- Department of Vascular and Endovascular Surgery, Second Affiliated Hospital of Naval Medical University, Shanghai, China
Background: Special instruments are needed for the revascularization of aortic branches in in situ fenestration during thoracic endovascular aortic repair (TEVAR). This prospective study compared the effectiveness and safety of three currently used fenestraters: laser, needle, and Quick Fenestrater (QF).
Methods: In all, 101 patients who underwent TEVAR for aortic disease (dissection, n = 62; aneurysm, n = 16, or ulcer, n = 23) were enrolled. All patients were randomly assigned to three groups: 34 were assigned to laser fenestration, 36 to needle fenestration, and 31 to QF fenestration. The epidemiological data, treatment, imaging findings, and follow-up outcomes were analyzed using data from the medical records.
Results: The technical success rates of the laser, needle, and QF fenestration groups were 94.1%, 94.4%, and 100% (p > 0.05). After correction of mixed factors such as age and gender, it was showed the average operative time (Laser group: 130.01 ± 9.36 min/ Needle group: 149.80 ± 10.18 min vs. QF group: 101.10 ± 6.75 min, p < 0.001), fluoroscopy time (Laser group: 30.16 ± 9.81 min/ Needle group: 40.20 ± 9.91 min vs. QF group: 19.91 ± 5.42 min, p < 0.001), fenestration time (Laser group 5.50 ± 3.10 min / Needle group 3.50 ± 1.50 min vs. QF group 0.67 ± 0.06 min, p < 0.001), and guide wire passage time after fenestration (Laser group 5.10 ± 1.70 min / Needle group 4.28 ± 1.60 min vs. QF group 0.07 ± 0.01 min, p < 0.001) were all shorter with QF fenestration than with the other two tools. The overall perioperative complication rates of the laser, needle, and QF fenestration groups were 5.9%, 5.6%, and 0% (p > 0.05): One case of sheath thermal injury and one case of vertebral artery ischemia occurred in the laser fenestration group; one case each of access site hematoma and brachial artery thrombosis were reported in the needle fenestration group. 89 (88.1%, 89/101) patients were followed for a median of 12.6 ± 1.6 months. The overall postoperative complication rates of the laser, needle, and QF fenestration groups were 3.3%, 6.5%, and 0% (p > 0.05): In the laser fenestration group, there was one death due to postoperative ST-segment elevation myocardial infarction; in the needle fenestration group, one patient developed occlusion of the bridge stent; no complications occurred in the QF group.
Conclusion: All three fenestration methods were effective in reconstructing supra-arch artery during TEVAR. QF fenestration required less contrast agent, with a shorter surgery duration and fewer complications than laser and needle fenestration.
1. Introduction
Aortic aneurysm, aortic ulcer, and aortic dissection are the major thoracic aortic diseases. These diseases greatly affect public health (1, 2). Thoracic endovascular aortic repair (TEVAR) is an effective method for the treatment of such diseases. However, aortic diseases involving supra arch branches are considered contraindications to TEVAR due to the retention of the supra arch branches (3–5). In situ fenestration can extend the sealing zone during TEVAR and has shown the potential for the revascularization of aortic branches (6–8). Three instruments are generally used in in situ fenestration during TEVAR—laser, needle, and “Quick Fenestrater” (QF) (9). In 2004, Mc Williams et al. (10) reported satisfactory clinical results with reconstruction of the left subclavian artery by in situ fenestration with a reversed end of a 0.018-inch guidewire. In 2009, Murphy et al. (11) first applied laser fenestration technology during TEVAR to preserve the left subclavian artery. There are increasing applications of in situ fenestration technology, and it is considered the most consistent technology with human anatomy and hemodynamics and is a safe and effective method (12, 13).
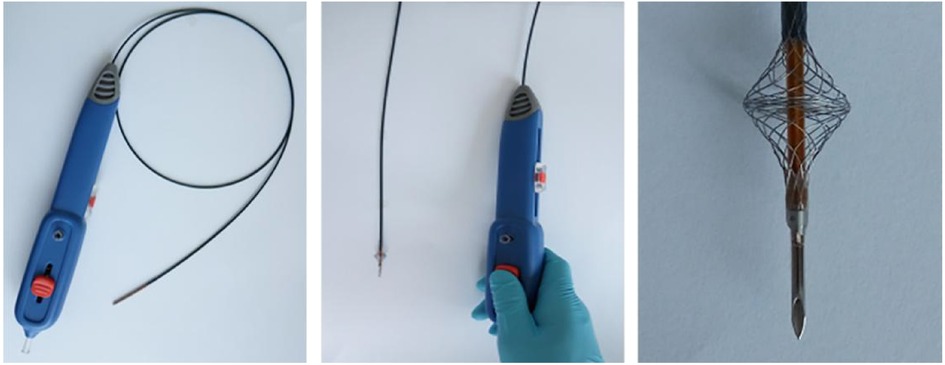
Both needle and laser in situ fenestration have limitations, such as injury to the contralateral aortic wall, long fenestration time, thermal injury, and invisible head end of the guide wire of laser fenestration (14). Therefore, our team pioneered a device, “Quick Fenestrater” (QF) specifically used for in situ fenestration of supra aortic vessels (Figure 1) (15). In our study, we showed that QF was a safe and effective method, but we did not compare the advantages and disadvantages of the three fenestration methods. In this study, we compared the effectiveness and safety of laser, needle, and QF in situ fenestration during the perioperative period and 1-year follow-up.
2. Methods
2.1. Patient enrollment
In this prospective cohort study, 101 patients were enrolled from December 2016 to October 2019 at the Department of Vascular Surgery in Shanghai Changzheng Hospital. The inclusion criteria were (1) age above 18 years, (2) at least 1 supra-aortic branch encroached by thoracic lesions, (3) landing zone not long enough (≤15 mm) for fixation of the aortic endograft so coverage of the LSA or LCCA had to be performed, (4) aortic disease confirmed by at least 1 radiologic examination (e.g., computed tomography angiography [CTA], magnetic resonance angiography [MRA]), (5) patent supra-aortic branch, and (6) type I/II aortic arch. The exclusion criteria were (1) cardiopulmonary and renal insufficiency contraindicating general anesthesia (according to the anesthesiologist), (2) severe infection causing high fever or organ dysfunction, (3) allergy to contrast medium, (4) adverse cardiovascular or cerebrovascular event within 3 months before intervention, (5) occlusion or stenosis of the supra-aortic branch or severe twisting of the arteries to be fenestrated, (6) no appropriate peripheral access, (7) Stanford A aortic lesions, (8) type III aortic arch and (9) Marfan syndrome. The protocol for this study was approved by the participating hospital’s Ethics Committee, and informed consent was obtained from each participant.
2.2. Surgical procedure
The enrolled patients underwent in situ laser, needle, or QF fenestration according to their order of admission. All interventions were performed by advanced interventional radiologists. The procedures were performed under general anesthesia. Two ProGlide vascular sutures (Perclose ProGlide, Abbott Inc, USA) were preset for right femoral artery puncture, and a 12-F sheath was inserted. A 7-F sheath (pre-left carotid artery fenestration) was punctured and inserted via the left common carotid artery, and a 6-F 55 cm renal artery sheath (Flexor® Check-Flo® Introducer, Cook, Inc, USA) was punctured and inserted into the left brachial artery. A 5-F labeled pig-tail catheter was introduced into the sheath of the right femoral artery to the ascending aorta, and the aortic size was measured. A Lunderquist super-stiff guide wire was inserted, and the pigtail catheter was withdrawn to the ascending aorta through the left brachial artery or carotid artery sheath for real-time intraoperative angiography. After angiographic evaluation of the aortic lesions, aortic stents of appropriate size (Capitivia, Medtronic, USA/Ankura, Lifetech, China) were introduced along the super stiff guide wire, accurately positioned, and released to repair thoracic aortic lesions and cover the supra arch branch arteries. Advanced through the 0.035 inch support catheter, the needle (reversed end of a 0.018 inch guidewire) or intravenous laser catheter (LFK-SLT30, Rafcon, China) or QF (Suzhou Innomed Medical Device, China) was introduced along the supra arch branch artery and guided to the branch artery for stent graft fenestration. After fenestration, the needle, intravenous laser catheter, or QF was advanced into the aortic lumen. The fenestration instruments were withdrawn, and another angiogram was made to confirm that the needle, intravenous laser catheter, or QF was positioned in the aortic cavity, and a 0.035 inch guide wire was introduced along the catheter to the ascending or descending aorta. A balloon (Mustang, Boston Scientific Corporation, USA) of appropriate size to expand the fenestration was positioned. The stent deployed in the super arch artery had a 5 mm protrusion in the aortic arch, and the distal end in left subclavian artery did not cover the orifice of the vertebral artery. Post dilatation was carried out if necessary. Finally, aortography was performed to check that the intervention was carried out successfully.
2.3. Outcomes
After the intervention, follow-up was conducted at 1 month, 6 months, and annually thereafter. The following perioperative outcomes were assessed: success rate of fenestration; average durations of intervention, fluoroscopy, rupture, and guide wire passage after fenestration (from guide wire touching the membrane to passing through the hole); and amount of contrast agent used. Follow-up outcomes included 30-day mortality, type I and III endoleaks, retrograde dissection, mortality, stent patency rate, stent morphology, secondary re-interventions, and severe cardiovascular and cerebrovascular events. Computed tomographic angiography of the aorta was performed to evaluate the rates of branch stenting, internal leakage rate, and secondary intervention.
2.4. Statistical analysis
The data were analyzed with SPSS 22.0 software. The data were expressed as mean values ± SD. Continuous variables were presented as median and range in the case of nonparametric distributions, and comparisons were made using the Mann-Whitney test. Continuous variables are presented as means ± standard deviations in cases of parametric distributions, and comparisons were made using the independent t-test. Categorical variables were compared using the Chi-square test or Fisher’s exact test and reported as frequencies (percentages). A p value of <0.05 was considered statistically significant.
3. Results
3.1. Patient demographics and presentation
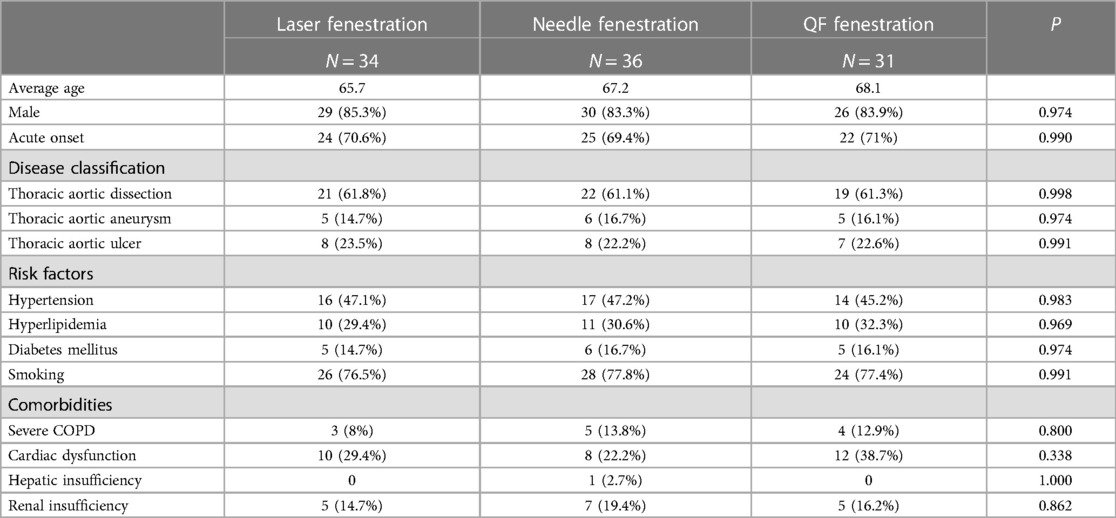
In all, 101 patients underwent in situ fenestration during TEVAR between May 2016 and October 2019 (85 males and 16 females; mean age, 67 ± 10 years; 71 acute onset). In all, 34 patients underwent 40 in situ laser fenestrations during TEVAR (aortic dissection: n = 21, aortic aneurysm: n = 5, aortic ulcer: n = 8); 36 patients underwent 43 in situ needle fenestrations during TEVAR (aortic dissection: n = 22, aortic aneurysm: n = 6, aortic ulcer: n = 8); and 31 patients underwent 37 in situ QF fenestrations during TEVAR (aortic dissection: n = 19, aortic aneurysm: n = 5, aortic ulcer: n = 7). The baseline characteristics, including risk factors and comorbidities, were not significantly different among the three groups (Table 1).
3.2. Operative data
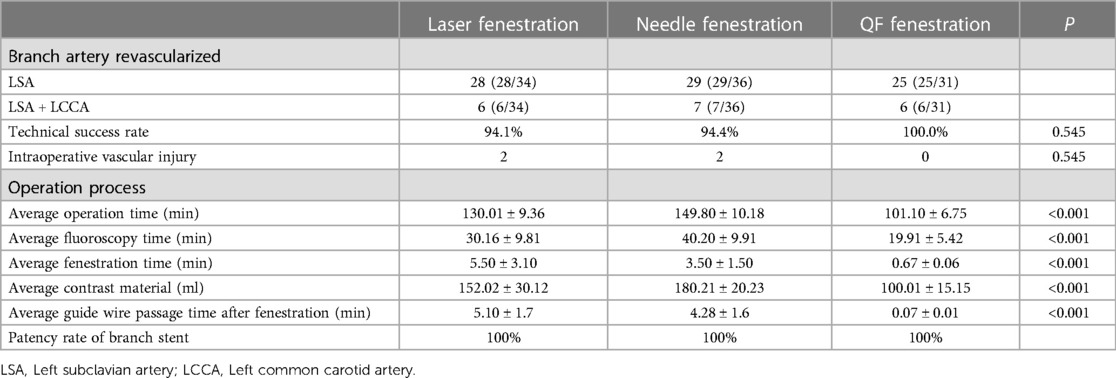
TEVAR was technically successful in 94 patients (Figure 2). The technical success rates of the laser, needle, and QF fenestration groups were 94.1%, 94.4%, and 100%, respectively, showing an insignificant difference (p > 0.05). In the in situ laser fenestration group, one procedure failed due to an awkward fenestration angle of the laser guide wire. In the in situ needle fenestration group, one procedure failed because the needle had failed to puncture the membrane. After correction of mixed factors such as age and gender, variance analysis and Kruskal-Wallis test results showed the average operation, fluoroscopy, fenestration, and guide wire passage durations after fenestration were shorter in the QF fenestration group (p < 0.001). Moreover, the average amount of contrast agent in the QF fenestration group was less than that in the needle fenestration and laser fenestration group (p < 0.001) (Table 2).
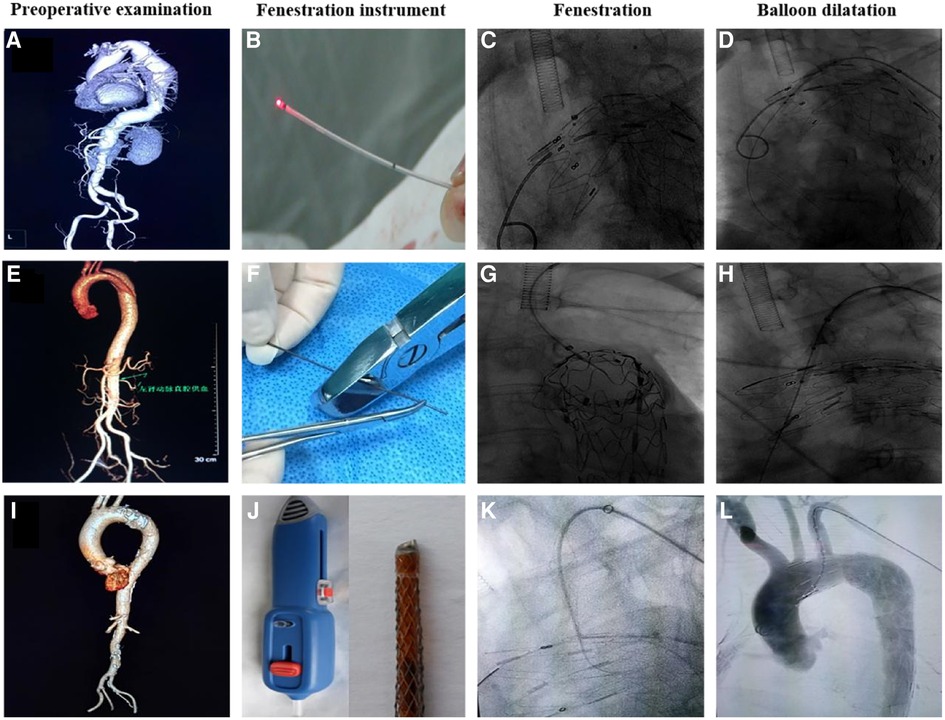
Figure 2. Preoperative examination, fenestration instrument, fenestration, and balloon dilatation using three different fenestration methods. (A–D) Laser fenestration, (E–H) needle fenestration, (I–L) QF fenestration.
All the in situ fenestrated arteries were found to be patent on postoperative follow-up CTA imaging and clinical symptoms.
3.3. Clinical follow-up
The overall Perioperative complication rates of the laser, needle, and QF fenestration groups were 5.9%, 5,6%, and 0% (p > 0.05). In the perioperative period, no cerebral infarction, myocardial infarction, transient ischemic attacks, or other neurologic complications occurred. In the in situ laser fenestration group, one patient had a sheath injury due to laser heat (Figure 3A), and one patient presented with intraoperative vertebral artery ischemia. In the in situ needle fenestration group, one patient presented with an access-site hematoma, and one patient presented with a femoral arterial thrombus. No endoleak (include type I and type III), false lumen thrombosis, or subsequent remodeling of the aorta was evident.
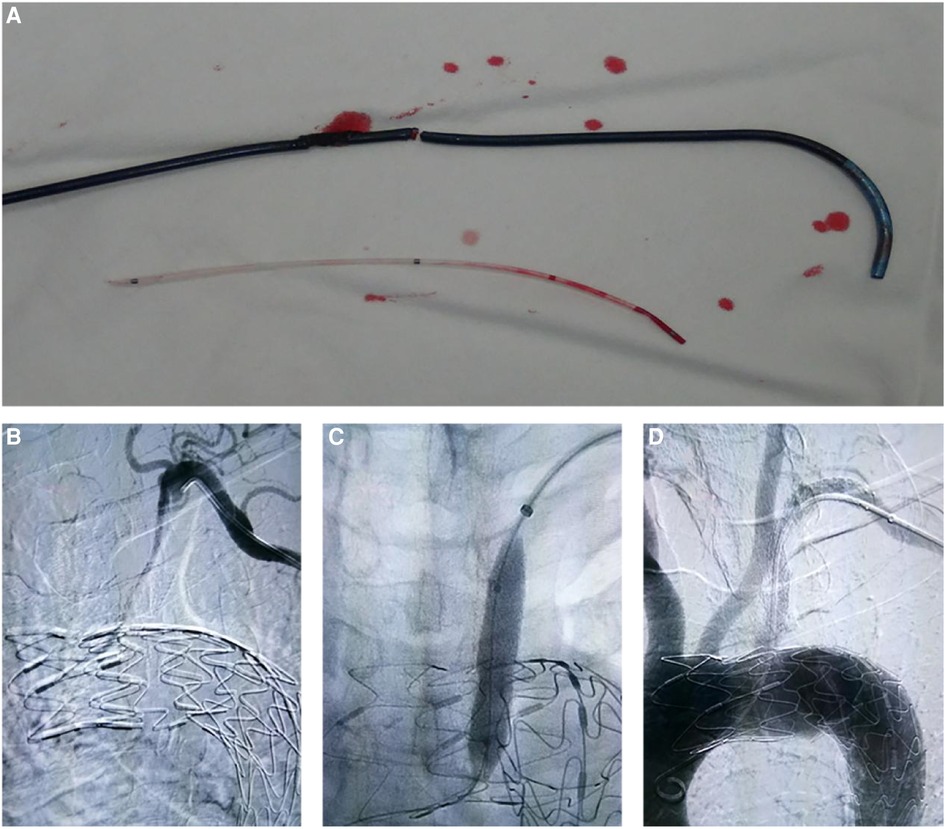
Figure 3. Complications in laser and needle fenestration groups. (A) In the laser fenestration group, one patient sustained sheath injury caused by laser ablation. (B–D) In the needle fenestration group, one patient developed subclavian artery occlusion after the intervention, but it was recanalized.
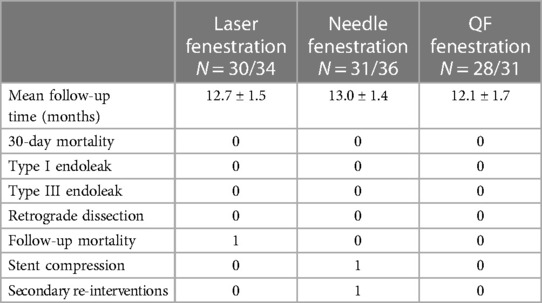
A total of 89 (88.1%, 89/101) patients were followed for a median of 12.6 ± 1.6 months. At the 1-year follow-up, the overall postoperative complication rates of the laser, needle, and QF fenestration groups were 3.3%, 6.5%, and 0% (p > 0.05). In the laser fenestration group, there was one death due to postoperative ST-segment elevation myocardial infarction, with no direct association with the intervention. In the needle fenestration group, one patient developed occlusion of the bridge stent at 3-month follow-up. The occlusion was recanalized after intervention (Figure 3B–D). There were no complications in the QF group. No reverse tear or endoleak (type I and type III) was observed in any of the three groups (Table 3, Figure 4).
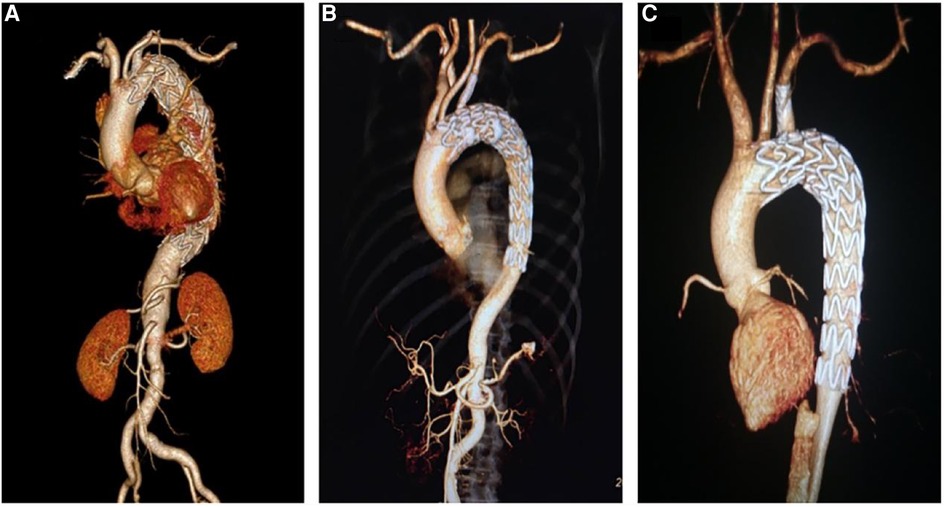
Figure 4. Postoperative computed tomography angiography of three different fenestration methods. (A) Laser fenestration, (B) needle fenestration, (C) quick fenestration.
4. Discussion
The fenestration methods used in TEVAR include mechanical fenestration, represented by self-made guide wires or puncture needles, and thermal fenestration, represented by laser/radio frequency. Our center developed a special fenestration instrument, the “Quick Fenestrater” (QF), which has demonstrated safety and efficacy in a previous single-center clinical study (15). On analyzing the data of the QF, laser, and needle fenestration groups, we found that (1) all three methods were effective for in situ fenestration of the superior arch artery, each with a technical success rate of over 90%; the QF fenestration rate was 100% and (2) while there was no significant difference in the average durations of operation, fluoroscopy, fenestration, and guide wire passage after fenestration and volume of contrast agent used between the needle and laser fenestration groups, the lowest measurements were observed in the QF group (P < 0.001). (3) There were no significant differences in the endoleak, postoperative all-cause mortality, secondary surgical intervention, and branch stent patency rates among the three groups during follow-up.
In situ fenestration during TEVAR is a safe and effective method for endovascular reconstruction of the branch arteries above the aortic arch (16–19). In 2016, in a meta-analysis of 118 articles conducted by Crawford et al., the success rate of in situ fenestration was 96%, and the rate of comprehensive complications (death, stroke, and paraplegia) was 7% (20). In situ fenestration has the advantages of rapid fenestration, repeatability, minimally invasiveness, and few complications.
Nevertheless, during the study of fenestration instruments, we found some undesirable features: needle fenestration requires manual or other types of puncture needles (e.g., biopsy needles); puncture needles have high rigidity and poor flexibility and cannot pass through the twisted subclavian artery, resulting in a relatively long fenestration time (21). Moreover, the direction of the puncture tip is difficult to adjust, and there is a risk of damaging the arterial intima and puncturing the opposite vessel wall (22–24). In this study, in the needle fenestration group, one patient developed an occlusion on the bridging stent at the 3-month follow-up; perhaps, because of the large torsion angle of the subclavian artery, the intima was damaged by the needle, resulting in a dissection. During recanalization, angiography showed that the stent itself was in good shape, and the dissection was seen at the tortuous subclavian artery at the distal end of the stent; the arterial dissection was repaired by balloon expansion and implantation of a bare stent.
Laser in situ fenestration is invisible under fluoroscopy at the head end, and the direction of the head end is difficult to adjust, resulting in the risk of thermal damage (25, 26). In the laser group, thermal injury of sheath due to the supporting catheter damaged the intima of the subclavian artery and caused thrombosis. After laser fenestration, the laser fiber must be pushed out and the guide wire must be exchanged; in this process, because the fenestration window is small and invisible, exchanging the guide wire often takes time; if and when it is difficult to pass through, the membrane must be ruptured with laser again to enlarge the window (27, 28). In the laser fenestration group, one patient, treated with local anesthesia, had sudden transient ischemia of the vertebral artery, and the symptoms resolved after the fenestration was completed. According to previous reports, the laser could lead to the formation of air bubbles, and the bubbles could enter cerebral blood vessels and cause a stroke, which could have occurred in our case (29).
In contrast to other fenestraters, QF can fix the access point in the central area of the blood vessel through the support bracket at the front end and ensure the fenestration is relatively parallel to the vessel wall. QF uses a needle with adjustable strength and depth control to quickly fenestrate, thus preventing vascular damage. After successful fenestration, the guide wire, preset from the needle lumen, can quickly enter the aortic lumen to complete the fenestration process. The whole process is simple, smooth, and time saving. In summary, QF has significant advantages over the other two fenestration methods.
The shape of the aortic arch and the angle of the branch blood vessels affect the success rate of the fenestration. Theoretically, fenestration perpendicular to the membrane has the highest success rate. For laser fenestration, the laser head must be placed on the stent graft. Given the arch type and angle of branch vessels, the laser head may slip off, resulting in failure of the fenestration. Needle fenestration faces the same problem. Moreover, the head ends of the needle and laser fibers are uncontrollable and their angles cannot be changed (24, 30). Because of these disadvantages of needle and laser fenestration, we developed the QF to fix the access point in the central area of the blood vessel through the support bracket at the front and use the membrane-perforating needle with adjustable strength and controllable depth to quickly fenestrate and minimize the risk of vascular damage. By optimizing the operation and simplifying the operation steps, the success rate of fenestration is increased, and the operation time is reduced. In the present comparison of QF, needle, and laser fenestration, we found that by reducing the intervention time, QF fenestration can reduce the amount of contrast agent used and the operator’s radiation exposure, so that complications in high-risk patients, such as renal insufficiency, are reduced.
5. Conclusions
Our comparative study of the effectiveness of the recently designed QF, needle, and laser as fenestration tools in endovascular aortic arch repair showed that the QF was associated with a shorter surgical duration, lower volume of contrast agent used, and better safety. At the follow-up, there were no complications in the patients who underwent endovascular aortic arch repair with the QF.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Clinical Test Ethics Committee of Shanghai Changzheng Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LQ contributed to the study conceptualization and design. XW, JB, and JW performed the data analysis and interpretation. XW, JB, and SZ collected the data. SZ conducted the statistical analysis. XW, JW, and KZ drafted the article. JB and LQ made critical revisions to the article. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (81870347, 82370498); Program of Shanghai Academic Research Leader (20XD1404900); Basic Research Cross Science Project of the Shanghai Science and Technology Commission’s “Science and Technology Innovation Action Plan” (21JC1406500); and High-level Achievement Training Program of Changzheng Hospital (2020YCGPZ-205).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor JF declared a shared parent affiliation with the authors at the time of the review.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kölbel T, Diener H, Larena-Avellaneda A, Debus S. Advanced endovascular techniques for thoracic and abdominal aortic dissections. J Cardiovasc Surg (Torino). (2013) 54(Suppl 1):81–90.
2. Waterford SD, Chou D, Bombien R, Uzun I, Shah A, Khoynezhad A. Left subclavian arterial coverage and stroke during thoracic aortic endografting: a systematic review. Ann Thorac Surg. (2016) 101:381–9. doi: 10.1016/j.athoracsur.2015.05.138
3. Hui DS, Fleischman F. Diagnostic and treatment dilemmas of the aortic arch. Cardiol Clin. (2017) 35:347–55. doi: 10.1016/j.ccl.2017.03.012
4. Girsowicz E, Steinmetz O. A challenging proximal aortic arch endovascular repair. Eur J Vasc Endovasc Surg. (2021) 61:190. doi: 10.1016/j.ejvs.2020.08.020
5. Harrington DK, Walker AS, Kaukuntla H, Bracewell RM, Clutton-Brock TH, Faroqui M, et al. Selective antegrade cerebral perfusion attenuates brain metabolic deficit in aortic arch surgery: a prospective randomized trial. Circulation. (2004) 110(Suppl 1):II231–6. doi: 10.1161/01.CIR.0000138945.78346.9c
6. Hongku K, Dias N, Sonesson B, Resch T. Techniques for aortic arch endovascular repair. J Cardiovasc Surg (Torino). (2016) 57:421–36.26940011
7. Riga CV, McWilliams RG, Cheshire NJ. In situ fenestrations for the aortic arch and visceral segment: advances and challenges. Perspect Vasc Surg Endovasc Ther. (2011) 23:161–5. doi: 10.1177/1531003510388421
8. Strauch JT, Spielvogel D, Lauten A, Galla JD, Lansman SL, McMurtry K, et al. Technical advances in total aortic arch replacement. Ann Thorac Surg. (2004) 77:581–9; discussion 589–90. doi: 10.1016/S0003-4975(03)01342-0
9. Bai J, Liu Y, Jin J, Li J, Ji X, Qu L. Single-stage endovascular management of complicated thoracic aorta coarctation concurrent with aortic arch aneurysm using a novel fenestration device. J Thorac Dis. (2018) 10:2474–80. doi: 10.21037/jtd.2018.03.162
10. McWilliams RG, Murphy M, Hartley D, Lawrence-Brown MM, Harris PL. In situ stent-graft fenestration to preserve the left subclavian artery. J Endovasc Ther. (2004) 11:170–4. doi: 10.1583/03-1180.1
11. Murphy EH, Dimaio JM, Dean W, Jessen ME, Arko FR. Endovascular repair of acute traumatic thoracic aortic transection with laser-assisted in-situ fenestration of a stent-graft covering the left subclavian artery. J Endovasc Ther. (2009) 16:457–63. doi: 10.1583/09-2746.1
12. Lin J, Rodriguez LE, Nutley M, Jun L, Mao Y, Parikh N, et al. Optimal in situ fenestration technique with laser perforation and balloon dilation for aortic stent-grafts. J Endovasc Ther. (2021) 28:300–8. doi: 10.1177/1526602820981980
13. Song J, Qian J, Duan Q, Dong A, Kong M. The effect of in situ laser fenestration for total endovascular arch repair in redo aortic dissection. Vascular. (2022) 30:1044–50. doi: 10.1177/17085381211041474
14. Zeng Q, Zhou X, He Y, Wang X, Shang T, He Y, et al. Experimental analysis of in situ fenestration of endovascular stent-grafts: comparison between needle and laser puncture. Ann Vasc Surg. (2021) 77:280–7. doi: 10.1016/j.avsg.2021.05.021
15. Bai J, Wang C, Liu Y, Jin J, Wu J, Ji X, et al. A novel fenestrating device: quick fenestrater for reconstructing supra-aortic arteries in situ during thoracic endovascular aortic repair. Can J Cardiol. (2021) 37:1539–46. doi: 10.1016/j.cjca.2021.04.024
16. Xiang Y, Wu Z, Zhang H. Progress on in situ fenestration during thoracic endovascular aortic repair. Zhejiang Da Xue Xue Bao Yi Xue Ban. (2018) 47:617–22. doi: 10.3785/j.issn.1008-9292.2018.12.09
17. Glorion M, Coscas R, McWilliams RG, Javerliat I, Goëau-Brissonniere O, Coggia M. A comprehensive review of in situ fenestration of aortic endografts. Eur J Vasc Endovasc Surg. (2016) 52:787–800. doi: 10.1016/j.ejvs.2016.10.001
18. Li Y, He C, Chen X, Yao J, Zhang T, Zhang H. Endovascular in situ fenestration technique of aortic arch pathology: a systematic review and meta-analysis. Ann Vasc Surg. (2021) 76:472–80. doi: 10.1016/j.avsg.2020.12.021
19. Shu C, Fan B, Luo M, Li Q, Fang K, Li M, et al. Endovascular treatment for aortic arch pathologies: chimney, on-the-table fenestration, and in-situ fenestration techniques. J Thorac Dis. (2020) 12:1437–48. doi: 10.21037/jtd.2020.03.10
20. Crawford SA, Sanford RM, Forbes TL, Amon CH, Doyle MG. Clinical outcomes and material properties of in situ fenestration of endovascular stent grafts. J Vasc Surg. (2016) 64:244–50. doi: 10.1016/j.jvs.2016.03.445
21. Li DL, Zeng QL, Xiang YL, Qiu CY, Li ZJ, He YY, et al. Experimental analysis of the quality of needle-assisted fenestration in aortic stent-grafts and the differences between gradual and rapid balloon dilation. J Endovasc Ther. (2021) 28:44–52. doi: 10.1177/1526602820947095
22. Luo M, Fang K, Fan B, Li Q, Li M, He H, et al. Midterm results of retrograde in situ needle fenestration during thoracic endovascular aortic repair of aortic arch pathologies. J Endovasc Ther. (2021) 28:36–43. doi: 10.1177/1526602820953406
23. Xiang Y, Qiu C, He Y, Li D, Shang T, Wu Z, et al. A single center experience of in situ needle fenestration of supra-aortic branches during thoracic endovascular aortic repair. Ann Vasc Surg. (2019) 61:107–15. doi: 10.1016/j.avsg.2019.03.016
24. Riga CV, Bicknell CD, Basra M, Hamady M, Cheshire NJ. In vitro fenestration of aortic stent-grafts: implications of puncture methods for in situ fenestration durability. J Endovasc Ther. (2013) 20:536–43. doi: 10.1583/12-4175.1
25. Sonesson B, Dias N, Abdulrasak M, Resch T. Midterm results of laser generated in situ fenestration of the left subclavian artery during thoracic endovascular aneurysm repair. J Vasc Surg. (2019) 69:1664–9. doi: 10.1016/j.jvs.2018.09.052
26. Liu G, Qin J, Cui C, Zhao Z, Ye K, Shi H, et al. Endovascular repair of aortic arch intramural hematoma and penetrating ulcers with 810nm in situ laser-assisted fenestration: preliminary results of a single-center. Lasers Surg Med. (2018) 50:837–43. doi: 10.1002/lsm.22937
27. Yan D, Shi H, Qin J, Zhao Z, Yin M, Liu X, et al. Outcomes of emergency in situ laser fenestration-assisted thoracic endovascular aortic repair in patients with acute stanford type A aortic dissection unfit for open surgery. J Vasc Surg. (2020) 71:1472–9.e1. doi: 10.1016/j.jvs.2019.08.233
28. Murphy EH, Stanley GA, Ilves M, Knowles M, Dimaio JM, Jessen ME, et al. Thoracic endovascular repair (TEVAR) in the management of aortic arch pathology. Ann Vasc Surg. (2012) 26:55–66. doi: 10.1016/j.avsg.2011.08.009
29. Qin J, Zhao Z, Wang R, Ye K, Li W, Liu X, et al. In situ laser fenestration is a feasible method for revascularization of aortic arch during thoracic endovascular aortic repair. J Am Heart Assoc. (2017) 6:e004542. doi: 10.1161/JAHA.116.004542
Keywords: aortic arch, aortic disease, in situ fenestration, thoracic endovascular aortic repair (TEVAR), quick fenestrater (QF)
Citation: Wang X, Wu J, Zhi K, Zou S, Jin J, Bai J and Qu L (2023) Comparative effectiveness and safety of laser, needle, and “quick fenestrater” in in situ fenestration during thoracic endovascular aortic repair. Front. Cardiovasc. Med. 10:1250177. doi: 10.3389/fcvm.2023.1250177
Received: 29 June 2023; Accepted: 15 September 2023;
Published: 29 September 2023.
Edited by:
Jiaxuan Feng, Second Military Medical University, ChinaReviewed by:
Jia Hu, Sichuan University, ChinaMaruti Haranal, U N Mehta Institute of Cardiology and Research, India
Wolf-Hans Eilenberg, Medical University of Vienna, Austria
© 2023 Wang, Wu, Zhi, Zou, Jin, Bai and Qu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Bai YmphdDIwMTBAeWVhaC5uZXQ= Lefeng Qu cXVsZWZlbmdAc21tdS5lZHUuY24=
†These authors contributed equally to this work
 Xiaokai Wang†
Xiaokai Wang† Jianjin Wu
Jianjin Wu Kangkang Zhi
Kangkang Zhi Sili Zou
Sili Zou Jie Jin
Jie Jin Jun Bai
Jun Bai Lefeng Qu
Lefeng Qu