23 November 2023.
Edited by:
Peter Wohlfahrt, Institute for Clinical and Experimental Medicine (IKEM), CzechiaReviewed by:
Sarawut Siwamogsatham, Chulalongkorn University, ThailandCharlotta Ljungman, Sahlgrenska University Hospital, Sweden
© 2023 Meani, Pagnoni, Mondellini, Fiorenza and Brunner-La Rocca. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. Pagnoni bWF0dGlhLnBhZ25vbmlAY2h1di5jaA==
†These authors have contributed equally to this work
 P. Meani
P. Meani M. Pagnoni
M. Pagnoni G. M. Mondellini
G. M. Mondellini S. Fiorenza
S. Fiorenza H. P. Brunner-La Rocca
H. P. Brunner-La Rocca