
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 25 September 2023
Sec. Heart Failure and Transplantation
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1265269
This article is part of the Research Topic Worsening Heart Failure: Challenges, Treatments, Future Perspectives View all 6 articles
Background: The benefits of rhythm control for atrial fibrillation (AF) in heart failure with preserved ejection fraction (HFpEF) have not been conclusively determined. We assessed the effects of rhythm control on left atrial (LA) structure remodeling and prognosis in patients with AF and HFpEF.
Methods: This was a retrospective, real-world, observational study involving patients diagnosed with AF and HFpEF. The cohort was divided into rhythm-control and rate-control groups depending on their treatment strategies. The primary outcomes were all-cause mortality, rehospitalization for any cause, HF-related rehospitalization, and stroke. Differences in follow-up LA structure parameters were also analyzed.
Results: Compared to the rate-control group, patients in the rhythm-control group had a lower risk of HF-related rehospitalization even after adjusting for potential confounders (adjusted HR 0.605, 95% CI 0.413–0.887, p = 0.010). Moreover, rhythm-control therapy led to marked reductions in LA echocardiographic indicators and a higher proportion of LA reverse remodeling (LARR).
Conclusions: Rhythm-control therapy reverses LA structure remodeling and is associated with improved clinical outcomes; therefore, it is an optimal treatment approach for AF in HFpEF patients.
Globally, approximately 64.3 million people are suffering from heart failure (HF), among whom, heart failure with preserved ejection fraction (HFpEF) accounts for about 36% of the total HF spectrum (1). Atrial fibrillation (AF) is one of the most common complications in HFpEF patients. The Olmsted County cohort study revealed that approximately two-thirds of HFpEF cases had AF during the disease process (2). In another study, AF patients were 4.8 times more likely to develop HFpEF than those without AF (3). These two diseases often share common risk factors, including advanced age, female sex, anemia, and comorbidities such as hypertension, obesity, chronic renal insufficiency, atherosclerosis, and sleep apnea. Additionally, AF and HFpEF interact and promote their progress through cardiac structure and electrophysiology remodeling, neurohumoral activation, inflammation, and other pathogenic mechanisms, resulting in a vicious cycle (4).
The latest therapeutic guidelines recommend that rhythm control therapy, particularly catheter ablation (CA), is a potentially effective strategy for treating symptomatic AF and HF (5, 6). There is sufficient evidence that patients with heart failure with reduced ejection failure (HFrEF) patients and AF who were subjected to catheter ablation (CA) to restore sinus rhythm (SR) exhibited better prognostic outcomes compared to those treated with rate-control therapy (7–12). However, little attention has been paid to HFpEF, and a limited number of studies have been conducted to assess the efficacy of rhythm control therapy (CA, electrocardioversion, or surgical ablation) for HFpEF and AF. According to the available evidence, HFpEF populations may benefit from rhythm control therapies. Thus, to verify it, we investigated the effect of rhythm-control therapy on prognosis and left atrial remodeling in patients with HFpEF and AF.
This was a single-center retrospective cohort study. Consecutive AF and HFpEF patients hospitalized at The First Affiliated Hospital of Dalian Medical University between January 2011 and December 2020 were enrolled. In this study, adult patients received rhythm control (CA, electrocardioversion, or surgical ablation) or rate control therapies (β-blockers or digoxin). The exclusion criteria were end-stage renal failure, absence of electrocardiogram (ECG) findings, and lack of follow-up data. This study was approved by the institutional review board of Dalian Medical University, Liaoning, China, and was conducted under the declaration of Helsinki. All participants involved provided informed consent.
The cohort was classified into rhythm and rate control groups depending on their treatment strategies. Patients treated with CA, electrocardioversion (ECV) (100J or 150J), or surgical ablation (SA) (COX Maze) were identified as the rhythm control group. CA is a standard procedure. Following reconstruction of the 3D model of the left atrium (LA) using the Carto 3 (Biosense Webster, USA) 3D mapping system, a catheter procedure was performed to isolate the pulmonary vein. After pulmonary vein isolation, other ablation strategies, such as the LA roof line, mitral isthmus line, tricuspid isthmus line, and BOX ablation, among others, were completed at physicians’ discretion. All patients in the rhythm group received standard anticoagulation (warfarin or novel oral anticoagulants) for at least three weeks and underwent transesophageal echocardiography to rule out the risk of cardiac thrombosis before the rhythm treatment. Postoperatively, patients were administered amiodarone (200 mg 3 times a day for the first week, 200 mg twice daily for the second week, and 200 mg once daily for the third week) to maintain sinus rhythms. By contrast, patients who had been administered with β-blockers or digoxin (without antiarrhythmic medications) to control their ventricular rates were assigned to the rate control group, with the target heart rate <80 bpm. at rest or <110 bpm. during moderate exercise. To reduce selection bias and potential confounding factors, a 1:1 optimal propensity-score (PS) matching with a 0.02 caliper and no replacement was conducted based on age, NYHA class IV, coronary artery disease, hypertension, diabetes, and warfarin. The flow chart of the study population is shown in Figure 1.
The diagnosis of HFpEF and AF is determined in accordance with the guideline of the European Society of Cardiology (6). The diagnostic criteria for HFpEF included: (i) typical signs and symptoms of HF; (ii) LVEF ≥50%; and (iii) evidence of spontaneous or provokable increased LV filling pressures (elevated brain natriuretic peptide, non-invasive and invasive hemodynamic measurements). Clinically, AF is defined as ≥30 s recorded in a standard 12-lead ECG or single-lead ECG, with no identifiable repetitive P waves and irregular RR intervals when atrioventricular conduction was not impaired.
Demographics, the New York Heart Association (NYHA) functional class, co-morbidities, laboratory tests, and information on drug therapies were collected. Some valuable echocardiographic parameters listed as follows were also recorded. Mitral Doppler early velocity/mitral annular early velocity (E/e'), the ratio of peak velocity of mitral inflow during early diastole (E), and over the average septal and lateral mitral annular early diastolic peak velocities (e'), reflects left ventricular (LV) stiffness and fibrosis. Left atrial volume index (LAVI), an essential indicator of chronic LA remodeling, is measured at end-systole and indexed to body surface area. Left ventricular mass index (LVMI) and relative wall thickness (RWT) are important markers of global LV remodeling or hypertrophy. LVMI is calculated using the Cube formula and indexed to body surface area. RWT is twice the LV posterior wall thickness divided by the LV internal diameter at end-diastole. Tricuspid regurgitation (TR) peak velocity is considered to be an indirect marker of LV diastolic dysfunctions (13). Left atrial reverse remodeling (LARR), a vital index of LA structure reverse remodeling, is defined by a ≥15% reduction in LA end-systolic volume (LAESV) (14).
The primary clinical outcomes in this study were all-cause mortality, rehospitalization for any cause, HF-related readmission, and stroke. Differences in follow-up LA structure parameters were calculated and compared. The time interval between the two echocardiogram examinations was at least 3 months. Postoperatively, all participants received at least three outpatient visits after ablation (3, 6, and 12 months within 1 year after surgery and once every 6 months after 1 year). During every patient visit or when they felt uncomfortable, an ECG or Holter examination was performed to monitor atrial arrhythmia. A telephone follow-up was made once they did not attend their scheduled appointments. The deadline for follow-up was December 30, 2021.
Normally or non-normally distributed continuous variables were expressed as mean ± standard deviation or median (interquartile range). They were analyzed using an independent-sample t-test or Mann-Whitney's U-test. Categorical variables were presented as percentages (%), and differences were assessed using a chi-square test. Cumulative incidences of prespecified endpoints were calculated through the Kaplan-Meier curve, with log-rank tests comparing the differences. Cox proportional hazard models were used to compare the risks of adverse events after adjustments, with hazard ratios (HR) and 95% confidence intervals (95% CI) presented. All factors with p < 0.05 in the univariate Cox analysis were entered into the multivariate model. p < 0.05 was the threshold for statistical significance. The Statistical Package for Social Sciences (SPSS) version 26.0 was used for analysis.
A total of 596 patients with HFpEF and AF were initially enrolled. Of these, 225 cases were assigned to the rhythm-control group and 371 to the rate-control group. A 1:1 optimal PS matching with a 0.02 caliper and no replacement was conducted to reduce selection bias and potential confounding factors, yielding 177 successfully matched pairs from the entire cohort.
In the pre-matched cohort, patients who received rate-control therapies were significantly older (72.09 ± 0.52 vs. 64.78 ± 0.66, p < 0.001), more likely to suffer from NYHA class IV (13.2% vs. 7.6%, p = 0.033), coronary artery disease (24.0% vs. 8.0%, p < 0.001), hypertension (65.0% vs. 52.0%, p = 0.002), diabetes (29.9% vs. 19.6%, p = 0.005), more often took β-blocker (88.1% vs. 45.8%, p < 0.001), digoxin (27.8% vs. 3.1%, p < 0.001). In contrast, those in the rhythm-control group exhibited higher frequencies of valvular disease (44.0% vs. 30.5%, p = 0.001), had higher hemoglobin levels (137.36 ± 1.23 vs. 130.74 ± 1.07, p < 0.001), and took more warfarin (65.8% vs. 39.4%, p < 0.001). After matching, most variables were comparable among the two groups. In the PS-matched rhythm-control subgroup, the number of cases treated with CA, ECV, and SA were 93, 50, and 34, respectively. The baseline characteristics before and after PS-matching are shown in Table 1.
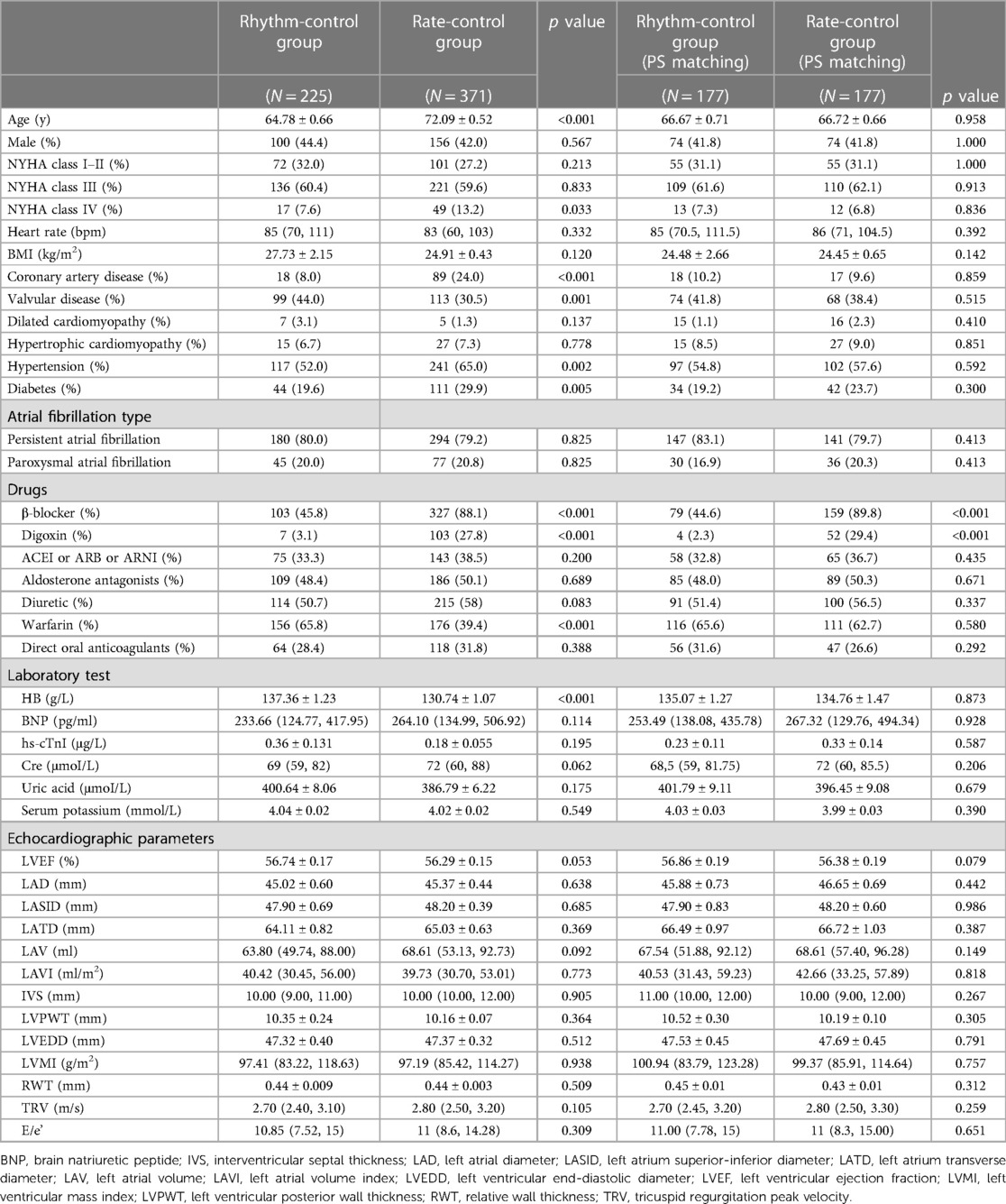
Table 1. Baseline characteristics between rhythm and rate-control groups before and after PS-matching.
Differences in follow-up echocardiographic parameters were compared between the two groups (Table 2). Compared with the rate-control group, the rhythm-control group showed more significant reductions in LA diameter (LAD) [rhythm control: from 45.88 ± 0.73 to 44.94 ± 0.68 vs. rate control: from 46.65 ± 0.69 to 48.41 ± 0.79, p = 0.001], LA superior-inferior diameter (LASID) [rhythm control: from 47.90 ± 0.83 to 47.86 ± 0.72 vs. rate control: from 48.20 ± 0.60 to 51.73 ± 0.76, p < 0.001], LA transverse diameter (LATD) [rhythm control: from 66.49 ± 0.97 to 62.50 ± 0.84 vs. rate control: from 66.72 ± 1.03 to 69.24 ± 0.99, p < 0.001], LAV [rhythm control: from 67.54 (51.88, 92.12) to 63.95 (50.07, 84.68) vs. rate control: from 68.61 (57.40, 96.28) to 82.44 (64.81, 108.86), p < 0.01] and LAVI [rhythm control: from 40.53 (31.43, 59.23) to 35.86 (30.18, 49.59) vs. rate control: from 42.66 (33.25, 57.89) to 45.68 (37.66, 62.34), p < 0.01] (Figure 2). Moreover, rhythm-control therapy was also associated with a higher incidence of LA reverse remodeling than traditional rate-control therapy [44 (24.90%) vs. 13 (7.30%), p < 0.001] (Figure 3). Other indicators, such as IVS, LVPWT, LVEDD, LVMI, E/e', TRV, and RWT, were similar between the two groups at follow-up.
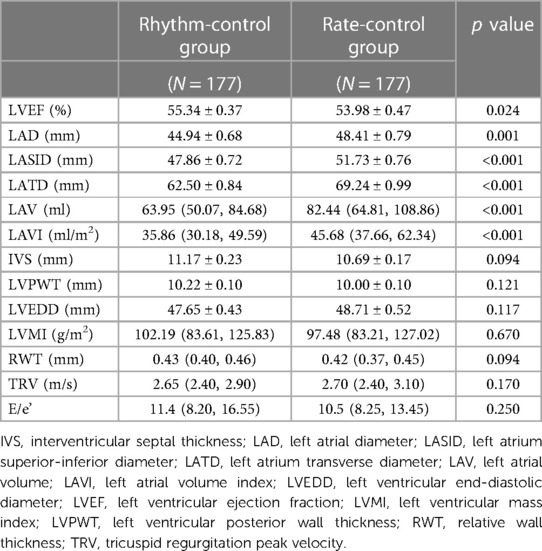
Table 2. Echocardiographic parameters between rhythm and rate-control groups after PS-matching on follow-up.
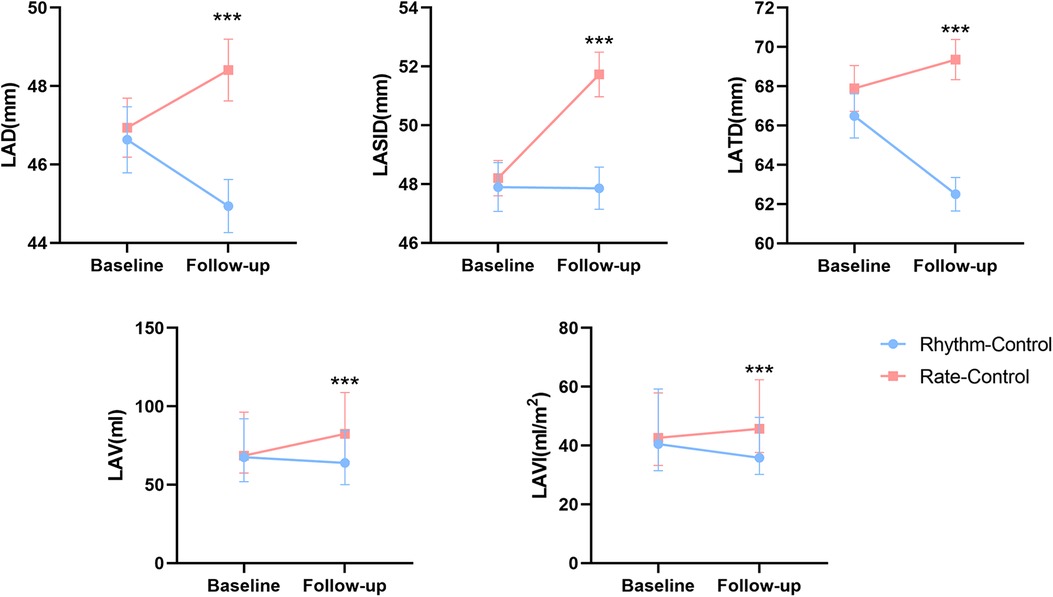
Figure 2. Changes in left atrial structure parameters between baseline and follow-up in rhythm and rate-control groups after PS-matching.
During follow-up, 25 patients died [rate-control: n = 15 (8.5%) vs. rhythm-control: n = 10 (5.6%)], and 42 cases developed stroke [rate-control: n = 27 (15.3%) vs. rhythm-control: n = 15 (8.5%)]. A total of 223 patients were rehospitalized [rate-control: n = 111 (62.7%) vs. rhythm-control: n = 112 (63.3%)], of whom 154 were readmitted for worsening HF [rate-control: n = 88 (45.8%) vs. rhythm-control: n = 66 (37.3%)]. Kaplan-Meier analysis showed that patients in the rhythm-control group had lower incidences of HF-related rehospitalization (p = 0.003) and stroke (p = 0.027) when compared to those in the rate-control group (Figure 4). Cox regression analysis also demonstrated that patients treated with rhythm-control therapy experienced lower risks of HF-related rehospitalization after adjusting for three potential confounders (adjusted HR 0.605, 95% CI 0.413–0.887, p = 0.010) but not with stroke (adjusted HR 0.589, 95% CI 0.279–1.242, p = 0.164) (Table 3). Differences in the risks of hospitalization for any cause and all-cause mortality among the two groups were insignificant either before or after adjustment (Table 3). Survival analysis was also performed to compare the efficacy of different rhythm control strategies for AF and HFpEF, aiming to identify a convenient and inexpensive approach for individuals unsuitable for CA. Surprisingly, CA did not show a remarkable superiority in reducing the risks of preset endpoints over ECV (data not shown).
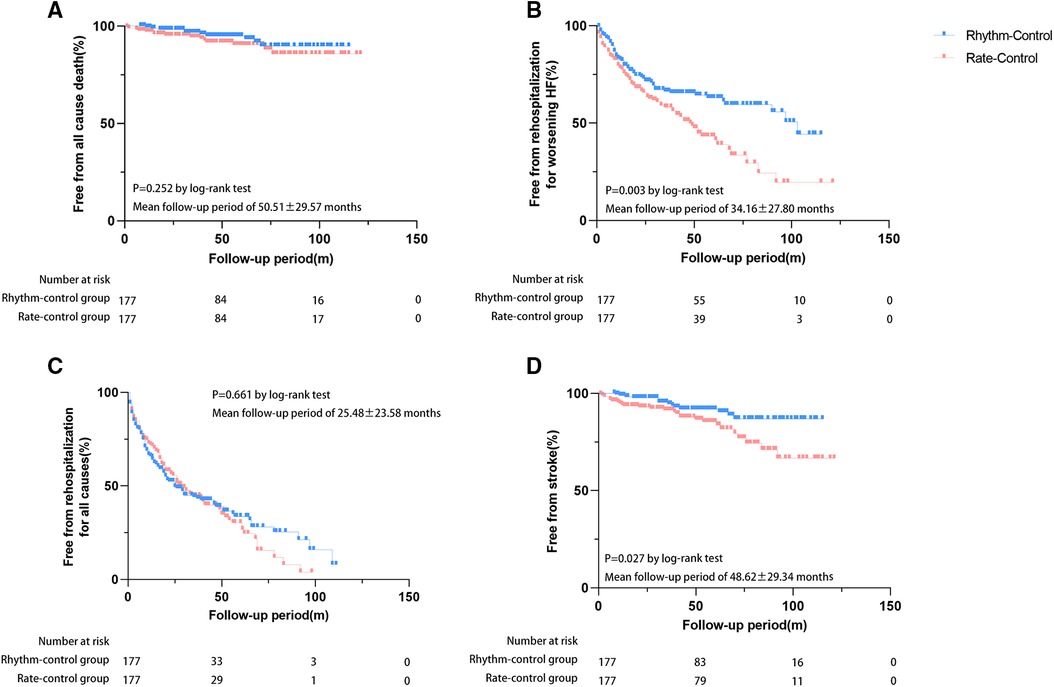
Figure 4. Risks of adverse events between rhythm and rate-control groups after PS-matching. Event-free proportions from (A) all-cause mortality, (B) HF-related rehospitalization, (C) rehospitalization for any cause, (D) stroke.
Among HFpEF and AF patients, rhythm-control treatment effectively reduced the risk of adverse endpoint. It significantly contributed to improved LA size, characterized by decreased LA volume and a higher rate of LA reverse remodeling compared to rate-control treatment.
Some extensive randomized controlled studies, such as CASTLE-AF, CABANA-HF, and EAST-AFNET4 trials, established that CA therapy for AF and HF, especially HFrEF, was superior to conventional ventricular rate medications in enhancing cardiac functions, improving the quality of life, and reducing the risks of death and admission (7, 8, 10, 12). Based on this, the latest guideline recommends that patients with deteriorating HF and symptomatic AF are encouraged to choose CA on the premise of comprehensive upstream therapy and stroke prevention (15). However, multiple pieces of evidence have shown that HFpEF significantly differs from HFrEF in pathogenesis, clinical manifestations, and prognosis (16–18). Patients with HFpEF are more female, have lower NP levels, and show a higher prevalence of atrial fibrillation and hypertension but a lower incidence of ischemic heart disease (16). Several large RCT trials have reported that, unlike HFrEF, most HFpEF patients respond poorly to conventional HF medical therapy (ACEI/ARB/ARNI, β-blocker, MRA) apart from loop diuretics and sodium-dependent glucose transporters-2 inhibitors (19–22). Recent basic research found that pyroptosis of adipocytes from epicardial adipose tissue was deeply involved in the myocardial damage in a mouse model of HFpEF, (23) implying the great importance of non-cardiac mechanisms in HFpEF. Therefore, there should also be more evidence of the clinical benefits of CA in HFpEF. Black-Maier et al. reported that CA significantly improved NYHA class and alleviated the symptoms in HFpEF and AF patients (24). A sizeable retrospective study revealed that compared with the rate control therapy, rhythm control was more effective in decreasing all-cause mortality in elderly AF and HFpEF patients after 1-year follow-up (25). Another study involving 85 HFpEF and AF patients showed that CA markedly reduced the rehospitalization risk for HF (26). Two meta-analyses showed that CA reduced the rehospitalization rate for HF and all-cause mortality in HFpEF and AF patients (27, 28). Our findings were consistent with these studies, further elucidating the clinical benefits of rhythm control in decreasing the incidence of HF-related rehospitalization for HFpEF and AF. Two other studies reported that CA decreased the risk of ischemic stroke [HR 0.69, 95% CI (0.51–0.93) and RR 0.22; CI (0.09–0.56)] (29, 30). Our study also confirmed that rhythm treatment was associated with a lower stroke hazard, even if there was no statistical difference after adjustment. However, no significant reduction in the risks of all-cause mortality and rehospitalization was observed for rhythm treatment in the study. This may be due to the small sample size, lower mortality rate, and relatively shorter follow-up period. Thus, large clinical trials are necessary to be carried out to detect the impact of rhythm treatments in these patients.
HFpEF and AF are closely associated with atrial failure, defined as any atrial dysfunction resulting in impaired HF-related symptoms and worsening life expectancy in the absence of significant valvular or ventricular abnormalities, which was proposed in 2020 (31). LA structure remodeling, manifested by LA dilation, is one of the most critical underlying mechanisms of atrial failure, leading to increased incidences of HFpEF and AF (4, 32). In turn, AF induces oxidative stress, atrial enlargement, calcium overload, inflammation, immune cell infiltration, microRNA expression, and myofibroblast activation, resulting in atrial remodeling, increasing the risk of HF and, ultimately, a vicious cycle (33–36). Thus, timely and effective intervention of AF may promote LA reverse remodeling and improve clinical endpoints. In this study, we analyzed some LA indicators and found that they were significantly decreased after rhythm-control therapy, with a higher proportion of LARR. These findings showed that rhythm treatment had a more favorable effect on reversing LA structure remodeling. Rhythm treatment may improve LA structure remodeling, greatly stabilizing hemodynamics and preventing HF deterioration.
In addition to CA, left atrial appendage closure (LAAC) is an effective strategy to reduce the risk of ischemic stroke/TIA/systemic embolism during follow-up (37). The combination of CA and LAAC has been proven beneficial to the long-term prognosis of patients with AF. A meta-analysis demonstrated that ablation with the vein of Marshall ethanol infusion (VOM-ABL) was associated with higher rates of successful mitral isthmus (MI) block and long-term freedom from AF/AT and was comparable safety compared with ablation alone in patients with AF followed over 1 year or more (38). Moreover, a significant proportion of patients cannot receive CA treatment due to various reasons (old age, over-dilated LA, multiple comorbidities, and worse cardiac functions) in clinical practice. Therefore, it is necessary to identify an alternative treatment strategy for this population. Interestingly, patients who received ECV and those subjected to CA exhibited comparable benefits in reducing the incidence of adverse events, indicating that ECV may be an effective alternative for those who cannot tolerate CA or may benefit less. These recent advancements also needed to be verified in the HFpEF population through large multi-center trials.
We admitted that there were several limitations involved in this study. Firstly, the study was neither a randomized controlled nor a large multi-center. Thus, our findings may not apply to all populations. Secondly, all participants were retrospectively identified; selection bias was inevitable. Thirdly, as there was no significant difference in the number of cases between the two groups, conducting a successful 1:1 match based on all potential confounders is unavailable, resulting in some difference in baseline data among the two groups. Fourthly, owing to financial factors and patients' wishes, implanting the cardiac monitor to record EKG for all patients may be difficult. Thus, some recurrent atrial arrhythmia might be missed. Lastly, the endpoints were mainly obtained by telephone interview, which may cause bias.
In conclusion, rhythm-control therapy reverses LA structure remodeling and is associated with improved clinical outcomes; therefore, it is an optimal therapeutic option for AF in HFpEF patients.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the institutional review board of Dalian Medical University, Liaoning, China. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired as part of a previous study for which ethical approval was obtained. All participants involved provided informed consent.
LB and YS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Writing – original draft, Writing review & editing. YS: Writing – original draft, Writing – review & editing. JS: Data curation, Formal analysis, Methodology, Writing – review & editing. ZD: Data curation, Writing – review & editing. XZ: Data curation, Writing – review & editing. YZ: Supervision, Writing – review & editing. YX: Project administration, Writing – review & editing. YL: Funding acquisition, Project administration, Resources, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This research was supported by the National Natural Science Foundation of China (grant numbers U1908209 and 82170385).
All authors have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats A. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. (2023) 118(17):3272–87. doi: 10.1093/cvr/cvac013
2. Zakeri R, Chamberlain AM, Roger VL, Redfield MM. Temporal relationship and prognostic significance of atrial fibrillation in heart failure patients with preserved ejection fraction: a community-based study. Circulation. (2013) 128(10):1085–93. doi: 10.1161/circulationaha.113.001475
3. Vermond RA, Geelhoed B, Verweij N, Tieleman RG, Van der Harst P, Hillege HL, et al. Incidence of atrial fibrillation and relationship with cardiovascular events, heart failure, and mortality: a community-based study from The Netherlands. J Am Coll Cardiol. (2015) 66(9):1000–7. doi: 10.1016/j.jacc.2015.06.1314
4. Kotecha D, Lam CS, Van Veldhuisen DJ, Van Gelder IC, Voors AA, Rienstra M. Heart failure with preserved ejection fraction and atrial fibrillation: vicious twins. J Am Coll Cardiol. (2016) 68(20):2217–28. doi: 10.1016/j.jacc.2016.08.048
5. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2022) 145(18):e895–1032. doi: 10.1161/cir.0000000000001063
6. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42(36):3599–726. doi: 10.1093/eurheartj/ehab368
7. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. (2018) 378(5):417–27. doi: 10.1056/NEJMoa1707855
8. Hunter RJ, Berriman TJ, Diab I, Kamdar R, Richmond L, Baker V, et al. A randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure (the CAMTAF trial). Circ Arrhythm Electrophysiol. (2014) 7 (1):31–8. doi: 10.1161/circep.113.000806
9. Jones DG, Haldar SK, Hussain W, Sharma R, Francis DP, Rahman-Haley SL, et al. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J Am Coll Cardiol. (2013) 61(18):1894–903. doi: 10.1016/j.jacc.2013.01.069
10. Rillig A, Magnussen C, Ozga AK, Suling A, Brandes A, Breithardt G, et al. Early rhythm control therapy in patients with atrial fibrillation and heart failure. Circulation. (2021) 144 (11):845–58. doi: 10.1161/circulationaha.121.056323
11. Prabhu S, Taylor AJ, Costello BT, Kaye DM, McLellan AJA, Voskoboinik A, et al. Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction: the CAMERA-MRI study. J Am Coll Cardiol. (2017) 70 (16):1949–61. doi: 10.1016/j.jacc.2017.08.041
12. Packer DL, Piccini JP, Monahan KH, Al-Khalidi HR, Silverstein AP, Noseworthy PA, et al. Ablation versus drug therapy for atrial fibrillation in heart failure: results from the CABANA trial. Circulation. (2021) 143 (14):1377–90. doi: 10.1161/circulationaha.120.050991
13. Pieske B, Tschöpe C, de Boer RA, Fraser AG, Anker SD, Donal E, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the heart failure association (HFA) of the European society of cardiology (ESC). Eur Heart J. (2019) 40(40):3297–317. doi: 10.1093/eurheartj/ehz641
14. Tops LF, Delgado V, Bertini M, Marsan NA, Den Uijl DW, Trines SA, et al. Left atrial strain predicts reverse remodeling after catheter ablation for atrial fibrillation. J Am Coll Cardiol. (2011) 57(3):324–31. doi: 10.1016/j.jacc.2010.05.063
15. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. (2021) 42(5):373–498. doi: 10.1093/eurheartj/ehaa612
16. Gevaert AB, Kataria R, Zannad F, Sauer AJ, Damman K, Sharma K, et al. Heart failure with preserved ejection fraction: recent concepts in diagnosis, mechanisms and management. Heart. (2022) 108(17):1342–50. doi: 10.1136/heartjnl-2021-319605
17. Drazner MH. Insights from the history and physical examination in HFpEF or HFrEF: similarities and differences. JACC Heart Fail. (2021) 9(5):398–400. doi: 10.1016/j.jchf.2021.02.009
18. Li Z, Shi Y, Xia Y, Wu L, Li H, Zhou R, et al. Disparate clinical characteristics and prognosis of HFpEF versus HFrEF phenotype of diabetic cardiomyopathy. J Clin Med. (2023) 12(4):1565. doi: 10.3390/jcm12041565
19. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, et al. Angiotensin-Neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. (2019) 381(17):1609–20.doi: 10.1056/NEJMoa1908655
20. Cleland JGF, Bunting KV, Flather MD, Altman DG, Holmes J, Coats AJS, et al. Beta-blockers for heart failure with reduced, mid-range, and preserved ejection fraction: an individual patient-level analysis of double-blind randomized trials. Eur Heart J. (2018) 39(1):26–35. doi: 10.1093/eurheartj/ehx564
21. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. (2014) 370(15):1383–92. doi: 10.1056/NEJMoa1313731
22. Vaduganathan M, Docherty KF, Claggett BL, Jhund PS, de Boer RA, Hernandez AF, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet. (2022) 400(10354):757–67. doi: 10.1016/s0140-6736(22)01429-5
23. Xia YY, Shi Y, Li Z, Li H, Wu LD, Zhou WY, et al. Involvement of pyroptosis pathway in epicardial adipose tissue—myocardium axis in experimental heart failure with preserved ejection fraction. Biochem Biophys Res Commun. (2022) 636(Pt 2):62–70. doi: 10.1016/j.bbrc.2022.10.109
24. Black-Maier E, Ren X, Steinberg BA, Green CL, Barnett AS, Rosa NS, et al. Catheter ablation of atrial fibrillation in patients with heart failure and preserved ejection fraction. Heart Rhythm. (2018) 15(5):651–7. doi: 10.1016/j.hrthm.2017.12.001
25. Kelly JP, DeVore AD, Wu JJ, Hammill BG, Sharma A, Cooper LB, et al. Rhythm control versus rate control in patients with atrial fibrillation and heart failure with preserved ejection fraction: insights from get with the guidelines-heart failure. J Am Heart Assoc. (2019) 8(24):e011560. doi: 10.1161/JAHA.118.011560
26. Fukui A, Tanino T, Yamaguchi T, Hirota K, Saito S, Okada N, et al. Catheter ablation of atrial fibrillation reduces heart failure rehospitalization in patients with heart failure with preserved ejection fraction. J Cardiovasc Electrophysiol. (2020) 31(3):682–8. doi: 10.1111/jce.14369
27. Gu G, Wu J, Gao X, Liu M, Jin C, Xu Y. Catheter ablation of atrial fibrillation in patients with heart failure and preserved ejection fraction: a meta-analysis. Clin Cardiol. (2022) 45(7):786–93. doi: 10.1002/clc.23841
28. Androulakis E, Sohrabi C, Briasoulis A, Bakogiannis C, Saberwal B, Siasos G, et al. Catheter ablation for atrial fibrillation in patients with heart failure with preserved ejection fraction: a systematic review and meta-analysis. J Clin Med. (2022) 11(2):288. doi: 10.3390/jcm11020288
29. Friberg L, Tabrizi F, Englund A. Catheter ablation for atrial fibrillation is associated with lower incidence of stroke and death: data from Swedish health registries. Eur Heart J. (2016) 37(31):2478–87. doi: 10.1093/eurheartj/ehw087
30. Rahman A, Hasani A, Moussa O, Kumar S, Jahufar F, Saeed O, et al. Efficacy of catheter ablation of atrial fibrillation in heart failure with preserved ejection fraction. J Card Fail. (2019) 25(8):S84–5. doi: 10.1016/j.cardfail.2019.07.241
31. Bisbal F, Baranchuk A, Braunwald E, Bayés de Luna A, Bayés-Genís A. Atrial failure as a clinical entity: JACC review topic of the week. J Am Coll Cardiol. (2020) 75(2):222–32. doi: 10.1016/j.jacc.2019.11.013
32. Reddy YNV, Obokata M, Verbrugge FH, Lin G, Borlaug BA. Atrial dysfunction in patients with heart failure with preserved ejection fraction and atrial fibrillation. J Am Coll Cardiol. (2020) 76(9):1051–64. doi: 10.1016/j.jacc.2020.07.009
33. Jalife J, Kaur K. Atrial remodeling, fibrosis, and atrial fibrillation. Trends Cardiovasc Med. (2015) 25(6):475–84.doi: 10.1016/j.tcm.2014.12.015
34. Gómez-Outes A, Lagunar-Ruíz J, Terleira-Fernández AI, Calvo-Rojas G, Suárez-Gea ML, Vargas-Castrillón E. Causes of death in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. (2016) 68(23):2508–21. doi: 10.1016/j.jacc.2016.09.944
35. Lam CS, Rienstra M, Tay WT, Liu LC, Hummel YM, van der Meer P, et al. Atrial fibrillation in heart failure with preserved ejection fraction: association with exercise capacity, left ventricular filling pressures, natriuretic peptides, and left atrial volume. JACC Heart Fail. (2017) 5(2):92–8. doi: 10.1016/j.jchf.2016.10.005
36. Wu LD, Li F, Chen JY, Zhang J, Qian LL, Wang RX. Analysis of potential genetic biomarkers using machine learning methods and immune infiltration regulatory mechanisms underlying atrial fibrillation. BMC Med Genomics. (2022) 15(1):64.doi: 10.1186/s12920-022-01212-0
37. Li F, Sun JY, Wu LD, Hao JF, Wang RX. The long-term efficacy and safety of combining ablation and left atrial appendage closure: a systematic review and meta-analysis. J Cardiovasc Electrophysiol. (2021) 32(11):3068–81. doi: 10.1111/jce.15230
Keywords: heart failure with preserved ejection fraction, rhythm control, atrial fibrillation, left atrial reverse remodeling, rate control
Citation: Bai L, Sun Y, Si J, Ding Z, Zhang X, Zhang Y, Xia Y and Liu Y (2023) Effects of rhythm control on left atrial structure remodeling in atrial fibrillation and heart failure with preserved ejection fraction. Front. Cardiovasc. Med. 10:1265269. doi: 10.3389/fcvm.2023.1265269
Received: 22 July 2023; Accepted: 11 September 2023;
Published: 25 September 2023.
Edited by:
Pietro Scicchitano, ASLBari—Azienda Sanitaria Localedella provincia di Bari (ASL BA), ItalyReviewed by:
Li-Da Wu, Nanjing Medical University, China© 2023 Bai, Sun, Si, Ding, Zhang, Zhang, Xia and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunlong Xia eXVubG9uZ194aWFAMTI2LmNvbQ== Ying Liu eWluZ2xpdS5tZWRAZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.