
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 19 September 2023
Sec. Heart Failure and Transplantation
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1248997
 Ruixia Liu1,†
Ruixia Liu1,† Jinbo Fang1,†
Jinbo Fang1,† Mei R. Fu2
Mei R. Fu2 Qingtong Meng3
Qingtong Meng3 Minlu Li4
Minlu Li4 Xiaoxia Zhang5
Xiaoxia Zhang5 Sarah R. Allred6
Sarah R. Allred6 Yuan Li7,8*
Yuan Li7,8*
Background: Abnormal interstitial fluid accumulation remains the major cause for patients with heart failure (HF) to endure a myriad of distressing symptoms and a decline in their health-related quality of life (HRQoL). The lymphatic system is essential in regulating fluid balance within the interstitial compartment and has recently been recognized as an important target for the prevention and mitigation of congestion. This study aimed to investigate the effects of exercises in activating lymphatic system on symptom distress and HRQoL among patients with HF.
Methods and results: This was a pre-determined, secondary analysis of the TOLF-HF [The-Optimal-Lymph-Flow for Heart Failure (TOLF-HF)] study, a two-arm pilot randomized controlled trial evaluating the preliminary effects of the lymphatic exercise intervention in enhancing interstitial decongestion among patients with HF. Participants were randomized to receive either a four-week TOLF-HF program in addition to standard care or standard care alone. The Chinese version of the Minnesota Living with Heart Failure Questionnaire (MLHFQ) was employed to measure symptom distress and HRQoL before and after the intervention. Data analyses included descriptive statistics, the independent sample t-test, Pearson’s chi-square test, the Mann-Whitney U test, and covariance analysis. Of the 66 patients enrolled, 60 completed the study. The study results exhibited that the TOLF-HF intervention were effective in alleviating both physical and psychological symptom distress. The intervention group yielded significantly lower MLHFQ total scores in comparison to the control group. The odd ratio of achieving meaningful improvement in HRQoL in TOLF-HF group was 2.157 times higher than those in the control group.
Conclusions: The TOLF-HF program focusing on activating lymphatic system was effective in alleviating physical and psychological symptom distress as well as improving HRQoL for patients with HF. The tolerability, feasibility, and effectiveness of the TOLF-HF intervention make it a promising intervention for patients to manage HF.
Clinical Trial Registration: http://www.chictr.org.cn/index.aspx, identifier (ChiCTR2000039121).
Heart failure (HF) is a significant public health challenge, affecting an estimated 64 million individuals worldwide (1). The prevalence of HF is age-dependent, ranging from under 2% among people younger than 60 years to exceeding 10% among those aged 80 years or older (2). With the rapidly aging global population, the prevalence of HF continues to increase worldwide. Abnormal interstitial fluid accumulation is central to the pathophysiology of HF, leading to hemodynamic congestion characterized by elevated central filling pressures and the subsequent onset of clinical congestion (3). Clinical congestion manifests as a myriad of congestive symptoms that elicit distress to the patient (4, 5). The occurrence or exacerbation of congestive symptoms constitutes a major contributor to diminished health-related quality of life (HRQoL) (6). According to time trade-off utility studies, patients suffering from HF would be willing to exchange their lifespan for an enhanced quality of life, with individuals experiencing severer symptoms being more inclined to prioritize HRQoL over longevity (7, 8). To help manage symptom distress and improve HRQoL in patients suffering from HF, it is imperative to provide feasible and effective interventions to prevent and mitigate congestion.
The lymphatic system, an integral part of the circulatory system, plays a crucial role in maintaining the balance of tissue fluid levels by taking up interstitial fluid in the form of lymph and returning it into the central circulation (9, 10). However, in the context of HF, the elevated central venous pressure leads to increased fluid accumulation in the interstitial space, and simultaneously impedes fluid to flow back into the venous system (9). Moreover, a reduction in the quantity of lymphatic vessels, accompanied by an expansion of their diameters in HF, also contributes to the accumulation of fluid within the interstitial space (11). Congestion occurs when the drainage of fluid fails to match the rate at which it permeates into the interstitial spaces (12). Therefore, the activation of the lymphatic system to facilitate lymphatic circulation with enhanced permeation holds great potential for averting and alleviating subclinical and clinical congestion. This potential is rooted in the fact that the removal of interstitial fluid relies exclusively on lymphatic pumping, and the lymphatic system has the capacity to augment fluid elimination by a minimum of ten-fold (9, 10, 13).
The-Optimal-Lymph-Flow (TOLF) intervention comprises a set of non-pharmacological therapeutic exercises that specifically target the activation of the lymphatic system (14–18). The fundamental TOLF intervention comprises lymphatic exercises which entail the contraction of muscles and pumping movements that are synchronized with deep breathing to simulate the physiological process of lymphatic pumping (14–18). TOLF lymphatic exercises are designed to elicit musculoskeletal contractions, skin tensions, arterial pulsations, postural adjustments, and modifications in breathing patterns; all of these collectively serve to activate the lymphatic system (14–18). As a result, the TOLF intervention possesses great promise for yielding favorable outcomes in the prevention and management of congestion.
The-Optimal-Lymph-Flow for Heart Failure (TOLF-HF) trial was conducted to assess the preliminary effects of the four-week lymphatic exercise training program among patients afflicted with HF. The finding revealed that the innovative implementation of TOLF-HF program was beneficial in alleviating the burden of congestive symptoms, enhancing physical functions, and maintaining stable body weight for this population (16). Nevertheless, it is unknown whether this strategy have positive impact on symptom distress and HRQoL, which were predefined as secondary outcomes in the TOLF-HF trial. In this analysis, we evaluated the effects of TOLF-HF intervention compared to standard care on the overall summary score and symptom distress items of the Minnesota Living with Heart Failure Questionnaire (MLHFQ).
The detailed information regarding the design of the TOLF-HF trial was previously published (16). In brief, the TOLF-HF trial was a single-center, two-arm, parallel-group, pilot randomized controlled trial (RCT) in which eligible patients were between the ages of 18 and 80, admitted to the hospital with a primary diagnosis of HF. The diagnosis of HF was made based on the Chinese guidelines for the diagnosis and treatment of heart failure 2018 (19). The detailed inclusion and exclusion criteria are given in Figure 1. Consecutive identification of eligible participants was carried out in the West China Hospital by reviewing inpatient census lists. Prospective participants were presented with the study details two days prior to their discharge. Patients who signed the informed consent form to participate in the study completed the baseline assessment.
The study randomly assigned participants in a 1:1 ratio to receive either standard care or a four-week TOLF-HF program in addition to standard care. The randomization assignment was determined using the SPSS random number generator by an independent researcher. Allocation concealment was assured through the use of sequentially numbered, sealed, opaque envelopes containing group assignment. Blinding of participants and interventionists was impossible given the inherent nature of the intervention in the study. Nevertheless, the outcome assessor, data collector, and data analyst were blinded to the group assignment throughout the entire duration of the study.
Participants allocated to the control group received standard care. Apart from the implementation of guideline-directed medical therapy for managing HF (19, 20), the standard care included providing patients with a comprehensive written summary containing essential information about their medical condition and treatment plan upon discharge. Moreover, nurses provided verbal guidance on adopting healthy lifestyle behaviors and adhering to prescribed medications. If it is deemed necessary, patients can be directed to post-discharge support services. Following their discharge, all participants were scheduled to attend a specialist clinic for a follow-up visit after four weeks. No additional educational or supportive post-discharge care was administered.
Participants assigned to the experimental group underwent the four-week TOLF-HF intervention plus standard care. The TOLF-HF program was fashioned based on physiological-cognitive-behavioral principles, featuring the activation of the lymphatic system through effective self-care strategies to ameliorate the pathological status of congestion in patients suffering from HF (14–18). Figure 2 provides an overview of the strategies employed in the TOLF-HF program, along with their corresponding physiological rationales and the recommended frequency of practice. Specifically, muscle-tightening deep breathing exercises serve to activate lymphatic ducts, thereby facilitating the drainage of lymph fluid. Muscle-tightening pumping exercises aid in promoting the flow of lymph fluid and mitigating fluid retention in the extremities. Furthermore, engaging in large muscle exercises enhances the flow and drainage of lymph fluid throughout the entire body (14–18).
The implementation of the TOLF-HF program was promptly initiated following the baseline assessment and conducted within two days prior to hospital discharge. The intervention was administered by a trained researcher during a dedicated 30-minute one-to-one session. Initially, a detailed video presentation was utilized to provide clear and standardized step-by-step instructions on the correct execution and frequency of the lymphatic exercises. Subsequently, patients were required to practice independently while being closely observed by the researcher, who promptly corrected any improper movements until patients were able to perform all exercises accurately. During the patients’ performance of lymphatic exercises, the researcher recorded a video clip, which was then shared with the patients for future reference as needed. The involvement of family members was encouraged throughout the process, with their active participation in accompanying and supporting the patients’ practice at home. Following hospital discharge, the researcher maintained weekly contact via WeChat to address any potential barriers, provide relevant advice, and encourage adherence to the TOLF-HF protocol.
A structured questionnaire was used to collect participants’ sociodemographic and clinical information. Sociodemographic information included age, gender, educational attainment, occupational status, and caregiver role. Clinical information included weight, height, body mass index, blood pressure, heart rate, length of hospital stays, duration of HF, HF etiology, HF type, number of co-morbidities, left ventricular ejection fraction, New York Heart Association function class, NT-proBNP levels, and dose of diuretics.
HRQoL was measured with the Minnesota Living with Heart Failure Questionnaire (MLHFQ), which is widely utilized as a disease-specific instrument that captures the impact of HF on patients’ daily life and functioning (21). This 21-item questionnaire, assessed on a 6-piont Likert scale of 0 (“no impact”) to 5 (“very high impact”), provides a summary score that ranges from 0 to 105, with higher scores indicating a lower HRQoL. Five points is generally considered as the minimal clinically important difference in overall MLHFQ score (22). The Chinese version of the MLHFQ has undergone validation and exhibited satisfactory reliability, with a Cronbach’s alpha coefficient of 0.95 (23).
Eight items within the MLHFQ were specifically designed to assess the degree of distress associated with HF symptoms, that is, to which extent these symptoms have prevented individuals from living as they wanted (24, 25). In the current analysis, these items were utilized to measure the patients’ symptom distress together with the evaluation of their overall HRQoL. Among these eight items, five specifically address physical symptoms (i.e., swelling in the ankles or legs, resting during days, sleeping difficult, shortness of breath, fatigue), while the remaining three focus on psychological symptoms (i.e., being worried, difficulty concentrating or remembering, being depressed) (24, 25). The scores for these items were derived from the overall MLHFQ assessment, where higher scores correspond to elevated levels of distress attributed to the symptoms (23).
Patient self-report and electronic medical records were utilized to gather baseline sociodemographic and clinical data. The outcome measures were completed by all participants both at baseline and during their routine visits to the specialist clinic, which occurred 4 weeks after discharge. An impartial research assistant conducted data collection without knowledge of the study’s hypothesis or participant allocation.
Descriptive analyses were conducted to present the baseline characteristics of the participants, stratified by treatment group. The Shapiro-Wilk test was employed to assess the normality of the data, while the Levene test was used to evaluate the homogeneity of variance. Continuous variables were reported as means with standard deviations (SDs) or medians with interquartile ranges (IQRs), depending on the distribution of the data. The comparison of the intervention group versus control group was conducted using the independent sample t-test for variables that exhibited a normal distribution. In cases where the variables did not adhere to normal distribution, the Mann-Whitney U test was employed instead. Where appropriate, covariance analysis (ANCOVA) was also performed to account for baseline outcome measures and significant demographic covariates. Categorical variables were reported as counts with percentages and analyzed using Pearson’s chi-square test. All statistical analyses were conducted using SPSS for Windows 26.0. Statistical significance was defined as a two-sided p value of <0.05.
The study was approved by the Biomedical Ethics Committee of the West China Hospital, Sichuan University [Approval number: 2019 (202)]. This study was registered with the Chinese Clinical Trial Registry (registration number ChiCTR2000039121) and adhered to the reporting guidelines outlined in the CONSORT statement (26). Detailed information of the study was provided with each potential participant, including the objectives, methods, anticipated duration, potential hazards, advantages, and the option to decline or withdraw from participation. Ample time was provided for potential participants to read the informed consent and ask questions. Participants were also assured that their personal data would remain confidential and not be disclosed to any third parties. Written informed consent was obtained from all participants.
Participant enrollment for this pilot study spanned from March 2019 to January 2020. The CONSORT flow diagram can be found in the first publication from the TOLF-HF study (16) and is provided in Supplementary Figure S1. Of the 85 patients assessed for eligibility, 66 met the inclusion criteria and were enrolled in the trial. During the study period, 6 patients were lost to follow-up. The final analysis was conducted using data from 60 patients (29 in TOLF-HF group and 31 in control group) who provided complete information.
The mean age was 58.07 years old [standard deviation (SD) = 12.79] for the TOLF-HF group and 61.65 years old (SD = 11.42) for the control group. Within each group, 65.5% and 64.5% were male, 75.9% and 64.5 were diagnosed as HF with reduced ejection fraction (HFrEF), and 37.9% and 58.1% of the participants were classified as NYHA class III, respectively. Detailed information on study participant characteristics is described in Supplementary Table S1 (16). No statistically significant differences in demographic and clinical characteristics were found between intervention and control groups at baseline, except that patients in intervention group had a higher prevalence of co-morbidities versus the control group (Z = 2.449, p = 0.014).
As indicated in Table 1, no statistically significant between-group differences were found at baseline in terms of median distress scores for each physical and psychological symptom. At four weeks post-intervention, the TOLF-HF group exhibited significantly lower median physical symptom distress scores compared to the standard care control group for swelling in the ankles or legs (p = 0.036), sleeping difficult (p = 0.041), and shortness of breath (p = 0.003). While no significant differences were noted in symptom distress for resting during days (p = 0.108) and fatigue (p = 0.113). Regarding psychological symptoms, the TOLF-HF group demonstrated significantly lower median psychological symptom distress scores for being worried (p = 0.002) and difficulty concentrating or remembering (p = 0.016) after the intervention. However, no significant differences were seen for being depressed (p = 0.292).
As illustrated in Figure 3, no significant differences were detected between the groups with regards to the mean MLHFQ total scores at baseline. At the study endpoint, the MLHFQ total scores were significantly lower in the TOLF-HF group compared to the standard care control group (F = 12.120, p = 0.001), indicating an improved HRQoL for patients who underwent the intervention. Additionally, the inter-individual variation in the change of MLHFQ total score from baseline to week 4 is shown as a waterfall plot in Figure 4. It was noted that 79.3% (23/29) of the patients in the TOLF-HF group achieved a clinically important improvement, compared to 54.8% (17/31) in the standard care group. The odds ratio of attaining a meaningful improvement in HRQoL in the TOLF-HF group was 2.157 times (95% CI 1.006–9.906; p = 0.044) higher than that in the standard care group.

Figure 3. Comparison of between-group differences in health-related quality of life (n = 60). *The data were compared using the independent sample t-test.†The data were compared using the analysis of covariance (ANCOVA) after controlling for the pre-intervention health-related quality of life values and the number of co-morbidities.
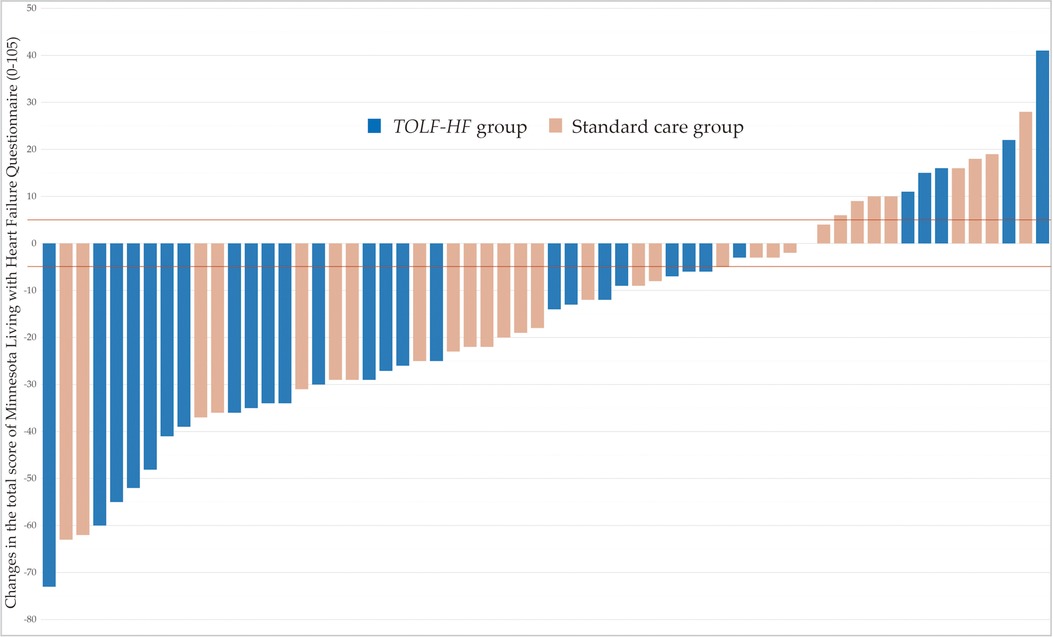
Figure 4. Inter-individual variation in the change of MLHFQ total score for each patient of the TOLF-HF and control groups (n = 60). The lines represent the minimal clinically important difference for improvement (−5 points) and worsening (+5 points) of health-related quality of life in patients with heart failure.
The challenge in visualizing the translucent lymphatic vessels has been historically led to overlook the significant role of the lymphatic system in regulating fluid homeostasis. Recent research progressions on the lymphatic network and the pathogenesis of cardiovascular diseases have established the importance of targeting the lymphatic circulation in the management and treatment of cardiovascular disorders (27, 28). The TOLF-HF trial innovatively applied lymphatic exercises among patients with HF. In this pre-determined secondary analysis of the pilot trial, we observed that patients who underwent TOLF-HF intervention demonstrated a reduction in physical and psychological symptom distress, as well as an improvement in HRQoL. While the original paper focused on the frequency, severity, and burden of congestion-related symptoms, this secondary analysis provides a more comprehensive look at the distress associated with overall HF symptoms. The findings were complementary to the primary analysis (16) and provided further support that the TOLF-HF program focusing on promoting lymph fluid flow through therapeutic lymphatic exercises is an effective adjunctive therapy for individuals with HF in terms of managing symptom distress and improving HRQoL.
Patients with HF commonly endure a multitude of distressing physical symptoms attributed to congestion (24, 29). The study findings showed that the integration of the four-week TOLF-HF lymphatic exercises, in conjunction with standard care, had beneficial effects on mitigating the levels of symptom distress associated with lower extremity swelling, sleep difficulty, and shortness of breath in individuals with HF. The amelioration of the aforementioned symptom distress can be attributed to the activation of lymphatic system and the consequent reduction in extracellular fluid retention. Specifically, the relief of distress related to lower extremity swelling can be ascribed to the enhanced clearance of fluid accumulation in the systemic circulation, while the alleviation of sleep difficulty and shortness of breath can be attributed to the improved resolution of volume overload in the pulmonary circulation (30). The TOLF-HF program, comprising muscle tightening deep breathing, muscle tightening pumping, and large muscle exercises developed to emulate the physiological mechanism of lymph propulsion (14–18), creates a synergistic effect in accelerating the removal of fluid volume in both the thoracic region and the whole body. Thus, the intervention confers benefits to patients by ameliorating physical symptoms associated with congestion.
Physical and psychological symptoms often co-exist in HF (24, 29). The study demonstrated that the TOLF-HF program yielded favorable outcomes in mitigating the levels of psychological symptom distress associated with being worried and difficulty concentrating or remembering. These psychological symptoms serve as indicators of the anxiety status in individuals with HF. The TOLF-HF lymphatic exercises encompass tightening and pumping movements that are coordinated with deep breathing. A previous RCT conducted by D’Silva et al. found that deep breathing exercises was beneficial in decreasing anxiety in patients with coronary heart disease (31). Another RCT also demonstrated the positive impact of deep breathing exercises on anxiety levels in patients with gestational diabetes (32). Furthermore, deep breathing constitutes an essential component of mindfulness-based interventions, which have consistently demonstrated effectiveness in alleviating anxiety across diverse populations (33–36). Accordingly, the relief of being worried and difficulty concentrating or remembering in the current study can be explained by the positive effect of deep breathing on psychological status. Furthermore, the beneficial effects of the TOLF-HF intervention on physical symptoms could potentially function as a mediator in alleviating psychological distress.
HRQoL is an important outcome that reflects the impact of HF on patients’ daily life (21). The study results indicated that the TOLF-HF intervention have positive effects on the overall HRQoL for patients suffering from HF. The likelihood of achieving clinically meaningful improvement from baseline in overall HRQoL was two times greater in patients who received the intervention than those received standard care alone. The finding is consistent with a previous Cochrane review, which provided a comprehensive review of exercise-based intervention for adults with HF and showed positive effects on HRQoL (37). The positive effects can be attributed to the beneficial impact of TOLF-HF exercises in ameliorating prevalent physical symptoms, which are widely recognized as independent predictors of HRQoL (38, 39). TOLF-HF exercises also provide psychological benefits among the participants and emotional well-being serves as an important aspect of HRQoL (39). Moreover, it is noteworthy that the intervention encourages these participating patients to pursue an active lifestyle, which is widely regarded as one of the most beneficial measures individuals can take to prevent illness, maintain good health, and improve their HRQoL (40, 41). Additionally, engaging in exercise-based interventions empowers HF patients to take a proactive role in managing their condition. As they experience improvements in symptom management, physical functioning, and psychological well-being, patients may gain a sense of control over their health, resulting in enhanced HRQoL (42, 43).
The TOLF-HF program exhibits multiple advantages. Firstly, the intervention is easy to learn and does not require the use of costly equipment or constant supervision of healthcare providers. Moreover, the TOLF lymphatic exercises can be performed in any desired location, granting individuals the flexibility to engage in the exercises according to their preferences. With proper training, virtually anyone can learn and perform TOLF lymphatic exercises in a preferred setting (e.g., indoor or outdoor) or preferred positions (e.g., sitting, standing, or lie-down position). This makes the intervention particularly suitable for the HF population, as a significant number of HF patients are elderly and may have physical and cognitive impairments which limit their ability to perform 30 min walking or exercises that need standing position. Additionally, the TOLF-HF lymphatic exercises are of relatively low intensity and do not impose high physical demands. Thus, even patients who are immobilized or bedridden can perform the majority of these exercises. The tolerability, feasibility, and effectiveness of the TOLF-HF intervention make it a promising candidate for integration into daily self-care regimens for HF management.
In addition, it is worth noting that the management of extravascular volume overload is progressively gaining recognition as a therapeutic target in HF (13). New device-based treatments are currently under development to restore interstitial fluid balance and alleviate congestive symptoms in HF patients (44). For example, the alfapump DS® (Sequana Medical NV, Belgium) (45) and the Reprieve System™ (Reprieve Cardiovascular, Milford, MA, USA) (46) exemplify such emerging technologies, which have demonstrated safety and tolerability in preliminary exploratory clinical trials. As we anticipate the translation of these innovative device-based treatments into clinical practice, it is imperative to underscore the advantages inherent in the TOLF-HF program. These advantages encompass enhanced convenience, in-home accessibility, cost-effectiveness, and preventive orientation compared to device-based volume removal treatments that necessitate invasive procedures and professional oversight.
We acknowledge the limitations of our study, including its single-center design, short follow-up duration, and small sample size. It is important to note that the beneficial effects of TOLF-HF were found in patients in the intervention group even though a higher proportion of individuals within the intervention group presented with a greater number of co-morbidities at the baseline and we also accounted for this using analysis of covariance. Another limitation of the study is the absence of real-time monitoring of the actual exercise dose administered, which hinders the trial’s ability to investigate the dose-effectiveness of the intervention. Further validation of the study findings is warranted through larger multicenter studies with a larger sample size, longer follow-up periods, and comprehensive exercise monitoring. Moreover, future studies may benefit from the incorporation of more objective metrics, such as echocardiography and chest x-ray, to directly quantify the decongestion impact of the TOLF-HF intervention.
The TOLF-HF program has demonstrated effectiveness in reducing both physical and psychological symptom distress and improving HRQoL in patients with HF, and shows promise as a candidate for integration into daily self-care regimens for HF management. The TOLF-HF trial represents an initial and important endeavor in targeting the lymphatic system as a potential approach to managing congestion in HF. Moving forward, further research involving larger samples is warranted to verify the benefits of TOLF-HF and explore its long-term clinical impacts. Nevertheless, this pilot trial has provided valuable preliminary evidence and laid a solid foundation for utilizing lymphatic activation as a new self-care modality for patients with HF.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by the Biomedical Ethics Committee of the West China Hospital, Sichuan University [Approval number: 2019 (202)]. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
RL—Data curation, Formal analysis, Visualization, Writing–original draft. JF—Conceptualization, Validation, Funding acquisition, Project administration, Resources. MF—Conceptualization, Validation, Writing–Review & Editing. QM—Conceptualization, Investigation, Data curation, Methodology. ML—Investigation, Data curation, Methodology. XZ—Validation, Writing–Review & Editing. SA—Methodology, Software, Formal analysis, Visualization, Validation. YL—Conceptualization, Formal analysis, Visualization, Validation, Writing–Original draft preparation, Writing–Review & Editing. All authors contributed to editorial changes in the manuscript. All authors contributed to the article and approved the submitted version.
This research was funded by the Science and Technology Plan Project of Sichuan Province, China, grant number 2020YFS0150. The funder had no involvement in the conduct of the study, data analysis or drafting of the manuscript.
We would like to extend our appreciation to the Sichuan Science and Technology Agency for funding this study. Additionally, we express our sincere gratitude to all the participants and their families for their valuable collaboration.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1248997/full#supplementary-material
1. Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. (2023) 118(17):3272–87. doi: 10.1093/cvr/cvac013
2. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics-2022 update: a report from the American Heart Association. Circulation. (2022) 145(8):e153–639. doi: 10.1161/cir.0000000000001052.
3. Miller WL. Fluid volume overload and congestion in heart failure: time to reconsider pathophysiology and how volume is assessed. Circ Heart Fail. (2016) 9(8):e002922. doi: 10.1161/circheartfailure.115.002922
4. Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. (2013) 34(11):835–43. doi: 10.1093/eurheartj/ehs444
5. Cooper LB, Lippmann SJ, DiBello JR, Gorsh B, Curtis LH, Sikirica V, et al. The burden of congestion in patients hospitalized with acute decompensated heart failure. Am J Cardiol. (2019) 124(4):545–53. doi: 10.1016/j.amjcard.2019.05.030
6. Freedland KE, Rich MW, Carney RM. Improving quality of life in heart failure. Curr Cardiol Rep. (2021) 23(11):159. doi: 10.1007/s11886-021-01588-y
7. Kraai IH, Vermeulen KM, Luttik ML, Hoekstra T, Jaarsma T, Hillege HL. Preferences of heart failure patients in daily clinical practice: quality of life or longevity? Eur J Heart Fail. (2013) 15(10):1113–21. doi: 10.1093/eurjhf/hft071
8. Stevenson LW, Hellkamp AS, Leier CV, Sopko G, Koelling T, Warnica JW, et al. Changing preferences for survival after hospitalization with advanced heart failure. J Am Coll Cardiol. (2008) 52(21):1702–8. doi: 10.1016/j.jacc.2008.08.028
9. Fudim M, Salah HM, Sathananthan J, Bernier M, Pabon-Ramos W, Schwartz RS, et al. Lymphatic dysregulation in patients with heart failure: JACC review topic of the week. J Am Coll Cardiol. (2021) 78(1):66–76. doi: 10.1016/j.jacc.2021.04.090
10. Moore JE Jr, Bertram CD. Lymphatic system flows. Annu Rev Fluid Mech. (2018) 50:459–82. doi: 10.1146/annurev-fluid-122316-045259
11. Rossitto G, Mary S, McAllister C, Neves KB, Haddow L, Rocchiccioli JP, et al. Reduced lymphatic reserve in heart failure with preserved ejection fraction. J Am Coll Cardiol. (2020) 76(24):2817–29. doi: 10.1016/j.jacc.2020.10.022
12. Itkin M, Rockson SG, Burkhoff D. Pathophysiology of the lymphatic system in patients with heart failure: JACC state-of-the-art review. J Am Coll Cardiol. (2021) 78(3):278–90. doi: 10.1016/j.jacc.2021.05.021
13. Aronson D. The interstitial compartment as a therapeutic target in heart failure. Front Cardiovasc Med. (2022) 9:933384. doi: 10.3389/fcvm.2022.933384
14. Fu MR, Axelrod D, Guth AA, Cartwright F, Qiu Z, Goldberg JD, et al. Proactive approach to lymphedema risk reduction: a prospective study. Ann Surg Oncol. (2014) 21(11):3481–9. doi: 10.1245/s10434-014-3761-z
15. Fu MR, McTernan ML, Qiu JM, Ko E, Yazicioglu S, Axelrod D, et al. The effects of kinect-enhanced lymphatic exercise intervention on lymphatic pain, swelling, and lymph fluid level. Integr Cancer Ther. (2021) 20:15347354211026757. doi: 10.1177/15347354211026757
16. Li Y, Meng Q, Luo B, Li M, Fang J, Allred S, et al. Exercises in activating lymphatic system on fluid overload symptoms, abnormal weight gains, and physical functions among patients with heart failure: a randomized controlled trial. Front Cardiovasc Med. (2023) 10:1094805. doi: 10.3389/fcvm.2023.1094805
17. Fu MR, Axelrod D, Guth AA, Scagliola J, Rampertaap K, El-Shammaa N, et al. A web- and mobile-based intervention for women treated for breast cancer to manage chronic pain and symptoms related to lymphedema: results of a randomized clinical trial. JMIR Cancer. (2022) 8(1):e29485. doi: 10.2196/29485
18. Liu F, Li F, Fu MR, Zhao Q, Wang Y, Pang D, et al. Self-management strategies for risk reduction of subclinical and mild stage of breast cancer-related lymphedema: a longitudinal, quasi-experimental study. Cancer Nurs. (2021) 44(6):E493–e502. doi: 10.1097/ncc.0000000000000919
19. Heart Failure Group of Chinese Society of Cardiology of Chinese Medical Association, Chinese Heart Failure Association of Chinese Medical Doctor Association, Editorial Board of Chinese Journal of Cardiology. Chinese guidelines for the diagnosis and treatment of heart failure 2018. Zhong Hua Xin Xue Guan Bing Za Zhi. (2018) 46:760–89. doi: 10.3760/cma.j.issn.0253-3758.2018.10.004
20. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart failure society of America. J Card Fail. (2017) 23(8):628–51. doi: 10.1016/j.cardfail.2017.04.014
21. Garin O, Herdman M, Vilagut G, Ferrer M, Ribera A, Rajmil L, et al. Assessing health-related quality of life in patients with heart failure: a systematic, standardized comparison of available measures. Heart Fail Rev. (2014) 19(3):359–67. doi: 10.1007/s10741-013-9394-7
22. Rector TS, Kubo SH, Cohn JN. Validity of the Minnesota living with heart failure questionnaire as a measure of therapeutic response to enalapril or placebo. Am J Cardiol. (1993) 71(12):1106–7. doi: 10.1016/0002-9149(93)90582-w
23. Ho CC, Clochesy JM, Madigan E, Liu CC. Psychometric evaluation of the Chinese version of the Minnesota living with heart failure questionnaire. Nurs Res. (2007) 56(6):441–8. doi: 10.1097/01.NNR.0000299849.21935.c4
24. Park J, Moser DK, Griffith K, Harring JR, Johantgen M. Exploring symptom clusters in people with heart failure. Clin Nurs Res. (2019) 28(2):165–81. doi: 10.1177/1054773817729606
25. Lee KS, Song EK, Lennie TA, Frazier SK, Chung ML, Heo S, et al. Symptom clusters in men and women with heart failure and their impact on cardiac event-free survival. J Cardiovasc Nurs. (2010) 25(4):263–72. doi: 10.1097/JCN.0b013e3181cfbb88
26. Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. Br Med J. (2010) 2010:340. doi: 10.1136/bmj.c869
27. Aspelund A, Robciuc MR, Karaman S, Makinen T, Alitalo K. Lymphatic system in cardiovascular medicine. Circ Res. (2016) 118(3):515–30. doi: 10.1161/circresaha.115.306544
28. Klaourakis K, Vieira JM, Riley PR. The evolving cardiac lymphatic vasculature in development, repair and regeneration. Nat Rev Cardiol. (2021) 18(5):368–79. doi: 10.1038/s41569-020-00489-x
29. Qiu C, Yu DS, Song D, Wang X. The prognostic impact of symptom clusters in patients with heart failure: a systematic review and meta-analysis. J Adv Nurs. (2022) 78(9):2713–30. doi: 10.1111/jan.15302
30. American Heart Failure Association. Heart failure signs and symptoms. Available at: https://www.heart.org/en/health-topics/heart-failure/warning-signs-of-heart-failure#.W09BCtgzZmA (Accessed June 1, 2023).
31. D’Silva F HV, Muninarayanappa NV. Effectiveness of deep breathing exercise (DBE) on the heart rate variability, BP, anxiety & depression of patients with coronary artery disease. J Health Allied Sci NU. (2014) 4(1):035–41. doi: 10.1055/s-0040-1703728
32. Fiskin G, Sahin N. Effect of diaphragmatic breathing exercise on psychological parameters in gestational diabetes: a randomised controlled trial. Eur J Integr Med. (2018) 23(7):50–6. doi: 10.1016/j.eujim.2018.09.006
33. Rechenberg K, Cousin L, Redwine L. Mindfulness, anxiety symptoms, and quality of life in heart failure. J Cardiovasc Nurs. (2020) 35(4):358–63. doi: 10.1097/jcn.0000000000000630
34. Zou H, Cao X, Geng J, Chair SY. Effects of mindfulness-based interventions on health-related outcomes for patients with heart failure: a systematic review. Eur J Cardiovasc Nurs. (2020) 19(1):44–54. doi: 10.1177/1474515119881947
35. Xunlin NG, Lau Y, Klainin-Yobas P. The effectiveness of mindfulness-based interventions among cancer patients and survivors: a systematic review and meta-analysis. Support Care Cancer. (2020) 28(4):1563–78. doi: 10.1007/s00520-019-05219-9
36. Ghawadra SF, Lim Abdullah K, Choo WY, Danaee M, Phang CK. The effect of mindfulness-based training on stress, anxiety, depression and job satisfaction among ward nurses: a randomized control trial. J Nurs Manag. (2020) 28(5):1088–97. doi: 10.1111/jonm.13049
37. Long L, Mordi IR, Bridges C, Sagar VA, Davies EJ, Coats AJ, et al. Exercise-based cardiac rehabilitation for adults with heart failure. Cochrane Database Syst Rev. (2019) 1(1):Cd003331. doi: 10.1002/14651858.CD003331.pub5
38. Johansson I, Balasubramanian K, Bangdiwala S, Mielniczuk L, Hage C, Sharma SK, et al. Factors associated with health-related quality of life in heart failure in 23,000 patients from 40 countries: results of the G-CHF research programme. Eur J Heart Fail. (2022) 24(9):1478–90. doi: 10.1002/ejhf.2535
39. Ye Y, Mei J, Zhang J, Zhao Q, Fan X. The heterogeneity of physical and anxiety symptoms and quality of life among patients with heart failure: a latent class analysis. J Cardiovasc Nurs. (2022) 37(6):558–69. doi: 10.1097/jcn.0000000000000867
40. Cunningham C ROS, Caserotti P, Tully MA. Consequences of physical inactivity in older adults: a systematic review of reviews and meta-analyses. Scand J Med Sci Sports. (2020) 30(5):816–27. doi: 10.1111/sms.13616
41. Li Y, Su S, Luo B, Wang J, Liao S. Physical activity and depressive symptoms among community-dwelling older adults in the COVID-19 pandemic era: a three-wave cross-lagged study. Int J Disaster Risk Reduct. (2022) 70:102793. doi: 10.1016/j.ijdrr.2022.102793
42. Taylor R, Walker S, Smart N, Piepoli M, Warren F, Ciani O, et al. Impact of exercise rehabilitation on exercise capacity and quality-of-life in heart failure. J Am Coll Cardiol. (2019) 73(12):1430–43. doi: 10.1016/j.jacc.2018.12.072
43. Jaarsma T, Hill L, Bayes-Genis A, La Rocca HB, Castiello T, Čelutkienė J, et al. Self-care of heart failure patients: practical management recommendations from the heart failure association of the European society of cardiology. Eur J Heart Fail. (2021) 23(1):157–74. doi: 10.1002/ejhf.2008
44. de Oliveira Cardoso C, Elgalad A, Li K, Perin EC. Device-based therapy for decompensated heart failure: an updated review of devices in development based on the DRI2P2S classification. Front Cardiovasc Med. (2022) 9:962839. doi: 10.3389/fcvm.2022.962839
45. Rao VS, Turner JM, Griffin M, Mahoney D, Asher J, Jeon S, et al. First-inhuman experience with peritoneal direct sodium removal using a zero-sodium solution: a new candidate therapy for volume overload. Circulation. (2020) 141:1043–53. doi: 10.1161/CIRCULATIONAHA.119.043062
Keywords: heart failure, lymphatic exercises, self-care, health related quality of life, symptom distress, randomized clinical trial
Citation: Liu R, Fang J, Fu MR, Meng Q, Li M, Zhang X, Allred SR and Li Y (2023) Strategies in activating lymphatic system on symptom distress and health-related quality of life in patients with heart failure: secondary analysis of a pilot randomized controlled trial. Front. Cardiovasc. Med. 10:1248997. doi: 10.3389/fcvm.2023.1248997
Received: 28 June 2023; Accepted: 6 September 2023;
Published: 19 September 2023.
Edited by:
Inna P. Gladysheva, University of Arizona College of Medicine—Phoenix, United StatesReviewed by:
Massimo Iacoviello, University of Foggia, Italy© 2023 Liu, Fang, Fu, Meng, Li, Zhang, Allred and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan Li bGkueXVhbkBzY3UuZWR1LmNu
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.