- 1Department of Ultrasound, Second Xiangya Hospital, Central South University, Changsha, China
- 2Department of Urology, Second Xiangya Hospital, Central South University, Changsha, China
Objective: Impaired elasticity of aorta has been observed in fetuses with congenital cardiac disease, while the orientation of left ventricle outflow tract has been found to influence the blood flow in the ascending aorta. Therefore, the objective of this study is to examine the left ventricle inflow and outflow tract angle (LIOA) in healthy fetuses.
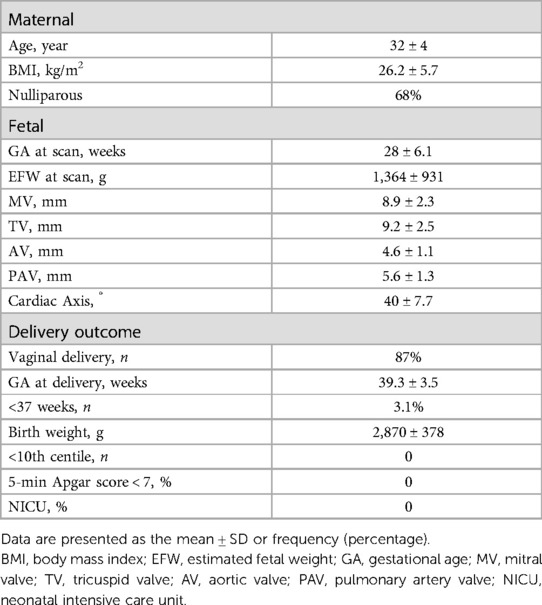
Method: A total of 668 fetuses were enrolled in this prospective study. The LIOA were measured with two-line method at left ventricle inflow and outflow tract view. Pearson's correlation coefficient was utilized to assess the associations between LIOA and estimated fetal weight (EFW) and cardiac dimensions, including cardiac axis and diameters of aortic valve (AV), pulmonary artery valve (PAV), mitral valve (MV) and tricuspid valve (TV).
Results: The LIOA was determined to be 44 ± 7.5° (mean ± SD). No significant difference was observed in the LIOA across different gestational ages (GAs). A mild positive correlation was observed between LIOA and cardiac axis. However, no significant associations were found between LIOA and parameters such as EFW, as well as diameters of AV, PAV, MV and TV.
Conclusion: The LIOA remained constant during the mid-third trimester and was mildly positively correlated with cardiac axis in normal fetuses.
1. Introduction
Impaired elasticity of ascending aorta has been demonstrated in fetuses with congenital heart defect (CHD). Our previous studies have showed decreased aortic strain (1), circumferential strain, fractional area change, and longitudinal strain (2) in the ascending aorta of fetuses with coarctation of aorta (CoA) as well as impaired aortic elastic properties in those with tetralogy of Fallot (TOF) (3). The underlying mechanism responsible for this impaired aortic elasticity in CHD fetuses is not yet fully understood. In addition to the significant histological abnormalities found in the ascending aorta (4, 5), hemodynamic disturbance caused by the inherent ascending geometry in CHD (6) may be an important contributing factor to the development of aorta elasticity.
The angel between LV inflow and outflow tract, known as the left ventricle inflow and outflow tract angle (LIOA), plays a crucial role in maintaining a normal blood flow pattern in the ascending aorta. Kauhanen et al. described this angle as the “heart-aortic angle” in adults with coronary artery disease using computed tomography angiograms. They found a smaller “heart-aortic angle” with increased total wall shear stress in the outer curvature of the proximal ascending aorta, and it was significantly associated with ascending aorta dilation (7). Besides, the curvature of ascending aorta impacted the blood flow patterns as well. Salmasi et al. found patients with left ventricular outflow tract aortic angles greater than 60o had marked asymmetric flow acceleration on the outer curvature in the proximal aorta. And higher angles associated with higher wall shear stress in the outer curve of the aorta (8). We hypothesized that this angle may also influence the elasticity of the aorta in fetal heart, but little is currently known about LIOA in fetuses. Therefore, the aim of this study was to document the normal LIOA in mid and third trimester fetuses and explore its relations with cardiac dimensions and fetal biometry.
2. Material and method
This prospective cross-sectional study was conducted in the Second Xiangya Hospital of Central South University in China. The study protocol received approval by the Ethics Committee of the Second Xiangya Hospital, and the written informed consent was obtained from all participating families. Recruitment of pregnant women took place at the Second Xiangya Hospital from January 2021 to January 2023.
The inclusive criteria for this study were as follows: (a) singleton pregnancy; (b) accurate determination of gestational age (GA) based on factors such as past menstrual regularity, precise recording of the last menstrual period, and ultrasound estimation in the first trimester consistent with the actual GA.
The exclusive criteria were as follows: (a) multiple pregnancy; (b) identifiable chromosomal abnormality; (c) cardiac or extracardiac malformations; (d) persistent fetal arrhythmia; (e) maternal complications that might change fetal hemodynamics, such as gestational diabetes, pregnancy-induced hypertension, preeclampsia, anemia, or thyroid disease; (f) fetal size outside the normal range for GA, defined as estimated fetal weight (EFW) below the 10th percentile or above the 90th percentile for fetuses of the same GA.
The obstetrical ultrasound and echocardiography were performed routinely for each fetus by a single operator using Voluson E8 or E10 system (GE Healthcare, Milwaukee, WI, USA) equipped with C2-9-D and C1-5 probes. Fetal biometry, including the biparietal diameter, head circumference, abdominal circumference, and femoral length, was measured and used to calculate the EFW (9). A standard fetal echocardiogram was conducted by an expert, who measure the dimensions of cardiac chambers and valves at their maximal size following the inner-to- inner edge model. These measurements were then automatically converted to z-scores based on GA (10). The mitral valve (MV) and tricuspid valve (TV) were obtained in the 4-chamber view (4CV) during diastole, while the aortic valve (AV) and pulmonary artery valve (PAV) were obtained in longitudinal view during systole.
The LIOA was measured at view of left ventricular inflow and outflow tract using the two-line method in systole. Briefly, the image was enlarged until the heart occupied two-thirds of the image with the apex preferably positioned upwards. The first line, indicating LV inflow tract, was positioned from the middle of the mitral valve to the apex. The second line, indicating LV outflow tract, was positioned from the center of aortic valve to the center of the ascending aorta. The acute angle formed between these two lines was recorded as LIOA (Figure 1). Additionally, the angle of fetal cardiac axis was measured using the two-line method at 4CV (11).
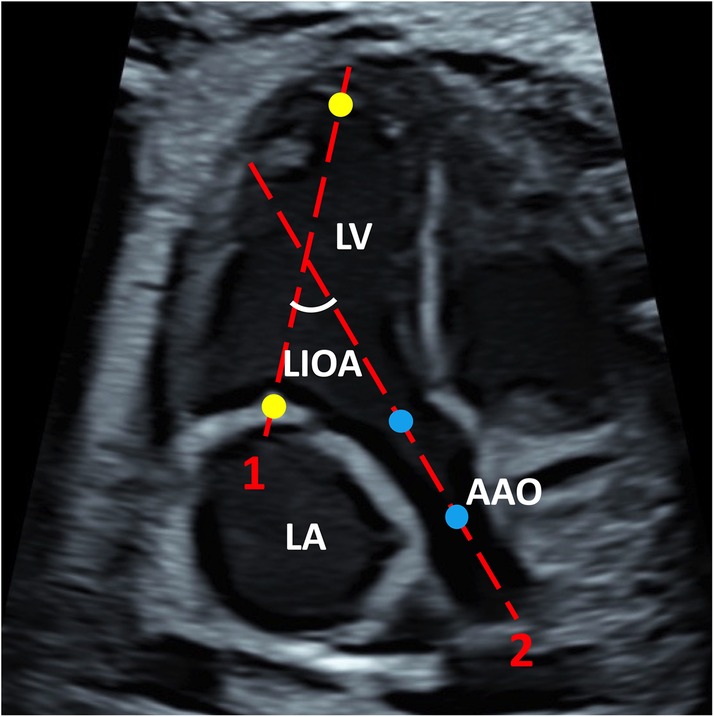
Figure 1. The representative image of LIOA measurement. The LIOA was measured at left ventricular inflow and outflow tract view. Line 1 was positioned from the middle of the mitral valve to the apex which were presented with yellow dots representatively. Line 2 was positioned from the center of aortic valve to the center of the ascending aorta which were presented with blue dots representatively. The acute angle between line 1 and 2 was recorded as LIOA. (LV, left ventricle; LA, left atrium; AAO, ascending aorta; LIOA, left ventricular inflow and outflow angle).
Continuous variables were presented as mean ± standard deviation (SD). To compare differences in the fetal LIOA among different GA, a one-way analysis of variance (ANOVA) was conducted. Pearson's correlation coefficient was utilized to examine the correlations between the fetal cardiac axis, EFW, diameters of AV, PAV, MV and TV and LIOA angle. The intra-observer and inter-observer agreement on LIOA was assessed using the intraclass correlation coefficient based on 30 randomly selected observations. A p-value of <0.05 was considered statistically significant. All statistical analyses were performed by GraphPad Prism 9 (GraphPad Software, Inc.).
3. Result
3.1. General condition of fetuses
A total of 691 fetuses, ranging in GA from 16 to 39 weeks, were initially included in this study. Out of these, 14 fetuses were excluded due to poor images quality and nine fetuses were lost to follow up, resulting in a final enrollment of 668 fetuses. The clinical characteristics of this cohort are summarized in Table 1.
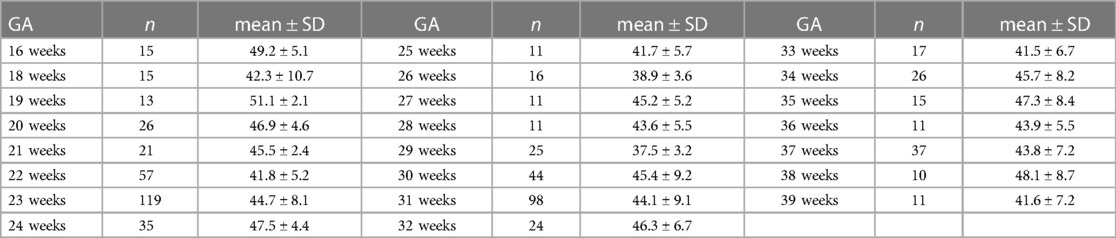
3.2. The LIOA value of fetuses in different Ga
The LIOA was successfully obtained from all fetuses, with the value of 44 ± 7.5° (mean ± SD). Table 2 presents the LIOA values for each GA from 16 to 39 weeks. By comparing the LIOA among different GAs, we observed no significant difference during the mid and third trimester (P > 0.05).
3.3. The correlation between LIOA and cardiac axis
The cardiac axis from the same group of fetuses was also documented, with value of 40 ± 7.7° (mean ± SD) (Table 1). There was no significant difference observed in the cardiac axis from 16 to 39 weeks which is consistent with previously published data (2, 3). Furthermore, the Pearson analysis suggests a mild positive correlation between cardiac axis and LIOA (r = 0.22, p < 0.0001, Figure 2).
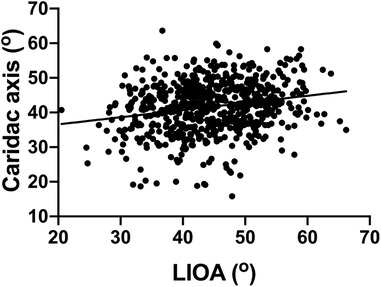
Figure 2. Positive correlation found in the LIOA and cardiac axis. The correlation between the LIOA and cardiac axis was analyzed using Pearson's correlation coefficient with the r and p-value as 0.22 and <0.0001, respectively.
3.4. No significant correlations between LIOA and EFW or cardiac dimensions
This study also investigated the correlation between LIOA and EFW as well as cardiac dimensions, including AV, PAV, MV and TV diameters. However, the findings indicated that there was no significant correlation between LIOA and either EFW or cardiac dimensions (Figure 3).
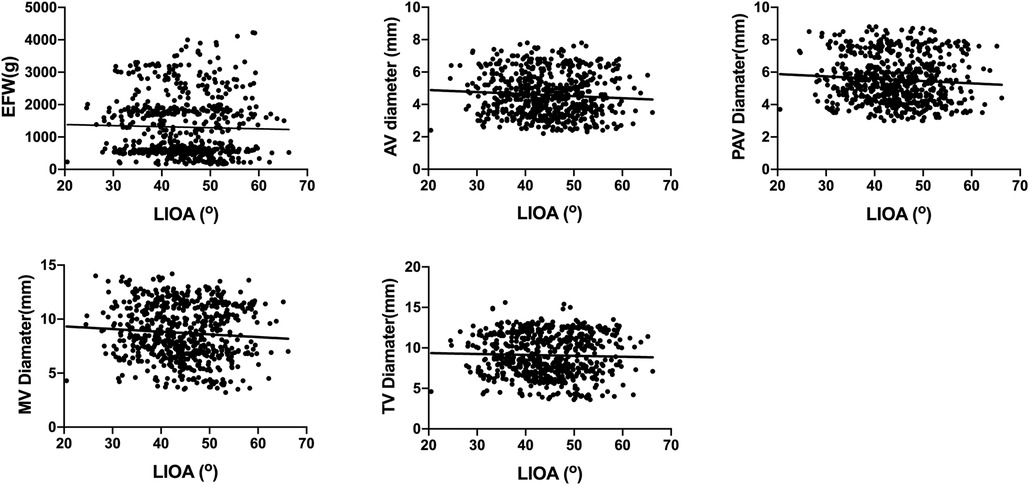
Figure 3. No significant correlations were found between LIOA and EFW or cardiac dimensions. The correlation between LIOA and EFW or cardiac dimensions was analyzed using Pearson's correlation coefficient. The r and p-value was r = −0.03, p > 0.05 for EFW, r = −0.08, p > 0.05 for AV diameter, r = −0.08, p > 0.05 for PAV diameter, r = −0.07, p > 0.05 for MV diameter and r = −0.03, p > 0.05 for TV diameter, respectively.
3.5. The intraclass correlation coefficients of inter-observer and intra-observer
The intraclass correlation coefficients of inter-observer and intra-observer for LIOA were 0.833 (95% CI, 0.721–0.910) and 0.861 (95% CI, 0.653–0.947) respectively.
4. Discussion
This is the first study to document the normal LIOA and explore its relationship with the cardiac axis in fetuses. We observed a consistent LIOA value of 44 ± 7.5° throughout gestation (16 to 39 weeks) in normal fetuses. Additionally, a mild positive correlation was found between LIOA and the cardiac axis, while no significant correlations were found between LIOA and EFW, as well as the diameters of AV, PAV, MV and TV.
In this study, the LIOA in normal fetuses was measured to be 44 ± 7.5°. However, there were several studies about the supplementary angle of LIOA in adults. For instance, Kwon et al. reported a LV-aortic root angle, which is the supplementary angle for LIOA, of 140 ± 7° in elderly healthy subjects. This angle was found to be decreased in patients with hypertrophic cardiomyopathy and showed an inverse correlation with age (12). Additionally, Kauhanen et al. investigated the heart-aorta-angle, also a supplementary angle for LIOA, and reported values of 127.9° in patients with dilated ascending aorta and 131.9° in patients with normal ascending aorta. They demonstrated that a smaller heart-aorta-angle correlated with increased total wall shear stress in the outer curvature of the proximal ascending aorta (7). The small difference observed among these angles might be attributed to variations in the age of the individual studies, as ages has been demonstrated to correlate with LV morphology (13), as well as the diameter (14) and length (15) of aorta.
In addition to our study, there was another study in fetuses on septal-aortic angle. Sternfeld et al. compared the angle between the interventricular septum and the ascending aorta between fetuses with and without cardiac anomalies (16). They reported a LVOT angle of 148.2° in normal fetuses. Interestingly, they found the LVOT angle was significantly wider in fetuses with D-transposition of the great arteries (D-TGA) and valvar aortic stenosis and narrower in fetuses with complete atrioventricular canal defect. The difference between their results and ours could attribute to the different measurement methods employed. And the LVOT angle in their study for D-TGA cases was the angle with pulmonary artery not aorta. However, there were two reasons why we choose to study the LIOA in this study rather than the septal-aortic angle. Firstly, the LIOA measurement avoids interference from the morphology of septum. Additionally, the measurement method used for LIOA in our study has been shown to have high intra-observer and inter-observer reproducibility (12). Secondly, the LIOA incorporates the orientation of blood flow from LV inflow tract, which means that LIOA may have greater impacts on hemodynamics than septal-aortic angle. However, the influence of LIOA in hemodynamics needs further investigation.
Interesting, this study indicated that the LIOA angle was mildly positively correlated to cardiac axis. The cardiac axis was 40 ± 7.7° in this study and was found to be independent of GA (11, 17, 18). The measurement of cardiac axis involved determining the angle between the long axis of septum and the antero-posterior axis of fetal chest, with the basal portion of septum being part of the LV outflow tract. Therefore, we speculated that alterations in cardiac axis may alter morphology of septum and LV outflow tract and then have an impact on LIOA.
There are certain limitations to this study should be acknowledged. Firstly, the investigate of LIOA was limited to the second and third trimesters, and data regarding LIOA in the first trimester were not included. Secondly, it's important to note that the sample sizes for certain GAs were relatively small, which may have influenced the generalizability of the findings.
5. Conclusion
In summary, this study reported a normal range of LIOA in fetuses and demonstrated its stability during the middle and third trimester. Furthermore, the LIOA was mildly positively correlated to cardiac axis and independent to EFW or cardiac dimensions, including AV, PAV, MV and TV diameters. These findings in this study may provide fundamental information for future research on aortic biomechanism in fetus with CHD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Second Xiangya Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YY: Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. RX: Conceptualization, Writing – review & editing. HT: Writing – review & editing, Data curation, Investigation, Methodology. DZ: Investigation, Methodology, Writing – review & editing. JZ: Investigation, Methodology, Writing – review & editing. SZ: Writing – review & editing, Conceptualization, Supervision.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study was supported by the National Natural Sciences Foundation of China (nos. 81871372), Natural Science Foundation of Changsha (kq2208341), and Natural Science Foundation of Hunan Province (no. 2023JJ30743). The funding body did not involve in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhou D, Xu R, Zhou J, Xie L, Xu G, Liu M, et al. Aortic elasticity and cardiac function in fetuses with aortic coarctation. Front Cardiovasc Med. (2022) 9:870683. doi: 10.3389/fcvm.2022.870683
2. Xu R, Zhou D, Liu Y, Yao L, Xie L, Liu M, et al. Impaired elastic properties of the ascending aorta in fetuses with coarctation of the aorta. J Am Heart Assoc. (2023) 12(2):e028015. doi: 10.1161/JAHA.122.028015
3. Xu R, Zhou D, Liu M, Zhou Q, Xie L, Zeng S. Impaired ascending aortic elasticity in fetuses with tetralogy of fallot. Ultrasound Obstet Gynecol. (2023) 61(4):497–503. doi: 10.1002/uog.26079
4. Niwa K, Perloff JK, Bhuta SM, Laks H, Drinkwater DC, Child JS, et al. Structural abnormalities of great arterial walls in congenital heart disease: light and electron microscopic analyses. Circulation. (2001) 103(3):393–400. doi: 10.1161/01.CIR.103.3.393
5. Tan JL, Davlouros PA, Mccarthy KP, Gatzoulis MA, Ho SY. Intrinsic histological abnormalities of aortic root and ascending aorta in tetralogy of fallot: evidence of causative mechanism for aortic dilatation and aortopathy. Circulation. (2005) 112(7):961–8. doi: 10.1161/CIRCULATIONAHA.105.537928
6. Xu R, Hou M, Zhou D, Liu Y, Xie L, Zeng S. Visualizable intracardiac flow pattern in fetuses with congenital heart defects: pilot study based on blood speckle-tracking echocardiography. Ultrasound Obstet Gynecol. (2023) 62(5):688–94. doi: 10.1002/uog.26243
7. Kauhanen SP, Liimatainen T, Kariniemi E, Korhonen M, Parkkonen J, Vienonen J, et al. A smaller heart-aorta-angle associates with ascending aortic dilatation and increases wall shear stress. Eur Radiol. (2020) 30(9):5149–57. doi: 10.1007/s00330-020-06852-3
8. Salmasi MY, Pirola S, Mahuttanatan S, Fisichella SM, Sengupta S, Jarral OA, et al. Geometry and flow in ascending aortic aneurysms are influenced by left ventricular outflow tract orientation: detecting increased wall shear stress on the outer curve of proximal aortic aneurysms. J Thorac Cardiovasc Surg. (2023) 166(1):11–21.e1. doi: 10.1016/j.jtcvs.2021.06.014
9. Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements–a prospective study. Am J Obstet Gynecol. (1985) 151(3):333–7. doi: 10.1016/0002-9378(85)90298-4
10. Schneider C, Mccrindle BW, Carvalho JS, Hornberger LK, Mccarthy KP, Daubeney PE. Development of Z-scores for fetal cardiac dimensions from echocardiography. Ultrasound Obstet Gynecol. (2005) 26(6):599–605. doi: 10.1002/uog.2597
11. Comstock CH. Normal fetal heart axis and position. Obstet Gynecol. (1987) 70(2):255–9. PMID: 3299186
12. Kwon DH, Smedira NG, Popovic ZB, Lytle BW, Setser RM, Thamilarasan M, et al. Steep left ventricle to aortic root angle and hypertrophic obstructive cardiomyopathy: study of a novel association using three-dimensional multimodality imaging. Heart. (2009) 95(21):1784–91. doi: 10.1136/hrt.2009.166777
13. Riffel JH, Mayo R, Mueller-Hennessen M, Giannitsis E, Katus HA, Andre F. Age- and gender-related reference values of cardiac morphology and function in cardiovascular magnetic resonance. Int J Cardiovasc Imaging. (2021) 37(6):2011–23. doi: 10.1007/s10554-021-02160-z
14. Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the European Society of Cardiology (ESC). Eur Heart J. (2014) 35(41):2873–926. doi: 10.1093/eurheartj/ehu281
15. Heuts S, Adriaans BP, Gerretsen S, Natour E, Vos R, Cheriex EC, et al. Aortic elongation part II: the risk of acute type a aortic dissection. Heart. (2018) 104(21):1778–82. doi: 10.1136/heartjnl-2017-312867
16. Raucher Sternfeld A, Betzer T, Tamir A, Mizrachi Y, Assa S, Bar J, et al. Can fetal echocardiographic measurements of the left ventricular outflow tract angle detect fetuses with conotruncal cardiac anomalies? Diagnostics (Basel). (2021) 11(7):1185. doi: 10.3390/diagnostics11071185
17. Mcbrien A, Howley L, Yamamoto Y, Hutchinson D, Hirose A, Sekar P, et al. Changes in fetal cardiac axis between 8 and 15 weeks’ gestation. Ultrasound Obstet Gynecol. (2013) 42(6):653–8. doi: 10.1002/uog.12478
Keywords: fetus, fetal cardiovascular, fetal echocardiography, mid-third trimester, left ventricle inflow and outflow angle
Citation: Yang Y, Xu R, Tan H, Zhou D, Zhou J and Zeng S (2023) Left ventricle inflow and outflow tract angle in normal fetuses. Front. Cardiovasc. Med. 10:1257475. doi: 10.3389/fcvm.2023.1257475
Received: 24 July 2023; Accepted: 14 November 2023;
Published: 24 November 2023.
Edited by:
Mingxing Xie, Huazhong University of Science and Technology, ChinaReviewed by:
Omar R. J. Tamimi, King Fahd Medical City, Saudi ArabiaZongjie Weng, Fujian Medical University, China
© 2023 Yang, Xu, Tan, Zhou, Zhou and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shi Zeng ZG9jdG9yenNoaUAxNjMuY29t
 Yang Yang1
Yang Yang1 Ran Xu
Ran Xu Dan Zhou
Dan Zhou Shi Zeng
Shi Zeng