- 1Department of Cardiology, Radboudumc, Nijmegen, Netherlands
- 2Department of Cardiology, Northwest Clinics, Alkmaar, Netherlands
- 3Department of Cardiology, University Medical Center Utrecht, Utrecht, Netherlands
- 4Dutch Network for Cardiovascular Research (WCN), Utrecht, Netherlands
- 5Department of Medicine, McMaster University, Hamilton, ON, Canada
- 6Department of Cardiology, Meander Medical Center, Amersfoort, Netherlands
- 7Heart and Vascular Research Institute of Western Australia, Perth, WA, Australia
- 8GenesisCare Western Australia, Perth, WA, Australia
- 9School of Medicine, University of Western Australia, Perth, WA, Australia
- 10Department of Cardiology, Amsterdam University Medical Centers, Amsterdam, Netherlands
- 11Cardialysis BV, Rotterdam, Netherlands
- 12Sir Charles Gairdner Hospital, Perth, WA, Australia
- 13Department of Internal Medicine, Radboudumc, Nijmegen, Netherlands
- 14Department of Internal Medicine, Northwest Clinics, Alkmaar, Netherlands
- 15Department of Internal Medicine, Amsterdam University Medical Centers, Amsterdam, Netherlands
Introduction: Despite optimal treatment, patients with chronic coronary artery disease (CAD) and diabetes mellitus (DM) are at high risk of cardiovascular events, emphasizing the need for new treatment options. The Low-Dose Colchicine 2 (LoDoCo2) trial demonstrated that colchicine reduces cardiovascular risk in patients with chronic CAD. This analysis determines the efficacy of colchicine in patients with chronic CAD and DM as well as the effect of colchicine on the development of new-onset type 2 diabetes mellitus (T2DM).
Methods: The LoDoCo2 trial randomized 5,522 patients to placebo or colchicine 0.5 mg once daily, with a median follow-up of 28.6 months. The primary composite endpoint was cardiovascular death, spontaneous myocardial infarction, ischemic stroke, or ischemia-driven revascularization. The effect of its treatment in patients with and without DM was evaluated by including an interaction term in the model.
Results: A total of 1,007 participants (18.2%) had T2DM at baseline. The adjusted hazard ratio (HR) [(95% confidence interval (CI)] for the primary endpoint in the T2DM group was 1.52 (1.15–2.01, p < 0.01) compared with the group without T2DM. The HR for the treatment effect on the primary endpoint was 0.87 (0.61–1.25) in participants with T2DM and 0.64 (0.51–0.80) in participants without diabetes (pinteraction = 0.14). The incidence of new-onset T2DM was 1.5% (34 out of 2,270) in the colchicine group and 2.2% (49 out of 2,245) in the placebo group (p = 0.10).
Discussion: In conclusion, based on the current evidence, the beneficial effects of colchicine on cardiovascular endpoints are consistent regardless of DM status. The potential benefits of colchicine in preventing new-onset DM need further investigation. These findings are only hypothesis-generating and require larger prospective trials to confirm the results.
Introduction
Atherosclerosis is an inflammatory disease (1). Patients with type 2 diabetes mellitus (T2DM) are at increased risk of developing atherosclerotic cardiovascular disease (2). Patients with chronic coronary artery disease (CAD) who also have diabetes mellitus are particularly at high risk for recurrent cardiovascular events, which underscores the need for new therapeutic options (3).
T2DM is a multifactorial disease characterized by insulin resistance and relative insulin deficiency attributed to islet beta-cell failure causing hyperglycemia and dyslipidemia (4). Obesity is a low-grade chronic inflammatory disease and is the most crucial factor in the development of T2DM and related metabolic disorders (5, 6). Therefore, the role of inflammation in general and oligomerization of nucleotide-binding domain-, leucine-rich repeat-, and pyrin domain-containing protein 3 (NLRP3) inflammasome in particular on the onset and progression of T2DM has been hypothesized (7, 8). Small studies suggest that therapy that targets the inflammatory cytokine interleukin-1 (IL-1) can improve glycemic control in T2DM (9, 10). However, in the larger substudy of the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS), targeting interleukin-1β (IL-1β) did not result in better glycemic control or a reduction in the incidence of new-onset T2DM (11).
The Colchicine Cardiovascular Outcomes Trial (COLCOT) and Low-Dose Colchicine 2 (LoDoCo2) trial demonstrated that low-dose colchicine reduces the risk for cardiovascular death, myocardial infarction, ischemic stroke, or ischemia-driven coronary revascularization in unselected patients with recent myocardial infarction and chronic CAD, respectively (12, 13). Colchicine has broad anti-inflammatory effects that include inhibition of the NLRP3 inflammasome and polymerization of tubulin that affects leukocyte function (14–16). In this study, we assessed the efficacy of colchicine on cardiovascular events and the effect of colchicine on the development of new-onset T2DM in patients with chronic CAD with and without diabetes mellitus (17).
Methods
The LoDoCo2 trial (ACTRN12614000093684) was a double-blind randomized clinical trial, with a total of 5,522 patients randomized to colchicine 0.5 mg (n = 2,762) or placebo once daily (n = 2,760). Recruitment started in 13 centers in Western Australia in 2014 and was expanded to 30 centers in the Netherlands in 2016. Enrollment ended in 2018. The median follow-up time was 28.6 months (interquartile range, 20.5–44.4). The patients were eligible if they were aged 35–82 years, had established chronic CAD, were clinically stable for at least 6 months prior to randomization, and were able to tolerate colchicine during a 30-day run-in period. Randomization was performed in a double-blind 1:1 fashion to colchicine or placebo that was performed by a computerized algorithm. The primary efficacy endpoint was composed of major adverse cardiovascular events (MACE+), namely, cardiovascular death, spontaneous myocardial infarction, ischemic stroke, or ischemia-driven revascularization. Secondary endpoints consisted of the aforementioned events, separately. The endpoints were revised several times before unblinding of the data. All cardiovascular events were judged by a clinical events committee blinded to treatment allocation.
Calculation of the sample size for the original trial has previously been published and details can be found in the study protocol (12, 18). To summarize, with 5,447 randomized participants, the study would achieve a beta of <0.10 at a two-sided alpha of 0.05 to detect a difference of 30% in the incidence of the primary composite endpoint between treatment groups. Diabetes status and insulin treatment were assessed at the time of randomization. A participant who was not on diabetes treatment at the time of randomization and subsequently started treatment was defined as having “new-onset T2DM.” The trial protocol was approved by a centralized institutional review board in each participating country (MEC-U, Nieuwegein, Netherlands; and Sir Charles Gairdner Group HREC, Perth, Australia). All patients provided written informed consent. Additional details of the design, statistical analyses, baseline characteristics of the participants, and primary results of LoDoCo2 have been published (12, 18).
Statistical analyses
The baseline characteristics stratified by diabetes status are shown as mean ± standard deviation for normally distributed variables, as median with the interquartile range if non-normally distributed, and as proportions with percentages. Normality was visually assessed using histograms and Q–Q plots. Differences between baseline characteristics were assessed with independent sample t-tests or chi-squared tests where applicable.
Cox proportional hazard models were used to investigate univariable relationships between DM status and endpoints in the placebo group. Multivariable adjustment was performed with baseline variable predictors of the primary outcome as previously reported by using the forward Wald method (19). The variables were as follows: age >70 years, current smoker, a history of both coronary artery bypass grafting and percutaneous coronary intervention, a combination of oral anticoagulants and dual antiplatelet therapy, and no statin use.
Treatment effects for primary and secondary efficacy endpoints were presented by diabetes status. Kaplan–Meier estimates were plotted by treatment group (colchicine or placebo) and diabetes status. The interactions between the treatment group and diabetes status were evaluated with the addition of treatment and the treatment-by-diabetes status variable interaction. The difference in the incidence of new-onset T2DM between treatment groups was calculated using Fisher's exact test because the time to new-onset T2DM was not registered in the LoDoCo2 trial.
Hazard ratio (HR) with 95% confidence intervals (CI) was calculated, and the calculated p-values were two-tailed.
Results
Baseline characteristics
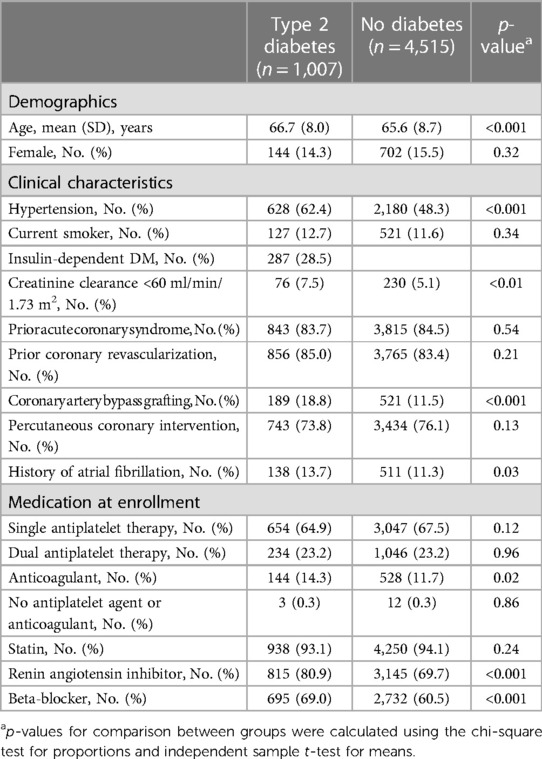
A total of 1,007 (18.2%) of the 5,522 participants in the LoDoCo2 trial had T2DM at baseline (Table 1), of whom 287 (28.5%) used insulin. The participants with T2DM were slightly older; more frequently had a history of atrial fibrillation, hypertension, and impaired renal function (eGFR of <60 ml/min/1.73 m2); and had more often undergone coronary artery bypass grafting (CABG) compared with participants without T2DM. Renin angiotensin inhibitors, beta-blockers, and anticoagulants were used more frequently by participants with T2DM.
Endpoints in relation to T2DM status at baseline
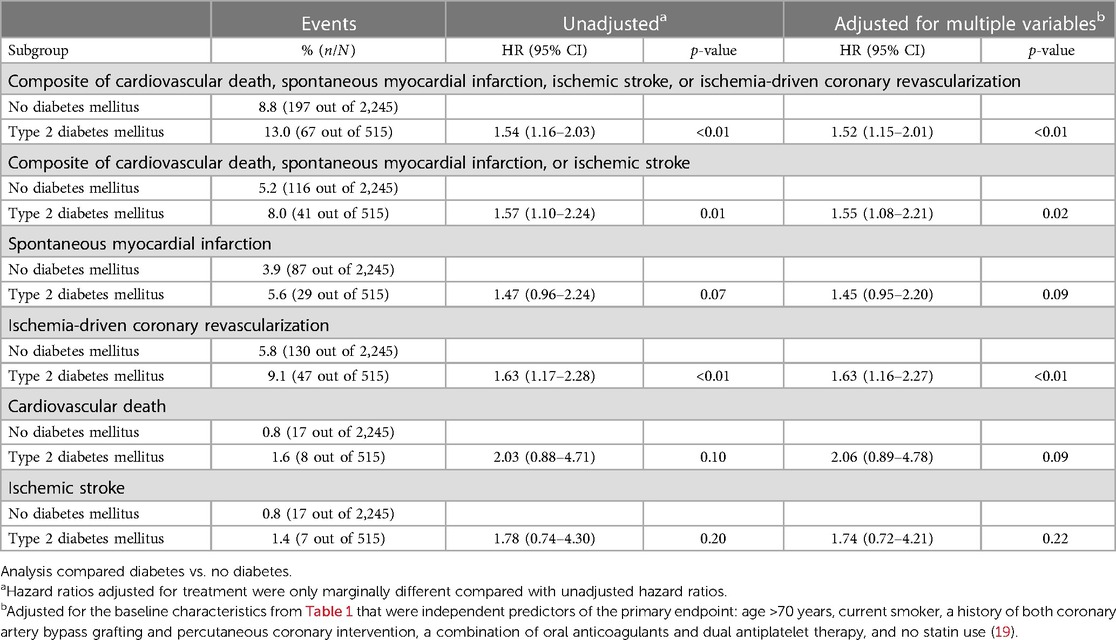
The primary composite endpoint of cardiovascular death, spontaneous myocardial infarction, ischemic stroke, or ischemia-driven coronary revascularization (MACE+) in the placebo group occurred in 13.0% (67 out of 515) participants with T2DM and in 8.8% (197 out of 2,245) of the participants without diabetes (Figure 1 and Table 2). Adjusted HR (95% CI) for MACE+ in the T2DM group was 1.52 (95% CI 1.15–2.01, p < 0.01) compared with the group without diabetes (Figure 1). The participants with T2DM had an increased hazard for all secondary endpoints compared with the participants without diabetes, although this did not reach statistical significance for spontaneous myocardial infarction, cardiovascular death, and ischemic stroke (Table 2).
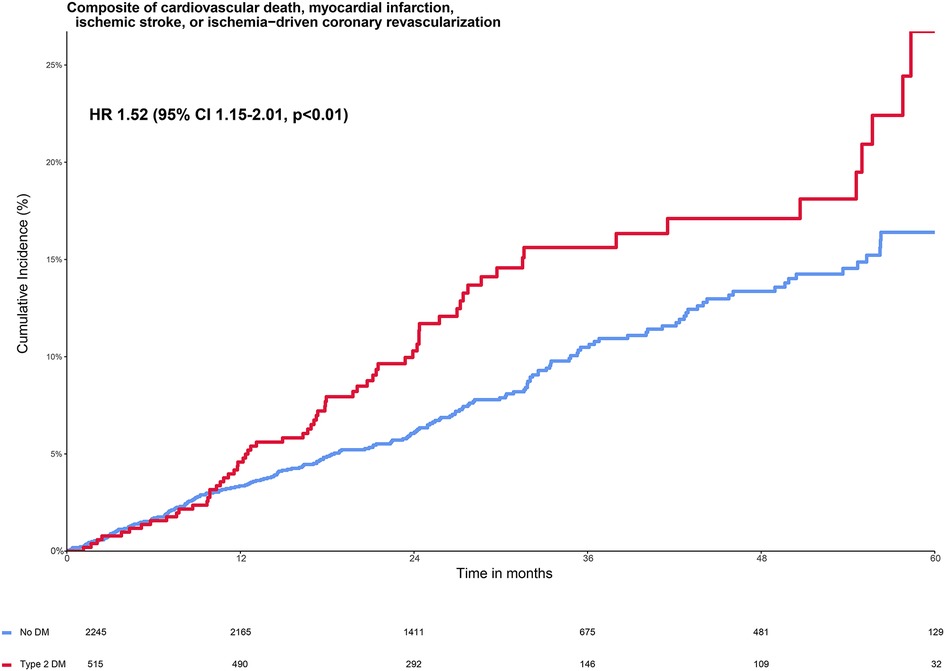
Figure 1. Cumulative incidence of primary composite endpoint stratified by diabetes status in the placebo group. The Kaplan–Meier curve shows the cumulative incidence of the primary composite endpoint of cardiovascular death, myocardial infarction, ischemic stroke, or ischemia-driven coronary revascularization in patients with type 2 diabetes (red line) and without type 2 diabetes (blue line). The figure shows the increased risk of patients with diabetes vs. without diabetes on the primary composite endpoint with a hazard ratio of 1.52 (95% 1.15–2.01, p < 0.01), adjusted for the baseline characteristics from Table 1 that were independent predictors of the primary endpoint: age >70 years, current smoker, a history of both coronary artery bypass grafting and percutaneous coronary intervention, a combination of oral anticoagulants and dual antiplatelet therapy, and no statin use (19).
Endpoints in relation to T2DM status and randomized treatment
The effects of colchicine on the primary composite endpoint and, separately, MACE, spontaneous myocardial infarction, and ischemia-driven coronary revascularization were consistent in the participants with and without T2DM (Figures 2, 3). No DM status-by-treatment interaction was found (Figure 3). The cumulative incidence of myocardial infarction and ischemia-driven coronary revascularization in the colchicine and placebo groups in the participants with diabetes at baseline and without diabetes are shown in the Supplementary material (Supplementary Figures S1, S2). Although the colchicine-treated participants had more non-cardiovascular death compared to placebo, no significant difference was reported in any single cause of non-cardiovascular fatalities across treatment groups.
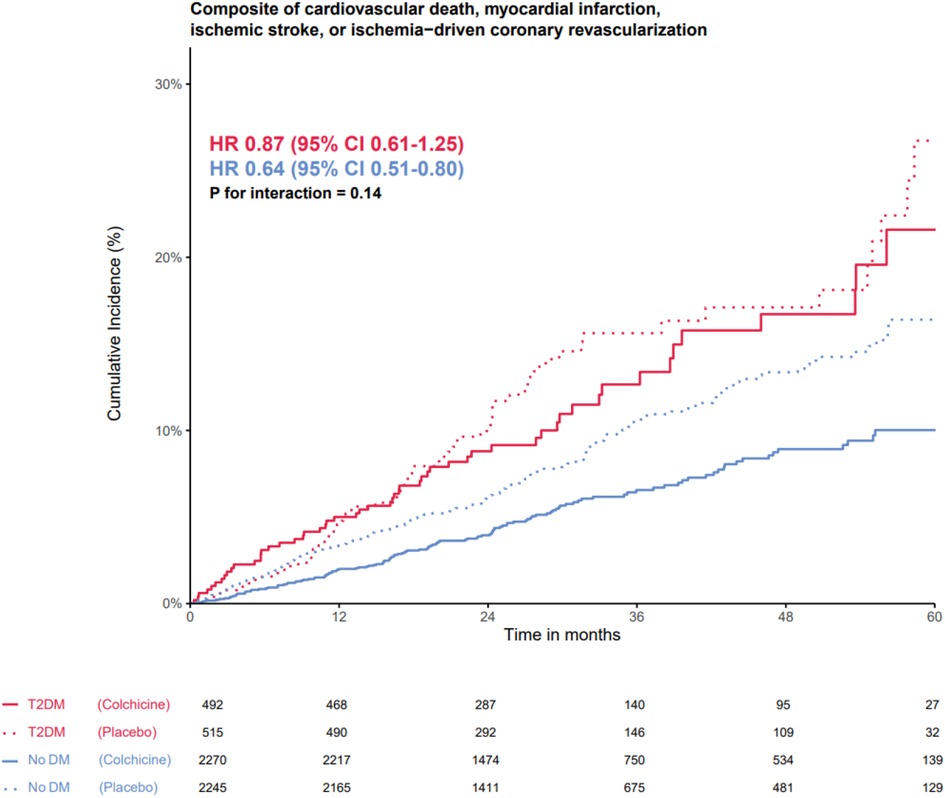
Figure 2. The efficacy of colchicine vs. placebo on the primary composite endpoint stratified by diabetes status. The Kaplan–Meier curve shows the effect of colchicine 0.5 mg (solid lines) once daily vs. placebo (dotted lines) on the primary composite endpoint of cardiovascular death, myocardial infarction, ischemic stroke, or ischemia-driven coronary revascularization in patients with type 2 diabetes (red lines) and without type 2 diabetes (blue lines). The hazard ratios for the treatment effect of colchicine did not show an interaction between the group with and without DM on the primary composite endpoint.
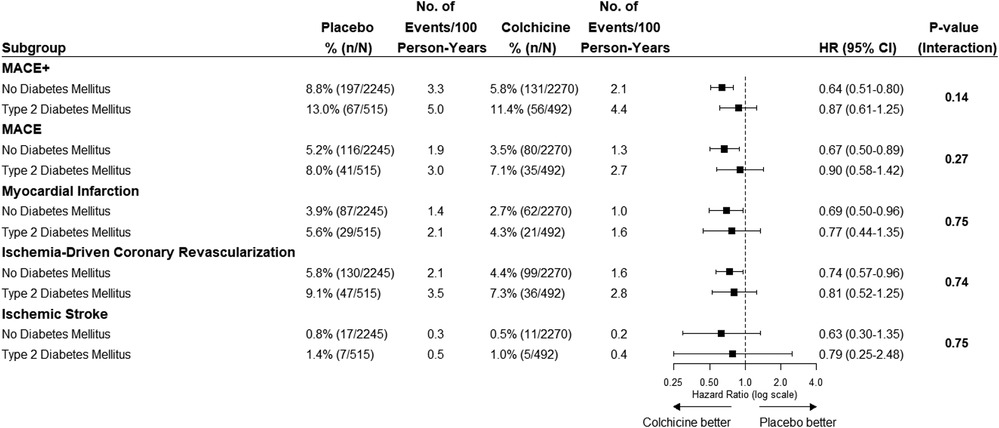
Figure 3. The efficacy of colchicine vs. placebo on the primary composite endpoint and secondary endpoints stratified by diabetes status. The figure shows the effect of colchicine 0.5 mg once daily vs. placebo on the primary composite endpoint of cardiovascular death, myocardial infarction, ischemic stroke, or ischemia-driven coronary revascularization and secondary outcomes. The hazard ratios for the treatment effect of colchicine did not show an interaction between the group with and without diabetes on the primary composite endpoint and secondary endpoints. MACE+, primary composite outcome of cardiovascular death, spontaneous myocardial infarction, ischemic stroke, or ischemia-driven coronary revascularization; MACE, cardiovascular death, spontaneous myocardial infarction, or ischemic stroke.
New-onset T2DM
New-onset T2DM occurred in 83 participants during follow-up. No significant difference was found in the baseline characteristics between the colchicine and placebo groups (Table 3). The incidence of new-onset T2DM was lower in the colchicine treatment-arm group (1.5%, 34 out of 2,270) compared with the placebo group (2.2%, 49 out of 2,245). However, no statistically significant difference was reported (p = 0.10).
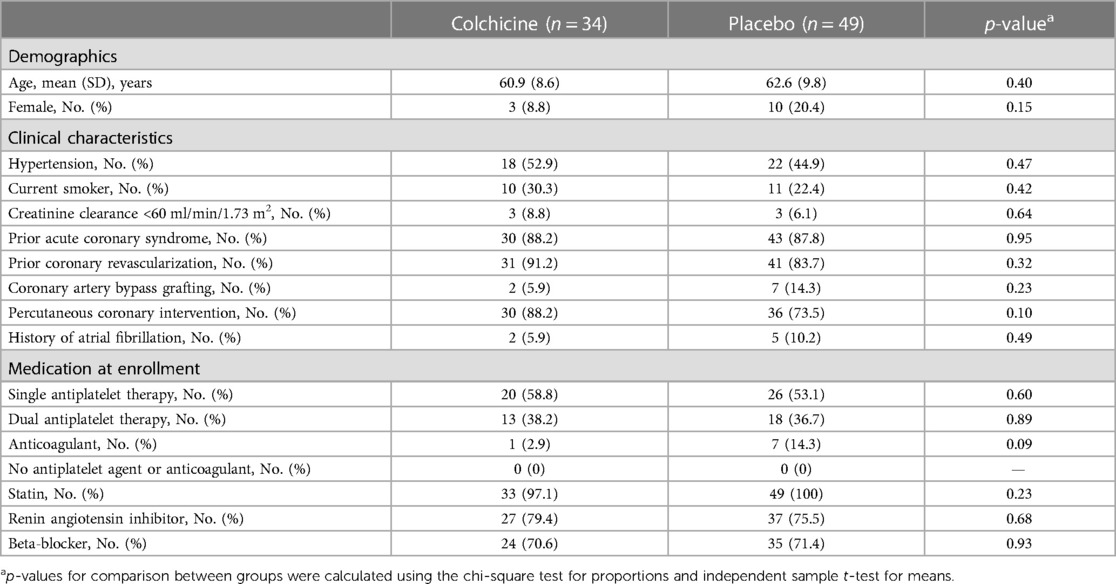
Table 3. Baseline characteristics by treatment group of participants that developed new-onset type 2 diabetes.
Premature permanent discontinuation of study medication
Of the participants with T2DM, 16 (3.3%) participants reported experiencing side effects that resulted in premature permanent discontinuation of colchicine compared with 14 (2.7%) participants in the placebo group (Supplementary Table S1).
Discussion
This LoDoCo2 substudy confirms that patients with chronic CAD who also have T2DM are at higher risk of MACE than patients without T2DM. It also demonstrates that colchicine produces consistent benefits in preventing recurrent MACE in patients with and without T2DM. While the incidence of new-onset T2DM in the colchicine group was numerically lower, this difference was not statistically significant.
The hypothesized underlying role of inflammation in T2DM relates to the belief that obesity results in the recruitment of macrophages into the adipose tissue and subsequent induction of a pro-inflammatory environment, which contributes to insulin resistance (20–22). Concomitant relative islet beta-cell dysfunction attributed to either inflammation or genetic predisposition results in insulin deficiency and further propels hyperglycemia contributing to the development or progression of T2DM (21–25). Therapy with IL-1 and IL-1β antibodies specifically targets these cytokines mediated by the NLRP3 inflammasome, whereas colchicine directly attenuates the inflammasome (26, 27). Also, the mechanisms of actions of colchicine reach beyond the IL-1 pathway (15, 28). Therefore, a wider therapeutic effect could be expected from colchicine, but we were unable to demonstrate this.
The effects of anti-inflammatory therapy on cardiovascular events had also been assessed in the pre-specified DM substudy of CANTOS. The study showed that the beneficial effect of the IL-1β inhibitor canakinumab in patients with a baseline high-sensitivity C-reactive protein of ≥2 mg/L and history of MI did not differ between participants with and without T2DM (11, 29). The current LoDoCo2 substudy adds to the accumulating evidence confirming the consistent reduction of the composite primary endpoint by the anti-inflammatory drug colchicine, independent of T2DM status. Because patients with DM are at higher risk of adverse cardiovascular events, they can be expected to derive greater absolute benefits from colchicine than patients without DM. This was also recently hypothesized in patients with type 1 diabetes (30).
The effects of anti-IL-1 therapy on glycemic control have also been studied in patients with T2DM. A study on the use of anti-IL-1 therapy in patients with rheumatoid arthritis and T2DM reported improved glycemic control (9). A clinical trial in 70 patients with an IL-1-receptor antagonist in T2DM improved glycemic control and reduced inflammation, although these findings have not been confirmed (10, 31). In CANTOS, the HbA1c values were not affected by IL-1β inhibition (11). This could not be explored in the LoDoCo2 study because glycemic control was not measured.
The effect of anti-inflammatories on the incidence of new-onset T2DM was only prospectively studied in CANTOS, showing no treatment difference between the treatment and placebo groups (11). For colchicine, two retrospective cohort studies compared new-onset T2DM in patients treated with colchicine or without colchicine for gout. Both studies showed a reduction in new-onset T2DM in the colchicine population, compared with the findings of the present study (32–34). The numerical lower rate of new-onset T2DM in the present study is consistent with the hypothesis that anti-inflammatory therapy by using colchicine possibly prevents or delays new-onset T2DM, although low numbers preclude any definitive conclusions. Statins also have anti-inflammatory properties, but meta-analyses have suggested an increase in the risk of new-onset T2DM with statins (34–36). This raises the possibility that different (inflammatory) pathways are involved in new-onset DM and the development of atherosclerosis.
Many currently available therapies for T2DM have anti-inflammatory properties, but it is not known whether their anti-inflammatory effects are beneficial (37). Several ongoing trials are investigating the role of anti-inflammatory therapy in patients with CAD and diabetes, such as the ZEUS trial with ziltivekimab (NCT05021835) and the CLEAR SYNERGY trial with colchicine (NCT03048825).
Limitations
The present study contains several limitations. First, the incidence of new-onset DM could have been underestimated in the LoDoCo2 trial population because new-onset DM was defined at the start of pharmacological treatment, whereas non-pharmacological (lifestyle) recommendations may precede pharmacological treatment. Second, the LoDoCo2 trial was not designed or powered to assess the effect of colchicine in patients with DM or the incidence of new-onset DM. Third, changes in the treatment of DM and measures of glycemic control were not routinely collected. Information on other variables influencing inflammation was also unavailable, such as body mass index, diet, and physical activity (5, 6, 37, 38). Lastly, the effects of colchicine on any specific cause of death were previously analyzed, showing no association with any cause (39). Subgroup analyses by DM status cannot further inform the effects of colchicine vs. placebo on any specific cause of death.
In conclusion, based on the current evidence, the beneficial effects of colchicine on cardiovascular endpoints are consistent regardless of DM status. The potential benefits of colchicine in preventing new-onset DM require further investigation. These findings are only hypothesis-generating and require larger prospective trials to confirm the results.
Data availability statement
The datasets presented in this article are not readily available because individual participant data that underlie the results reported in this article, after de-identification (text, tables, figures, and supplements), will be made accessible for analyses approved by the LoDoCo2 steering committee. The data will be accessible for researchers who provide a methodologically sound proposal, approved by the steering committee to avoid overlap with planned or ongoing analyses. All requests for data can be done via the steering committee, to be contacted viaYS5tb3N0ZXJkQG1lYW5kZXJtYy5ubA==. Requests to access the datasets should be directed toYS5tb3N0ZXJkQG1lYW5kZXJtYy5ubA==.
Ethics statement
The studies involving humans were approved by a centralized institutional review board in each participating country (MEC-U, Nieuwegein, Netherlands, and Sir Charles Gairdner Group HREC, Perth, Australia). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization of the analysis was conducted by JC, SM, NM, and JT. The data analysis was performed by NM, TO, JT, CB, JC, and SM. The data were fully accessible to all authors. The original draft was written by NM, JL, JE, JT, CT, SS, WB, JC, and SM. All authors were involved in the interpretation of the data and writing of the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
The LoDoCo2 trial was supported by the National Health Medical Research Council of Australia, a grant from the Sir Charles Gairdner Research Advisory Committee, the Withering Foundation the Netherlands, the Netherlands Heart Foundation, the Netherlands Organization for Health Research and Development, and a consortium of Teva, Disphar, and Tiofarma in the Netherlands.
Acknowledgments
We thank all the patients for their participation in the trial, the trial investigators, and the coordinators at all the centers.
Conflict of interest
AM reports membership of advisory boards of and/or consultancy for AstraZeneca, Bayer, Boehringer Ingelheim, and Novartis. AM will not accept personal fees; these fees will be donated to research. JE reports consulting/honoraria support from AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, GlaxoSmithKline, Pfizer, Janssen, Sanofi-Aventis, and Servier, and grants and/or in-kind support from AstraZeneca, Bayer, Boehringer-Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Pfizer, Janssen, and Sanofi-Aventis. PT reports grants, travel support, and honoraria from Amarin, Amgen, AstraZeneca, Bristol-Myers Squibb, Merck, and Pfizer. CT reports membership of advisory boards and/or consultancy for AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk. WB reports membership of advisory boards and/or honoraria from Amgen, AstraZeneca, Boehringer Ingelheim, Daiichi-Sankyo, Novo Nordisk, and Sanofi-Aventis. JC reports membership in advisory boards with Amgen and AstraZeneca. Author JT was employed by Cardialysis BV.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1244529/full#supplementary-material
References
1. Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. (2009) 54(23):2129–38. doi: 10.1016/j.jacc.2009.09.009
2. Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. (2018) 17(1):83. doi: 10.1186/s12933-018-0728-6
3. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes: the task force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J. (2019) 41(3):407–77. doi: 10.1093/eurheartj/ehz425
4. Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. (2017) 389(10085):2239–51. doi: 10.1016/S0140-6736(17)30058-2
5. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. (2006) 444(7121):840–6. doi: 10.1038/nature05482
6. Hotamisligil GS. Inflammation and metabolic disorders. Nature. (2006) 444(7121):860–7. doi: 10.1038/nature05485
7. Stienstra R, Tack CJ, Kanneganti TD, Joosten LA, Netea MG. The inflammasome puts obesity in the danger zone. Cell Metab. (2012) 15(1):10–8. doi: 10.1016/j.cmet.2011.10.011
8. Stienstra R, van Diepen JA, Tack CJ, Zaki MH, van de Veerdonk FL, Perera D, et al. Inflammasome is a central player in the induction of obesity and insulin resistance. Proc Natl Acad Sci U S A. (2011) 108(37):15324–9. doi: 10.1073/pnas.1100255108
9. Ruscitti P, Masedu F, Alvaro S, Airò P, Battafarano N, Cantarini L, et al. Anti-interleukin-1 treatment in patients with rheumatoid arthritis and type 2 diabetes (track): a multicentre, open-label, randomised controlled trial. PLoS Med. (2019) 16(9):e1002901. doi: 10.1371/journal.pmed.1002901
10. Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, et al. Interleukin-1–receptor antagonist in type 2 diabetes mellitus. N Engl J Med. (2007) 356(15):1517–26. doi: 10.1056/NEJMoa065213
11. Everett BM, Donath MY, Pradhan AD, Thuren T, Pais P, Nicolau JC, et al. Anti-inflammatory therapy with canakinumab for the prevention and management of diabetes. J Am Coll Cardiol. (2018) 71(21):2392–401. doi: 10.1016/j.jacc.2018.03.002
12. Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, et al. Colchicine in patients with chronic coronary disease. N Engl J Med. (2020) 383(19):1838–47. doi: 10.1056/NEJMoa2021372
13. Tardif J-C, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. (2019) 381(26):2497–505. doi: 10.1056/NEJMoa1912388
14. Cronstein BN, Molad Y, Reibman J, Balakhane E, Levin RI, Weissmann G. Colchicine alters the quantitative and qualitative display of selectins on endothelial cells and neutrophils. J Clin Invest. (1995) 96(2):994–1002. doi: 10.1172/jci118147
15. Leung YY, Yao Hui LL, Kraus VB. Colchicine—update on mechanisms of action and therapeutic uses. Semin Arthritis Rheum. (2015) 45(3):341–50. doi: 10.1016/j.semarthrit.2015.06.013
16. Robertson S, Martínez GJ, Payet CA, Barraclough JY, Celermajer DS, Bursill C, et al. Colchicine therapy in acute coronary syndrome patients acts on caspase-1 to suppress NLRP3 inflammasome monocyte activation. Clin Sci (Lond). (2016) 130(14):1237–46. doi: 10.1042/cs20160090
17. Mohammadnia N, Los J, Opstal TSJ, Fiolet ATL, Eikelboom JW, Mosterd A, et al. The effects of colchicine in patients with diabetes mellitus and chronic coronary artery disease: a post-hoc analysis of the Lodoco2-trial. Eur Heart J. (2022) 43(Supplement_2). doi: 10.1093/eurheartj/ehac544.2401
18. Nidorf SM, Fiolet ATL, Eikelboom JW, Schut A, Opstal TSJ, Bax WA, et al. The effect of low-dose colchicine in patients with stable coronary artery disease: the Lodoco2 trial rationale, design, and baseline characteristics. Am Heart J. (2019) 218:46–56. doi: 10.1016/j.ahj.2019.09.011
19. Opstal TSJ, Fiolet ATL, Broekhoven AV, Mosterd A, Eikelboom JW, Nidorf SM, et al. Colchicine in patients with chronic coronary disease in relation to prior acute coronary syndrome. J Am Coll Cardiol. (2021) 78(9):859–66. doi: 10.1016/j.jacc.2021.06.037
20. Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. (2011) 11(11):738–49. doi: 10.1038/nri3071
21. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. (2011) 11(2):98–107. doi: 10.1038/nri2925
22. de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett. (2008) 582(1):97–105. doi: 10.1016/j.febslet.2007.11.057
23. Cerf M. Beta cell dysfunction and insulin resistance. Front Endocrinol. (2013) 4:37. doi: 10.3389/fendo.2013.00037
24. Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. (2002) 110(6):851–60. doi: 10.1172/jci15318
25. Florez JC. Newly identified loci highlight beta cell dysfunction as a key cause of type 2 diabetes: where are the insulin resistance genes? Diabetologia. (2008) 51(7):1100. doi: 10.1007/s00125-008-1025-9
26. Grebe A, Hoss F, Latz E. NLRP3 inflammasome and the IL-1 pathway in atherosclerosis. Circ Res. (2018) 122(12):1722–40. doi: 10.1161/circresaha.118.311362
27. Opstal TSJ, Hoogeveen RM, Fiolet ATL, Silvis MJM, The SHK, Bax WA, et al. Colchicine attenuates inflammation beyond the inflammasome in chronic coronary artery disease. Circulation. (2020) 142(20):1996–8. doi: 10.1161/CIRCULATIONAHA.120.050560
28. Herder C, Dalmas E, Böni-Schnetzler M, Donath MY. The IL-1 pathway in type 2 diabetes and cardiovascular complications. Trends Endocrinol Metab. (2015) 26(10):551–63. doi: 10.1016/j.tem.2015.08.001
29. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. (2017) 377(12):1119–31. doi: 10.1056/NEJMoa1707914
30. Johansen NJ, Knop FK. The potential of colchicine for lowering the risk of cardiovascular events in type 1 diabetes. Eur Heart J Cardiovasc Pharmacother. (2023) 9(4):311–7. doi: 10.1093/ehjcvp/pvad005
31. van Asseldonk EJ, Stienstra R, Koenen TB, Joosten LA, Netea MG, Tack CJ. Treatment with anakinra improves disposition index but not insulin sensitivity in non-diabetic subjects with the metabolic syndrome: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. (2011) 96(7):2119–26. doi: 10.1210/jc.2010-2992
32. Wang L, Sawhney M, Zhao Y, Carpio GR, Fonseca V, Shi L. Association between colchicine and risk of diabetes among the veterans affairs population with gout. Clin Ther. (2015) 37(6):1206–15. doi: 10.1016/j.clinthera.2015.03.010
33. Chu C-C, Chen Y-C, Lin M-H, Wu W-T, Liu F-C, Chen H-C, et al. Association between clinical use of colchicine and risk of type 2 diabetes mellitus among gouty patients: a nationwide cohort study. Int J Environ Res Public Health. (2022) 19(6):3395. doi: 10.3390/ijerph19063395
34. Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. (2001) 286(1):64–70. doi: 10.1001/jama.286.1.64
35. Rajpathak SN, Kumbhani DJ, Crandall J, Barzilai N, Alderman M, Ridker PM. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care. (2009) 32(10):1924–9. doi: 10.2337/dc09-0738
36. Preiss D, Seshasai SRK, Welsh P, Murphy SA, Ho JE, Waters DD, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. (2011) 305(24):2556–64. doi: 10.1001/jama.2011.860
37. Hamer M, Sabia S, Batty GD, Shipley MJ, Tabák AG, Singh-Manoux A, et al. Physical activity and inflammatory markers over 10 years. Circulation. (2012) 126(8):928–33. doi: 10.1161/CIRCULATIONAHA.112.103879
38. Giugliano D, Ceriello A, Esposito K. The effects of diet on inflammation. J Am Coll Cardiol. (2006) 48(4):677–85. doi: 10.1016/j.jacc.2006.03.052
Keywords: diabetes mellitus, coronary artery disease, colchicine, inflammation, prevention
Citation: Mohammadnia N, Los J, Opstal TSJ, Fiolet ATL, Eikelboom JW, Mosterd A, Nidorf SM, Budgeon CA, Tijssen JGP, Thompson PL, Tack CJ, Simsek S, Bax WA, Cornel JH and El Messaoudi S (2023) Colchicine and diabetes in patients with chronic coronary artery disease: insights from the LoDoCo2 randomized controlled trial. Front. Cardiovasc. Med. 10:1244529. doi: 10.3389/fcvm.2023.1244529
Received: 22 June 2023; Accepted: 12 September 2023;
Published: 6 October 2023.
Edited by:
Isabella Russo, University of Turin, ItalyReviewed by:
Panagiotis Theofilis, Hippokration General Hospital, GreeceMattia Galli, Agostino Gemelli University Polyclinic (IRCCS), Italy
© 2023 Mohammadnia, Los, Opstal, Fiolet, Eikelboom, Mosterd, Nidorf, Budgeon, Tijssen, Thompson, Tack, Simsek, Bax, Cornel and El Messaoudi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jan H. Cornel amFuaGVpbi5jb3JuZWxAcmFkYm91ZHVtYy5ubA==
†These authors have contributed equally to this work and share last authorship
 Niekbachsh Mohammadnia1
Niekbachsh Mohammadnia1 Jan Los
Jan Los John W. Eikelboom
John W. Eikelboom Stefan M. Nidorf
Stefan M. Nidorf Peter L. Thompson
Peter L. Thompson Suat Simsek
Suat Simsek Jan H. Cornel
Jan H. Cornel Saloua El Messaoudi
Saloua El Messaoudi