
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Cardiovasc. Med. , 12 September 2023
Sec. General Cardiovascular Medicine
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1244275
This article is part of the Research Topic Cardiorenal Disease: A practical approach View all 8 articles
 Yosef Manla1,2*
Yosef Manla1,2* Obada Kholoki1
Obada Kholoki1 Feras Bader1
Feras Bader1 Oshin Kanwar2
Oshin Kanwar2 Emna Abidi2,3
Emna Abidi2,3 Wasim S. El Nekidy3
Wasim S. El Nekidy3 Fadi Hijazi4
Fadi Hijazi4 Nizar Attallah4
Nizar Attallah4
Background and aim: Little is known about the burden of cardiorenal syndrome (CRS) and cardiorenal anemia syndrome (CRAS) in the Middle East Region. Furthermore, whether the occurrence rates of CRAS differ across heart failure (HF) phenotypes is not widely investigated. We aimed to examine the prevalence of CRS and CRAS in patients with HF, compare characteristics of patients with CRAS-HFrEF vs. CRAS-HFpEF, and investigate anemia association with 1-year all-cause hospitalizations.
Methods: HF patients who visited a multidisciplinary HF clinic at a single center between 10-2015 and 06-2022 (n = 968) were retrospectively included. Differences in rates of CRAS prevalence, and patients’ characteristics of those with CRAS-HFrEF vs. CRAS-HFpEF were determined using appropriate testing methods. Generalized estimating equation (GEE) models were used to determine if anemia was associated with higher rates of hospitalization.
Results: CRS was prevalent in 34.4% of subjects, while 25.3% had CRAS. CRAS prevalence rates among patients with HFpEF vs. HFrEF were comparable (27.2% vs. 24.2%, p = 0.3). Compared to patients with HFrEF-CRAS, those with HFpEF-CRAS were more likely females (p < 0.001), had a higher burden of hypertension (p = 0.01), and lower hemoglobin (p = 0.02). In an adjusted GEE model, anemia was associated with an average increase of 1.8 admissions in CRS patients (p = 0.015).
Conclusion: In patients with HF, 1 in 3 patients presented with CRS, and 1 in 4 patients had CRAS. The prevalence of CRAS was comparable among those HFpEF and HFrEF. Anemia was associated with an increased rate of 1-year all-cause hospitalization in CRS patients.
Cardiorenal syndrome (CRS) is a comprehensive term encompassing the intricate relationship between simultaneous cardiac and renal impairments, wherein the deterioration of one organ initiates, perpetuates, or accelerates the decline in the other (1–3). This occurs due to a vicious cycle of feedback mechanisms comprising neuroregulatory hormones, oxidative stressors, and inflammatory cytokines (3, 4). Patients with CRS may develop comorbid anemia, resulting in cardiorenal anemia syndrome (CRAS) (4). CRAS is attributed to numerous factors, including erythropoietin resistance and/or deficiency, iron deficiency, or due to the occurring inflammatory changes. As a result, patients develop impaired oxygen transport and tissue hypoxia, contributing to a worse prognosis and poor quality of life (4–7). While the current management of CRAS is multifactorial, there is a lack of specific, evidence-based recommendations for patients with this syndrome. The use of guideline-directed medical therapy for heart failure (HF) as well as erythropoietin-stimulating agents for the management of anemia in chronic kidney disease (CKD) has not been shown to be as effective in patients with CRAS as in isolated conditions (2, 6, 7). There is a need for multidisciplinary inputs to incorporate and enforce all available screening and treatment modalities.
Furthermore, heart failure with preserved ejection fraction (HFpEF) encompasses complicated pathophysiology that involves multiple systems and secondary organ dysfunction, demonstrating a considerable association between HFpEF and CRS (8, 9). One can assume a stronger tendency towards developing comorbid anemia in patients with HFpEF compared to heart failure with reduced ejection fraction (HFrEF).
There are currently limited data on the burden of CRS or CRAS in the Middle East Region. Therefore, we aimed to investigate the prevalence of CRS and CRAS in patients with HF followed in an outpatient setting, compare the characteristics of patients with CRAS-HFrEF vs. CRAS-HFpEF, and investigate the association of anemia with 1-year all-cause hospitalization in patients with CRS at a single-center in the United Arab Emirates.
Consecutive patients with chronic HF who visited a multidisciplinary HF clinic at a single center in the United Arab Emirates between October 2015 and June 2022 (n = 968) were retrospectively included in this analysis; Patients included in this study established care in our HF clinic following either a referral from another clinic with a diagnosis of HF based on recent HF guidelines or after an event of hospitalization for acute HF. A flow diagram of patients included in this analysis is shown in Figure 1.
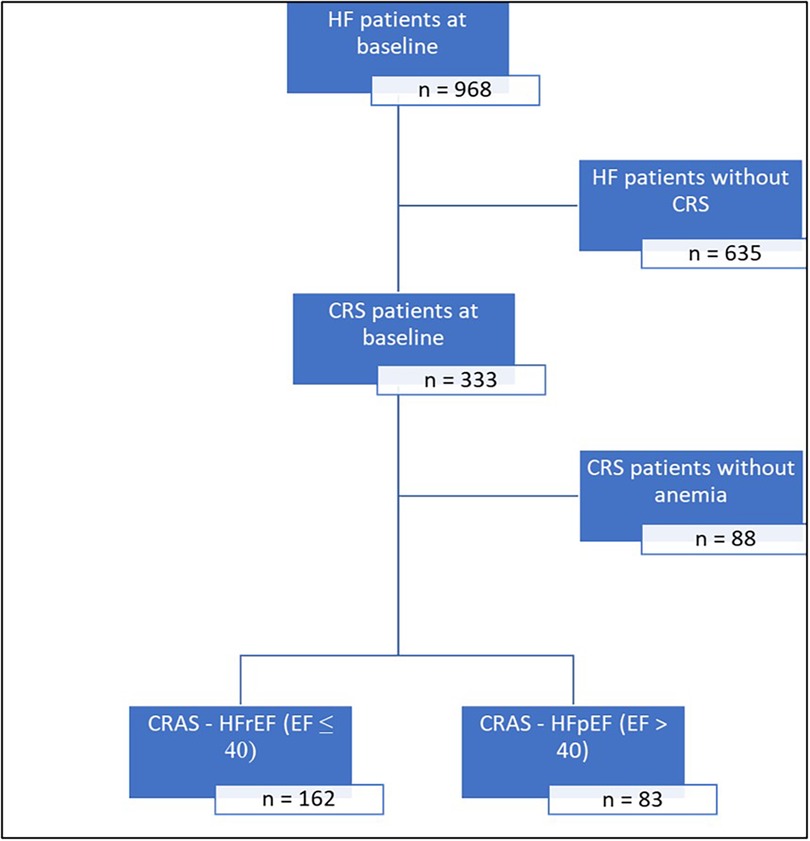
Figure 1. Flow diagram of patients included in this analysis. CRS, cardiorenal syndrome; CRAS, cardiorenal anemia syndrome; EF, ejection fraction; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction.
Data on patient demographics, baseline comorbidities, vital signs, and laboratory findings were collected using electronic medical records. Patients were determined to have CRS (referring to type II or IV) if they were diagnosed with HF and had an (eGFR) of <60 ml/min/1.73 m2 calculated using the Modification of Diet in Renal Disease (MDRD) study equation (10). Patients were determined to have CRAS if they had CRS and a hemoglobin level of <130 g/L for males and <120 g/L for females. HF phenotypes were defined as having an ejection fraction (EF) of ≤40 and >40 for HFrEF and HFpEF, respectively. The study was approved by the local Research Ethics Committee, and informed consent was waived due to the retrospective nature of the study.
Descriptive statistics for categorical variables were reported as absolute numbers (%), and continuous variables were reported as a mean ± SD or median [IQR] and were compared between CRAS-HFrEF and CRAS-HFpEF groups using Chi-square test and t-test (or Mann-Whitney U-test for non-normally distributed variables) as appropriate. Unadjusted and adjusted generalized estimating equation (GEE) models were used to test the hypothesis that, among CRS patients, the presence of anemia was associated with higher counts of hospital inpatient admission. We arrived at a final model that optimized the combination of model fit and parsimony, compared to a fully adjusted GEE model containing all relevant independent variables of clinical significance (age, gender, hypertension, diabetes, hyperlipidemia, ischemic disease and baseline ejection fraction) each possible model's performance using the Quasi-Likelihood under Independence Criterion. A p-value <0.05 was considered to be statistically significant. All statistical analyses were performed with JMP® Data Analysis (Software Version 16, SAS Institute Inc., Cary, NC, USA), Microsoft R Open [Microsoft Corporation (2020). Microsoft R: Microsoft R umbrella package. R package version 4.0.2.] and R Studio [RStudio Team (2021). RStudio: Integrated Development Environment for R. RStudio, PBC, Boston, MA].
CRS was prevalent in 34.4% (333/968) of subjects, while 25.3% (245/968) had CRAS. Among patients with CRS, 73.6% (245/333) had CRAS (Figure 1). Among patients with CRAS, the mean age was 67.7 ± 12.6, and 33% were females. The mean hemoglobin was 105.3 ± 13.4 g/L, and the mean eGFR was 36.2 ± 14.5 ml/min/1.73 m2. These patients also featured a high burden of CV comorbidities, including ischemic heart disease (IHD) (62.0%), hypertension (87.8%), hyperlipidemia (77.6%), and diabetes mellitus (79.2%). The mean ejection fraction was 37.4 ± 15.86%, and the mean BMI measured 29.2 ± 7 Kg/m2 (Table 1).

Table 1. Baseline characteristics of patients with cardiorenal anemia syndrome according to their heart failure phenotype.
Interestingly, when stratifying patients with HF according to HF phenotypes (HFrEF vs. HFpEF), rates of CRAS among patients with HFpEF vs. HFrEF were comparable (27.2% vs. 24.2%, p = 0.3). When comparing patient characteristics in the CRAS-HFpEF and CRAS-HFrEF groups, our study demonstrated that patients with HFpEF-CRAS were more likely females (49.4% vs. 24.7%, p < 0.001), had a higher burden of hypertension (95.2% vs. 84%, p = 0.01), and a lower burden of IHD (50.6% vs. 67.9%, p = 0.01). Patients with CRAS-HFpEF also had higher baseline systolic blood pressure (133.3 ± 24.6 vs. 120.1 ± 20.8 mmHg, p < 0.01) and higher BMI values (31.7 ± 7.5 vs. 27.9 ± 6.4 kg/m2, p < 0.001). Patients with CRAS-HFpEF also had lower hemoglobin levels (102.8 ± 14.5 vs. 106.6 ± 12.7 g/L, p = 0.02). There was no significant difference in the degree of renal dysfunction between both groups (p = 0.1).
At 1-year follow-up, 171 patients with CRS completed their follow-up visit (mean follow-up period of 369.7 ± 47.2 days). During the follow-up period, 262 events of hospitalizations occurred with an average of 1.7 and 1.1 admissions in the CRAS and non-anemic CRS patient groups, respectively. Unadjusted GEE model tested the hypothesis that the presence of anemia in CRS patients was associated with higher counts of inpatient hospitalizations. In this small sample size, the unadjusted model estimates showed an association between anemia and hospitalization that did not reach statistical significance (β = 0.43, p = 0.06). However, upon adjusting the model for patient age and the history of IHD, the model estimated that the presence of anemia was associated with an average increase of 1.8 hospital admission events (p = 0.015) (Table 2).
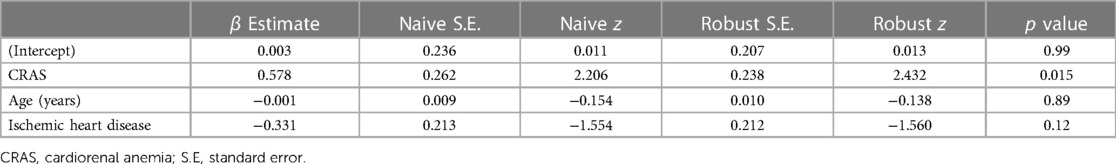
Table 2. Adjusted generalized estimating equations (GEE) model testing the association between the anemia and 1-year all-cause hospitalizations in patients with cardiorenal syndrome.
The present study showed that almost one-third of HF patients (34.4%) followed up at an outpatient clinic in the Middle East presented with CRS, aligning with previously published reports around the globe (1). In addition, our study revealed that 25.3% (245/968) of HF patients had CRAS, and 73.6% (245/333) of patients with CRS had concomitant anemia. Generally, the prevalence of CRAS among patients with HF ranges from 19% to 44%, while the prevalence of anemia in patients with CRS ranges from 39% to 45% (4). In a prospective, multi-center registry of 4,934 patients admitted with acute HF to 47 hospitals in seven Middle Eastern countries (Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, United Arab Emirates, and Yemen), CRAS was evident in 27% of the cases (6). This is comparable to the findings of our study despite it being conducted in outpatient settings.
In our paper, we aimed to explore the association between CRAS and HF phenotypes. However, our results showed no difference in the prevalence of CRAS among patients with HFpEF vs. HFrEF, despite HFpEF representing a more complicated and systematic syndrome. We found that patients with CRAS-HFpEF were more likely females, obese, and had hypertension, in accordance with the typical clinical picture of HFpEF patients (8). In addition, patients with HFpEF CRAS had lower hemoglobin, highlighting the profound effect of undergoing mechanisms in HFpEF on developing anemia (11). Patients in advanced stages of HF and CKD manifest anemia more severely (12).
Silverberg et al. showcased the positive correlation between worsening cardiovascular and renal function on the development of anemia, causing up to 70% and 49% increase in the prevalence of anemia, respectively (12).
Our study also evaluated the association of having anemia with the incidence of 1-year all-cause hospitalization in CRS patients, and we found that patients with CRAS were hospitalized an additional 1.8 times, on average, after adjusting for age and history of IHD. Previous studies have strongly linked anemia with poor outcomes and quality of life. Al-Jarallah et al. reported in their multi-center study from the Middle East that patients with CRAS had higher odds of all-cause mortality during hospital admission [adjusted odds ratio (aOR), 2.10; 95% confidence interval (CI): 1.34–3.31], and at 12 months follow-up (aOR, 1.45; 95% CI: [1.12–1.87]) (6). Another study conducted by Kim et al. showed that patients with CRAS were more likely to develop non-fatal myocardial infarctions, be re-hospitalized due to HF exacerbations, and die from cardiovascular causes compared to patients without CRAS (7). While hospitalized, patients with such a morbidity profile should benefit from multidisciplinary interventions during hospitalization, which can further improve patient outcomes, including reducing rehospitalization rates (13). Moreover, we noticed that the number of patients with CRS evaluated at 1-year follow-up was significantly lower than those seen at initial visits. This loss of patient follow-up might be attributed to visiting multiple providers and patient non-adherence, highlighting the need for multidisciplinary cardio-renal metabolic clinics that address these comorbidities at once (14, 15).
Our study had several limitations. This is a retrospective analysis of patients' baseline kidney function and hemoglobin at their first visit to the HF clinic, and being a single-point estimation of eGFR might underestimate the burden of CRAS as it fluctuates due to many factors (e.g., Medication). In addition, given the high prevalence of DM in our region (16), the resultant hyperfiltration in the early stage of diabetic nephropathy might have masked the worsening in renal function. Furthermore, GFR was estimated using the MDRD equation, which is less accurate at higher GFR values (10). Also, the presence of dilutional anemia among patients with volume overload might overestimate the prevalence of CRAS. Moreover, being a single-center study might limit the study's external validity, which calls for multi-center studies to evaluate the true burden of the disease. Lastly, we did not evaluate other clinical outcomes, such as mortality or quality of life measures, due to the study's retrospective nature and the study's sample size.
In this single-center study, approximately 1 in 3 patients with HF presented with CRS, and 1 in 4 patients with HF had CRAS. Interestingly, the prevalence of CRAS was comparable between HFpEF and HFrEF patients. In addition, anemia was associated with an increased rate of 1-year all-cause hospitalization in patients with CRS. Future multi-center studies are warranted to better estimate and tackle the burden of CRAS in the region.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Cleveland Clinic Abu Dhabi Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because of the retrospective nature of the study.
Conceptualization: YM, FB, and NA; Manuscript drafting: YM, OKh; Data collection: YM, and OKh; Statistical analysis: YM, and OKa; Methodology: EA, WN, NA, and FB; Reviewing and editing: EA, WN, FH, FB, and NA. All authors contributed to the article and approved the submitted version.
Preliminary data from this study was presented at the American Society of Nephrology meeting “Kidney Week 2021” and the presented abstract was published in the meeting online supplement.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hashmani S, Madhyastha R, El Nekidy W, Atallah B, Bader F, Attallah N. Polypharmacy in cardiorenal syndrome patients. Clin Nephrol. (2023) 99:141–8. doi: 10.5414/CN110989
2. Bader FM, Attallah N. Insights into cardiorenal interactions in acute decompensated heart failure. Curr Opin Cardiol. (2017) 32:203–8. doi: 10.1097/HCO.0000000000000378
3. Rangaswami J, Bhalla V, Blair JEA, Chang TI, Costa S, Lentine KL, et al. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American heart association. Circulation. (2019) 139:e840–78. doi: 10.1161/CIR.0000000000000664
4. McCullough PA. Anemia of cardiorenal syndrome. Kidney Int Suppl. (2021) 11:35–45. doi: 10.1016/j.kisu.2020.12.001
5. Silverberg DS, Wexler D, Iaina A, Steinbruch S, Wollman Y, Schwartz D. Anemia, chronic renal disease and congestive heart failure—the cardio renal anemia syndrome: the need for cooperation between cardiologists and nephrologists. Int Urol Nephrol. (2006) 38:295–310. doi: 10.1007/s11255-006-0064-8
6. Al-Jarallah M, Rajan R, Al-Zakwani I, Dashti R, Bulbanat B, Sulaiman K, et al. Incidence and impact of cardiorenal anaemia syndrome on all-cause mortality in acute heart failure patients stratified by left ventricular ejection fraction in the Middle East. ESC Heart Fail. (2019) 6:103–10. doi: 10.1002/ehf2.12351
7. Kim CJ, Choi I-J, Park H-J, Kim TH, Kim P-J, Chang K, et al. Impact of cardiorenal anemia syndrome on short-and long-term clinical outcomes in patients hospitalized with heart failure. Cardiorenal Med. (2016) 6:269–78. doi: 10.1159/000443339
8. Pfeffer MA, Shah AM, Borlaug BA. Heart failure with preserved ejection fraction in perspective. Circ Res. (2019) 124:1598–617. doi: 10.1161/CIRCRESAHA.119.313572
9. Agrawal A, Naranjo M, Kanjanahattakij N, Rangaswami J, Gupta S. Cardiorenal syndrome in heart failure with preserved ejection fraction—an under-recognized clinical entity. Heart Fail Rev. (2019) 24:421–37. doi: 10.1007/s10741-018-09768-9
10. Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF III, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
11. Okuno K, Naito Y, Asakura M, Sugahara M, Horimatsu T, Yasumura S, et al. Anemia has an impact on prognosis in heart failure with preserved ejection fraction with mild chronic kidney disease. IJC Heart & Vasculature. (2021) 34:100796. doi: 10.1016/j.ijcha.2021.100796
12. Silverberg DS, Wexler D, Blum M, Keren G, Sheps D, Leibovitch E, et al. The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiol. (2000) 35:1737–44. doi: 10.1016/S0735-1097(00)00613-6
13. Manla Y, Ghalib HH, Al Badarin F, Ferrer R, John TL-S, Abdalla K, et al. Implementation of a multidisciplinary inpatient heart failure service and its association with hospitalized patient outcomes: first experience from the Middle East and north Africa region. Heart Lung. (2023) 61:92–7. doi: 10.1016/j.hrtlng.2023.05.004
14. Manla Y, Almahmeed W. Cardiometabolic clinics: is there a need for a multidisciplinary clinic? Front Clin Diabetes Healthc. (2022) 3:880468. doi: 10.3389/fcdhc.2022.880468
15. Manla Y, Almahmeed W. The pandemic of coronary heart disease in the Middle East and North Africa: What clinicians need to know. Curr Atheroscler Rep. (2023) 25:543–57. doi: 10.1007/s11883-023-01126-x
16. Malekpour MR, Abbasi-Kangevari M, Ghamari SH, Khanali J, Heidari-Foroozan M, Moghaddam SS, et al. The burden of metabolic risk factors in North Africa and the Middle East, 1990-2019: findings from the Global Burden of Disease Study. EClinicalMedicine. (2023) 60:102022. doi: 10.1016/j.eclinm.2023.102022
Keywords: cardiorenal anemia syndrome, cardiorenal syndrome, heart failure, Middle East, chronic kidney disease
Citation: Manla Y, Kholoki O, Bader F, Kanwar O, Abidi E, El Nekidy WS, Hijazi F and Attallah N (2023) The prevalence of cardiorenal anemia syndrome among patients with heart failure and its association with all-cause hospitalizations: a retrospective single-center study from the Middle East. Front. Cardiovasc. Med. 10:1244275. doi: 10.3389/fcvm.2023.1244275
Received: 22 June 2023; Accepted: 25 August 2023;
Published: 12 September 2023.
Edited by:
Rafael De La Espriella, Hospital Clínico Universitario de Valencia, SpainReviewed by:
Alvaro Aceña, University Hospital Fundación Jiménez Díaz, Spain© 2023 Manla, Kholoki, Bader, Kanwar, Abidi, El Nekidy, Hijazi and Attallah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yosef Manla eW9zZWYubWFubGExQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.