
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 31 October 2023
Sec. Coronary Artery Disease
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1242339
Objective: YKL-40, previously known as chitinase-3-like protein 1 (CHI3L1), is an inflammation-related glycoprotein that promotes atherosclerosis, but its application and optimal cut-off value as a prognostic biomarker in coronary heart disease (CHD) require more clinical evidence. Thus, this prospective study aimed to evaluate the linkage of serum YKL-40 with disease features, inflammatory cytokines, and major adverse cardiovascular events (MACEs) in CHD patients.
Methods: A total of 410 CHD patients were enrolled for serum YKL-40 determination via enzyme-linked immunosorbent assay. Meanwhile, serum YKL-40 levels in 100 healthy controls (HCs) were also quantified.
Results: YKL-40 level was higher in CHD patients compared with that in HCs (P < 0.001). YKL-40 was positively linked with hyperlipidemia (P = 0.014), diabetes mellitus (P = 0.001), fasting blood glucose (P = 0.045), C-reactive protein (P < 0.001), the Gensini score (P < 0.001), and stenosis degree (graded by the Gensini score) (P < 0.001) in CHD patients. In addition, an elevated YKL-40 level was associated with increased levels of tumor necrosis factor alpha (P = 0.001), interleukin (IL)-1β (P = 0.001), IL-6 (P < 0.001), and IL-17A (P = 0.002) in CHD patients. The 1-/2-/3-year cumulative MACE rates of CHD patients were 5.5%, 14.4%, and 25.0%, respectively. Regarding the prognostic capability, YKL-40 ≥100 ng/ml (the median cut-off value) (P = 0.003) and YKL-40 ≥150 ng/ml (the third interquartile cut-off value) (P = 0.021) reflected an elevated accumulating MACE rate, whereas accumulating MACE was not different between CHD patients with YKL-40 ≥80 and <80 ng/ml (the first interquartile cut-off value) (P = 0.083).
Conclusion: Serum YKL-40 is positively linked with inflammatory cytokines and the Gensini score, whose high expression cut-off by 100 and 150 ng/ml estimates a higher MACE risk in CHD patients.
Coronary heart disease (CHD), which consists of stable angina and acute coronary syndromes, is a series of life-threatening diseases that account for approximately 7 million deaths globally each year (1, 2). CHD is caused by the interaction of complex pathogenic factors, such as cholesterol-rich apolipoprotein B (ApoB) accumulation within the arterial intima, chronic inflammation, vascular endothelial dysfunction, and other factors contributing to atherogenesis and CHD (3–5). Despite appropriate application of lifestyle, pharmacological, or surgical interventions, the major cardiovascular outcomes of CHD remain unsatisfactory (6, 7). Thus, continued efforts in seeking biomarkers that estimate the clinical prognosis of CHD patients are still necessary.
YKL-40, previously named chitinase-3-like protein 1 (CHI3L1), is an inflammation-related glycoprotein that belongs to the glycoside hydrolase family and is involved in CHD progression as noted in several studies (8–10). A previous study showed that YKL-40 increases the lesion area of atherosclerotic plaques in an apolipoprotein E-deficient (ApoE−/−) mouse model (9). Another study revealed that YKL-40 exacerbates atherosclerosis by inducing endothelial cell inflammation and activation of vascular smooth muscle cells (10).
Some clinical studies have identified the ability of YKL-40 to identify the disease risk and severity of CHD (11–14). In addition, the prognostic value of YKL-40 for CHD patients cannot be ignored (15–17). For example, one study determined YKL-40 in patients with ST-segment elevation myocardial infarction (STEMI) and found that it is positively linked with in-hospital major adverse cardiovascular events (MACEs) in these patients (16). Another study revealed the predictive value of YKL-40 for mortality in stable CHD patients (17). Nonetheless, these studies focused on either stable CHD or STEMI (15–17), and the optimal cut-off value of YKL-40 requires more clinical evidence.
Hence, this prospective study quantified serum YKL-40 in 410 CHD patients to observe the linkage of serum YKL-40 with disease features, inflammatory cytokines, and MACE in these patients.
A total of 410 patients with CHD diagnosed by coronary angiography between January 2019 and October 2022 were consecutively enrolled in this research. The patients were included if they were (i) diagnosed with CHD via coronary angiography; (ii) aged more than 18 years old; and (iii) about to and able to participate. The patients were excluded if they (i) had malignant diseases, (ii) had pronounced infections, or (iii) were pregnant or lactating. In addition, 100 people who recently underwent physical examination in our hospital were enrolled as healthy controls (HCs). The inclusion criteria of HCs were as follows: (i) those without abnormalities in the physical examination; (ii) those who were age-matched and gender-matched with the CHD patients and aged from 45 to 79 years old with a male-to-female ratio of 7:3; and (iii) those who were ready to cooperate with this research. HCs who have histories of drug abuse, are pregnant, or are lactating were excluded. The Ethics Committee of the First Affiliated Hospital, Harbin Medical University, supported this research (approval No. 2018098). Informed consent was obtained from each subject.
Demographics, medical history, biochemical indexes, and disease characteristics were collected from the CHD patients. In addition, the Gensini score was collected by coronary arteriography to assess the luminal stenosis, which was the sum of all lesion scores, and was further graded into mild (<32), moderate (32–56), and severe (>56). Each lesion score was completed by multiplying the stenosis score and the lesion site score (18).
Peripheral blood (PB) samples were gathered from the CHD patients at enrollment during the acute phase; meanwhile, PB samples of HCs were also obtained at enrollment. Thereafter, PB samples were isolated for serum analysis to detect YKL-40. Serum YKL-40 was detected via enzyme-linked immunosorbent assay (ELISA) using a commercial kit (Cat. No. DY2599, R&D, USA). For the majority of the CHD patients (n = 354), inflammatory cytokines including interleukin (IL)-1β, tumor necrosis factor alpha (TNF-α), IL-6, and IL-17A were also measured by ELISA. The ELISA kits used were obtained from the R&D Systems (USA), and the catalog numbers of the kits were DTA00D, HSLB00D, D6050, and QK317. All tests were performed in triplicate and were strictly conducted in accordance with the instructions of the kit.
The CHD patients underwent routine follow-ups (median, 15.5 months; range, 1.1–43.7 months). During follow-ups (last follow-up date: December 2022), MACEs were recorded and defined similarly in previous research (19), including cardiovascular death, myocardial infarction, unplanned coronary revascularization, and hospital admission for cardiovascular causes. In addition, the cumulative MACE risk was calculated for evaluation.
For the CHD patients, the median value of YKL-40 was approximately 100 ng/ml, the first interquartile (Q1) value of YKL-40 was approximately 80 ng/ml, and the third interquartile (Q3) value of YKL-40 was approximately 150 ng/ml. For prognosis analysis, YKL-40 was classified into different levels with the following cut-off values: (1) median cut-off value, ≥100 and <100 ng/ml levels; (2) Q1 cut-off value, ≥80 and <80 ng/ml levels; and (3) Q3 cut-off value, ≥150 and <150 ng/ml levels.
SPSS 26.0 (IBM, USA) and GraphPad Prism 7.0.1 (GraphPad Software, USA) were utilized for data processing and figure plotting, respectively. The Wilcoxon rank-sum test or Kruskal–Wallis test was used to estimate the difference between the two or among multiple groups as appropriate. Student’s t-test and χ2 test were used to compare age and gender between the CHD patients and HCs, respectively. The ability of YKL-40 to distinguish between CHD patients and HCs was recognized by a plotted receiver operating characteristic (ROC) curve. In addition, the ability of YKL-40 to predict 1-year, 2-year, and 3-year MACE risks was also evaluated by ROC curves. Correlation analysis was conducted using the Spearman test. Kaplan–Meier curves were performed to show the accumulating MACE rates, in which the log-rank test was used to compare the accumulating MACE rates between patients with different YKL-40 levels. Univariate and stepwise forward multivariate Cox regression analyses were conducted to identify the influence factors of MACE. P < 0.050 indicated statistical significance.
There were 122 (29.8%) females and 288 (70.2%) males among the 410 CHD patients whose mean age was 62.9 ± 9.9 years. The median [interquartile range (IQR)] Gensini score was 32.3 (17.0–50.1). In 104 CHD patients with diabetes mellitus (DM), the body mass index (BMI) of 61 (58.7%) and 23 (22.1%) patients was ≥24 kg/m2 and ≥28 kg/m2, respectively. In addition, 199 (48.5%), 124 (30.2%), and 87 (21.3%) patients were classified as having mild, moderate, and severe stenosis degrees, respectively, based on the Gensini score. In addition, 410 (100.0%), 289 (70.5%), 116 (28.3%), 177 (43.2%), and 157 (38.3%) CHD patients received antiplatelet therapy, β-blocker, calcium channel blockers, statin or other lipid-lowering therapy, and angiotensin-converting enzyme inhibitor (ACEI) or angiotensin II receptor blocker (ARB), respectively. Table 1 provides detailed information on the CHD patients.
In addition, there were 30 (30.0%) females and 70 (70.0%) males in HCs, with a mean age of 61.6 ± 8.2 years. Meanwhile, age (P = 0.222) and gender (P = 0.962) had no significant difference between the CHD patients and HCs (Supplementary Table S1).
Serum YKL-40 level was higher in the CHD patients compared with that in HCs [median (IQR): 100.2 (79.6–148.6) ng/ml vs. 51.7 (31.5–65.9) ng/ml, P < 0.001] (Figure 1A). Meanwhile, serum YKL-40 possessed a pleasing ability to distinguish CHD patients from HCs [area under the curve (AUC): 0.892, 95% confidence interval (CI): 0.860–0.925] (Figure 1B).
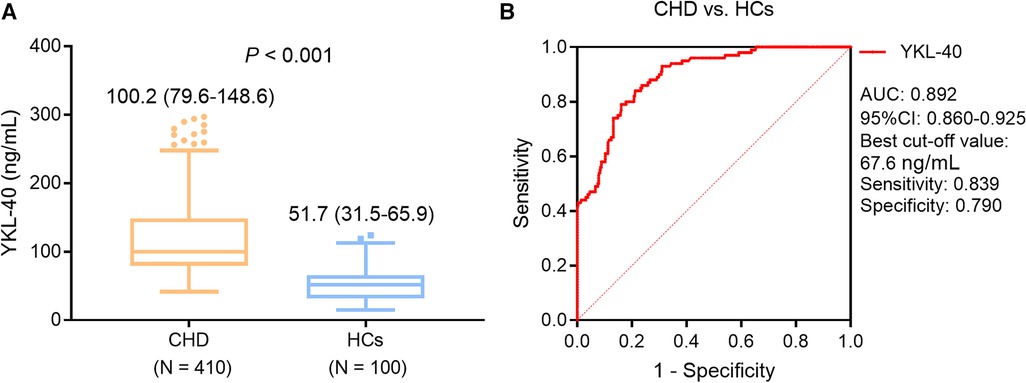
Figure 1. Serum YKL-40 level was higher in CHD patients compared with that in HCs. Comparison of serum YKL-40 between CHD patients and HCs. (A) ROC curve for the ability of serum YKL-40 to differentiate CHD patients from HCs (B).
Serum YKL-40 was positively linked with hyperlipidemia (P = 0.014) and DM (P = 0.001) but not with age (P = 0.622), sex (P = 0.622), BMI (P = 0.077), smoking (P = 0.703), hypertension (P = 0.095), or chronic kidney disease (CKD) (P = 0.296) in CHD patients (Figures 2A–H). In addition, serum YKL-40 was positively correlated with fasting blood glucose (FBG) (P = 0.045) and C-reactive protein (CRP) (P < 0.001) but not with the other biochemical indexes in CHD patients (all P > 0.050) (Table 2). Moreover, serum YKL-40 was not related to β-blocker (P = 0.273), calcium channel blockers (P = 0.296), statin or other lipid-lowering therapy (P = 0.061), or ACEI or ARB (P = 0.186) in CHD patients (Supplementary Table S2).
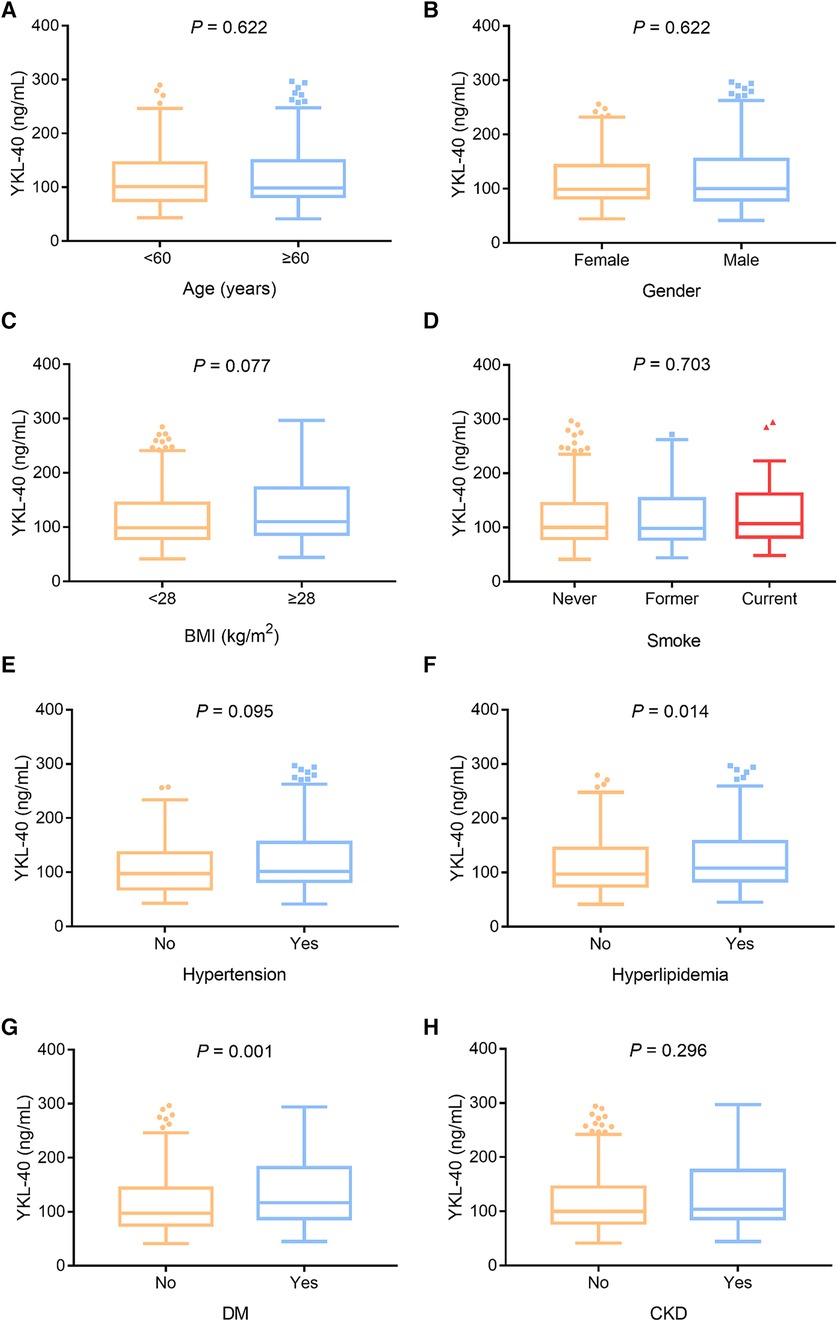
Figure 2. Serum YKL-40 was positively linked with hyperlipidemia and DM in CHD patients. Correlation of serum YKL-40 with age (A), sex (B), BMI (C), smoking (D), hypertension (E), hyperlipidemia (F), DM (G), and CKD (H) in CHD patients.
Serum YKL-40 was positively associated with the Gensini score (P < 0.001) (Figure 3A) and stenosis degree (P < 0.001) (Figure 3B) in CHD patients.
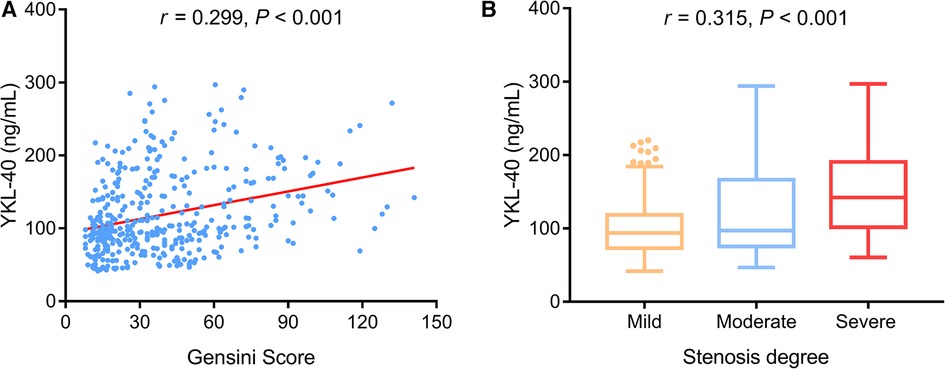
Figure 3. Serum YKL-40 was positively related to the Gensini score and stenosis degree in CHD patients. Association of serum YKL-40 with Gensini score (A) and stenosis degree (B) in CHD patients.
With regard to the inflammatory cytokines, elevated serum YKL-40 level was linked with increased TNF-α (P = 0.001) (Figure 4A), IL-1β (P = 0.001) (Figure 4B), IL-6 (P < 0.001) (Figure 4C), and IL-17A (P = 0.002) (Figure 4D) in CHD patients.
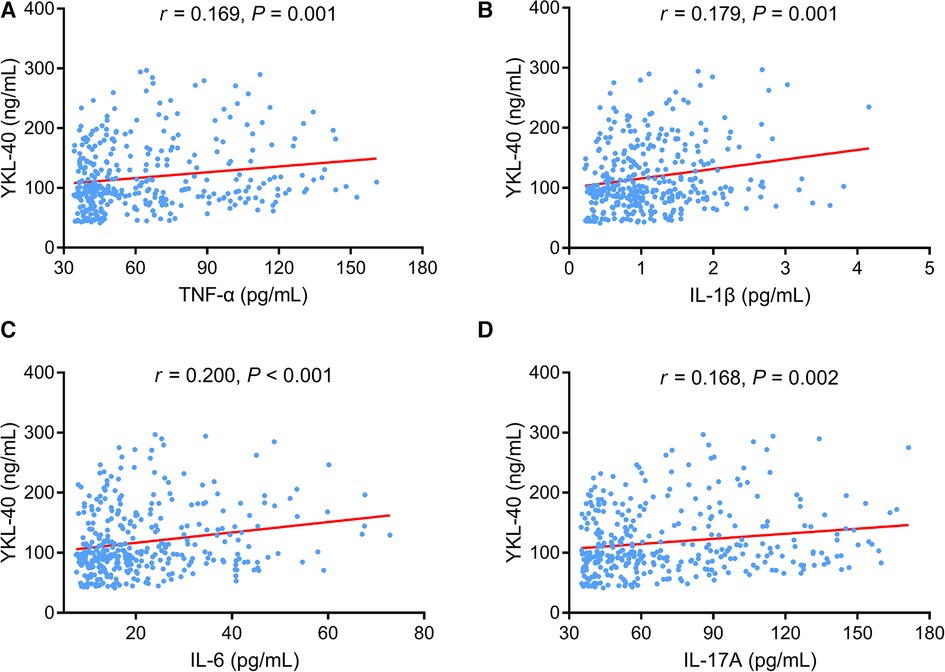
Figure 4. Serum YKL-40 positively correlated with TNF-α, (IL-1β, IL-6, and IL-17A in CHD patients. Linkage of serum YKL-40 with TNF-α (A), IL-1β (B), IL-6 (C), and IL-17A (D) in CHD patients.
Among the 41 CHD patients who had MACE, there were five patients with cardiovascular death, 11 patients with myocardial infarction, 17 patients with unplanned coronary revascularization, and eight patients with hospital admission for cardiovascular causes. The 1-/2-/3-year cumulative MACE rates of CHD patients were 5.5%, 14.4%, and 25.0%, respectively (Figure 5A). Moreover, serum YKL-40 was cut off by its Q1 (80 ng/ml), median (100 ng/ml), and Q3 (150 ng/ml) values in CHD patients to explore its prognostic value. Accumulating MACE rates did not vary between CHD patients with serum YKL-40 levels of ≥80 and <80 ng/ml (P = 0.083). In detail, the 1-/2-/3-year accumulating MACE rates were 7.4%, 16.6%, and 26.1%, respectively, in patients with a serum YKL-40 level of ≥80 ng/ml and 0.0%, 7.7%, and 22.1%, respectively, in patients with a serum YKL-40 level of <80 ng/ml (Figure 5B). In contrast, a serum YKL-40 level of ≥100 ng/ml was linked with elevated MACE in CHD patients (P = 0.003). The 1-/2-/3-year accumulating MACE rates were 8.6%, 20.8%, and 35.3%, respectively, in patients with a serum YKL-40 level of ≥100 ng/ml and 2.3%, 7.3%, and 16.0%, respectively, in patients with a serum YKL-40 level of <100 ng/ml (Figure 5C). In addition, a serum YKL-40 level of ≥150 ng/ml was correlated with increased MACE risk in CHD patients (P = 0.021). Specifically, the 1-, 2-, and 3-year cumulative MACE rates were 8.7%, 22.2%, and 36.4%, respectively, in patients with a serum YKL-40 level of ≥150 ng/ml and 4.5%, 11.5%, and 20.5%, respectively, in patients with a serum YKL-40 level of <150 ng/ml (Figure 5D). Furthermore, when YKL-40 was considered as a continuous variable, elevated YKL-40 level was correlated with increased MACE risk (hazard ratio: 1.007, 95% CI: 1.002–1.011, P = 0.006).
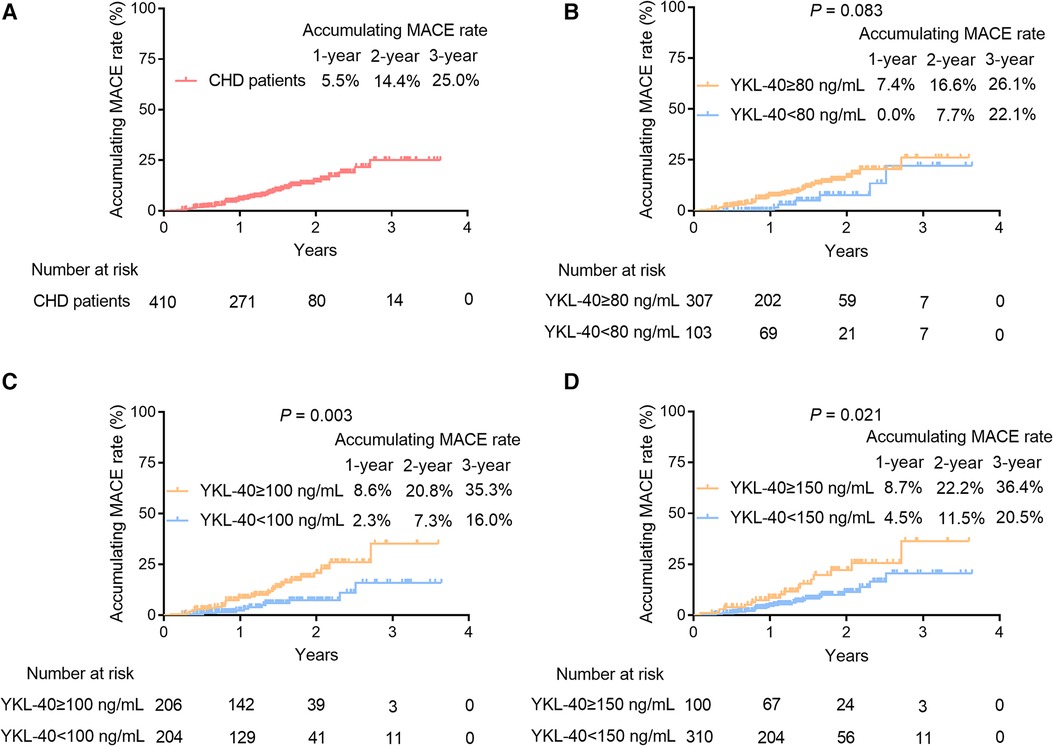
Figure 5. Serum YKL-40 ≥100 and ≥150 ng/ml were associated with increased MACE accumulation in CHD patients. The 1-/2-/3-year cumulative MACE rate in CHD patients (A) Prognostic value of serum YKL-40 cut-off values of 80 ng/ml (B), 100 ng/ml (C), and 150 ng/ml (D) in CHD patients.
Furthermore, ROC curves showed that serum YKL-40 had a good value for predicting 1-year MACE risk (AUC, 0.729; best cut-off value, 109.0 ng/ml; sensitivity, 0.800; specificity, 0.672) (Figure 6A); meanwhile, serum YKL-40 disclosed a general capability for estimating 2-year (AUC, 0.671; best cut-off value, 104.6 ng/ml; sensitivity, 0.750; specificity, 0.578) (Figure 6B) and 3-year (AUC, 0.643; best cut-off value, 104.6 ng/ml; sensitivity, 0.732; specificity, 0.569) (Figure 6C) MACE risk in CHD patients.
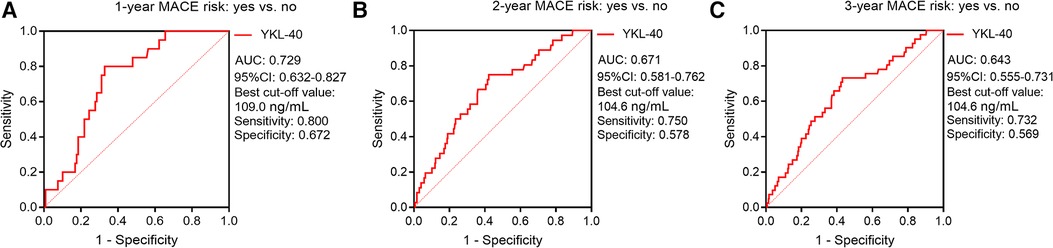
Figure 6. Serum YKL-40 possessed a good value for predicting 1-/2-/3-year MACE risks. ROC curves for the ability of serum YKL-40 to estimate 1-year (A), 2-year (B), and 3-year (C) MACE risks in CHD patients.
The univariate Cox regression analysis revealed that YKL-40 ≥100 ng/ml (P = 0.005) and YKL-40 ≥150 ng/ml (P = 0.024) were linked with increased MACE risk in CHD patients. However, the stepwise forward multivariate Cox regression analysis exhibited that YKL-40 was not an independent influence factor for MACE in CHD patients (P > 0.050). History of smoke (former or current vs. never) (P = 0.014), CRP ≥5 mg/L (P = 0.024), and higher Gensini score (P = 0.002) were independently associated with increased MACE risk, while serum uric acid (SUA) >350 μmol/L (P = 0.049) and low-density lipoprotein cholesterol (LDL-C) ≥2.60 mmol/L (P = 0.015) were related to declined MACE risk in CHD patients (Supplementary Table S3).
Moreover, the combination of YKL-40 ≥100 ng/ml, history of smoke, SUA >350 μmol/L, LDL-C ≥2.60 mmol/L, CRP ≥5 mg/L, and higher Gensini score disclosed a general value for estimating 3-year MACE risk in CHD patients (AUC, 0.620) (Supplementary Figure S1A), so did the combination of YKL-40 ≥50 ng/ml, history of smoke, SUA >350 μmol/L, LDL-C ≥2.60 mmol/L, CRP ≥5 mg/L, and higher Gensini score (AUC, 0.603) (Supplementary Figure S1B).
The engagement of YKL-40 in several chronic diseases has been reported before (20, 21). A previous study reported that elevated YKL-40 is related to the presence and severity of metabolic syndrome (20). Another study suggested that YKL-40 is associated with all lipoprotein subclasses in patients with type I DM (21). CHD patients are often complicated with dyslipidemia and metabolic syndrome (22). The present study revealed that serum YKL-40 is positively related to hyperlipidemia, DM, and FBG in CHD patients. These results could be explained as follows: (1) YKL-40 increased the concentration of lipoprotein subclasses, which are crucial in the pathological process of hyperlipidemia (21, 23). Hence, serum YKL-40 was positively linked with hyperlipidemia in CHD patients. (2) YKL-40 suppressed insulin-mediated glucose metabolism by cross talk with intestinal fatty acid binding proteins (24, 25). Therefore, increased serum YKL-40 was linked with DM and increased FBG in CHD patients. These findings indicated that targeting YKL-40 might contribute to the reduction of the risk of complications (such as dyslipidemia and metabolic syndrome) in CHD patients.
Moreover, this study also showed that serum YKL-40 was positively linked with the Gensini score and stenosis degree in CHD patients, which could be explained by the fact that YKL-40 accelerated atherosclerotic plaque initiation and deterioration by inhibiting macrophage apoptosis, which aggravated luminal stenosis (9, 26, 27). As a result, serum YKL-40 was positively related to the Gensini score and stenosis degree in CHD patients.
YKL-40, secreted by locally activated macrophages and neutrophils, is a well-recognized inflammatory glycoprotein involved in both chronic and acute inflammation, and its positive correlation with many inflammatory cytokines, such as IL-6 and IL-18, has been observed previously (28–30). Also, it is exhibited that YKL-40 activates the nuclear transcription factor-kappa B (NF-κB) signaling pathway by promoting the NF-κB subunit nuclear translocation (31, 32). This study showed that serum YKL-40 was positively linked with TNF-α, IL-1β, IL-6, IL-17A, and CRP in CHD patients. The probable explanations were that (1) YKL-40 facilitated vascular inflammation, and thereafter, proinflammatory cytokines all surged in CHD patients (10, 33). As a result, serum YKL-40 was positively related to these inflammatory cytokines in CHD patients. (2) CRP is a systemic inflammatory marker secreted by hepatocytes in response to IL-6, and in this study, IL-6 was positively associated with serum YKL-40 in CHD patients (34). Consequently, a positive correlation was seen between serum YKL-40 and CRP in CHD patients.
The prognostic value of YKL-40 in cardiovascular diseases has been elucidated in some previous studies (35–37). For instance, one study found a positive linkage of YKL-40 with all-cause mortality in aortic stenosis patients (35). Similarly, another study showed that elevated YKL-40 is a risk factor for cardiovascular death in chronic CHD patients (36). The present study showed that increased serum YKL-40 was related to elevated MACE in CHD patients. The probable explanations are as follows: (1) Elevated YKL-40 was linked with aggravated inflammatory lesions and a more severe degree of artery stenosis, and the latter were considered risk factors for elevated MACE in CHD patients (16). (2) YKL-40 was a mediator of plaque vulnerability that increased the rupture risk of the fibrous cap, and then the MACE risk was elevated (38). Combining the above two reasons, serum YKL-40 was positively related to MACE risk in CHD patients. However, the stepwise forward multivariate Cox regression analysis exhibited that YKL-40 was not an independent influence factor for MACE in CHD patients, indicating that YKL-40 might exert its prognostic value with the interaction with lipid and inflammation.
Consistent with the previous studies (39, 40), this study set the cut-off value of YKL-40 by the median and interquartile values. Interestingly, serum YKL-40 cut-offs of 100 ng/ml (median) and 150 ng/ml (3/4 interquartile) had a good ability to estimate MACE in CHD patients, but when it was a cut-off of 80 ng/ml (1/4 interquartile), it lacked predictive value. The findings suggested that 100 ng/ml and 150 ng/ml serum YKL-40 levels appeared to be candidate prognostic biomarkers, and patients with YKL-40 levels of ≥100 and 150 ng/ml needed more close monitoring. Further verification was necessary.
There are some shortcomings in the current study. First, the follow-up period (median, 15.5 months) was too short to collect enough MACEs, which might result in underestimated MACE rate. Second, the measurement of serum YKL-40 in CHD patients was only conducted at enrollment during the acute phase, but its fluctuation during the CHD course was unknown, which might be an additional limitation in the current study. Third, because the comparison of serum YKL-40 between CHD patients and HCs was not the main objective of this study, the ratio of CHD patients and HCs was not balanced. Fourth, though YKL-40 disclosed good discriminating value between CHD patients and HCs, whether it was superior to a common blood test was unclear. Fifth, in vitro and in vivo experiments were not conducted in this study to investigate the underlying mechanism of YKL-40 in CHD. Sixth, this study lacked external validation in independent cohorts, which affected the generalization of the findings. Seventh, this study did not enroll disease controls, and confounders might exist. Eighth, according to the previous study (41), the YKL-40 level gradually decreased after exercise in CHD patients, but this issue was not explored in the current study.
Conclusively, increased serum YKL-40 level relates to elevated inflammatory cytokines and exacerbated artery stenosis, whose high expression cut-offs of 100 and 150 ng/ml reflect higher MACE risk in CHD patients. The findings indicate that serum YKL-40 may serve as a potential biomarker for identifying CHD patients with a high risk of unfavorable prognosis, which helps provide individualized management to these patients.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital, Harbin Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
MS and GZ contributed to the conception and design of the study. LD and HoS contributed to the acquisition of data. MS and GZ drafted the article. All authors contributed to the analysis and interpretation of data and revising it critically for important intellectual content. All authors contributed to the article and approved the submitted version.
This study was supported by Postdoctor Science Foundation of Heilongjiang Province (2022), Project of Guangdong Provincial Chinese Medicine Bureau (20231401, 20241383), and the Research Project Number of Maoming Science and Technology Bureau (2021184).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1242339/full#supplementary-material
Supplementary Figure S1
Combination of serum YKL-40 and independent factors showed a general value for estimating 3-year MACE risks. The ROC curve for the ability of the combination of YKL-40 ≥100 ng/mL, smoke, SUA >350 μmol/L, LDL-C ≥2.60 mmol/L, CRP ≥5 mg/L, and higher Gensini score to predict 3-year MACE risk in CHD patients (A). The ROC curve for the ability of the combination of YKL-40 ≥150 ng/mL, smoke, SUA >350 μmol/L, LDL-C ≥2.60 mmol/L, CRP ≥5 mg/L, and higher Gensini score to predict 3-year MACE risk in CHD patients (B). Fix correct style.
1. Agrawal H, Choy HK, Liu J, Auyoung M, Albert MA. Coronary artery disease. Arterioscler Thromb Vasc Biol. (2020) 40(7):e185–92. doi: 10.1161/ATVBAHA.120.313608
2. Ralapanawa U, Sivakanesan R. Epidemiology and the magnitude of coronary artery disease and acute coronary syndrome: a narrative review. J Epidemiol Glob Health. (2021) 11(2):169–77. doi: 10.2991/jegh.k.201217.001
3. Shao C, Wang J, Tian J, Tang YD. Coronary artery disease: from mechanism to clinical practice. Adv Exp Med Biol. (2020) 1177:1–36. doi: 10.1007/978-981-15-2517-9_1
4. Libby P. Inflammation during the life cycle of the atherosclerotic plaque. Cardiovasc Res. (2021) 117(13):2525–36. doi: 10.1093/cvr/cvab303
5. Medina-Leyte DJ, Zepeda-Garcia O, Dominguez-Perez M, Gonzalez-Garrido A, Villarreal-Molina T, Jacobo-Albavera L. Endothelial dysfunction, inflammation and coronary artery disease: potential biomarkers and promising therapeutical approaches. Int J Mol Sci. (2021) 22(8):3850. doi: 10.3390/ijms22083850
6. Reynolds HR, Shaw LJ, Min JK, Page CB, Berman DS, Chaitman BR, et al. Outcomes in the ISCHEMIA trial based on coronary artery disease and ischemia severity. Circulation. (2021) 144(13):1024–38. doi: 10.1161/CIRCULATIONAHA.120.049755
7. Stergiopoulos K, Boden WE, Hartigan P, Mobius-Winkler S, Hambrecht R, Hueb W, et al. Percutaneous coronary intervention outcomes in patients with stable obstructive coronary artery disease and myocardial ischemia: a collaborative meta-analysis of contemporary randomized clinical trials. JAMA Intern Med. (2014) 174(2):232–40. doi: 10.1001/jamainternmed.2013.12855
8. Zhao T, Su Z, Li Y, Zhang X, You Q. Chitinase-3 like-protein-1 function and its role in diseases. Signal Transduct Target Ther. (2020) 5(1):201. doi: 10.1038/s41392-020-00303-7
9. Chen L, Zheng J, Xue Q, Zhao Y. YKL-40 promotes the progress of atherosclerosis independent of lipid metabolism in apolipoprotein E(−/−) mice fed a high-fat diet. Heart Vessels. (2019) 34(11):1874–81. doi: 10.1007/s00380-019-01434-w
10. Jung YY, Kim KC, Park MH, Seo Y, Park H, Park MH, et al. Atherosclerosis is exacerbated by chitinase-3-like-1 in amyloid precursor protein transgenic mice. Theranostics. (2018) 8(3):749–66. doi: 10.7150/thno.20183
11. Song CL, Bin L, Diao HY, Wang JH, Shi YF, Lu Y, et al. Diagnostic value of serum YKL-40 level for coronary artery disease: a meta-analysis. J Clin Lab Anal. (2016) 30(1):23–31. doi: 10.1002/jcla.21804
12. Fang C, Chen Z, Zhang J, Pan J, Jin X, Yang M, et al. The value of serum YKL-40 and TNF-alpha in the diagnosis of acute ST-segment elevation myocardial infarction. Cardiol Res Pract. (2022) 2022:4905954. doi: 10.1155/2022/4905954
13. Sciborski K, Kuliczkowski W, Karolko B, Bednarczyk D, Protasiewicz M, Mysiak A, et al. Plasma YKL-40 levels correlate with the severity of coronary atherosclerosis assessed with the SYNTAX score. Pol Arch Intern Med. (2018) 128(11):644–8. doi: 10.20452/pamw.4345
14. Jin Y, Cao JN, Wang CX, Feng QT, Ye XH, Xu X, et al. High serum YKL-40 level positively correlates with coronary artery disease. Biomark Med. (2017) 11(2):133–9. doi: 10.2217/bmm-2016-0240
15. Schroder J, Jakobsen JC, Winkel P, Hilden J, Jensen GB, Sajadieh A, et al. Prognosis and reclassification by YKL-40 in stable coronary artery disease. J Am Heart Assoc. (2020) 9(5):e014634. doi: 10.1161/JAHA.119.014634
16. Cetin M, Kocaman SA, Canga A, Kirbas A, Yilmaz A, Erdogan T, et al. Elevated serum YKL-40 level predicts myocardial reperfusion and in-hospital MACE in patients with STEMI. Herz. (2013) 38(2):202–9. doi: 10.1007/s00059-012-3671-4
17. Harutyunyan M, Gotze JP, Winkel P, Johansen JS, Hansen JF, Jensen GB, et al. Serum YKL-40 predicts long-term mortality in patients with stable coronary disease: a prognostic study within the CLARICOR trial. Immunobiology. (2013) 218(7):945–51. doi: 10.1016/j.imbio.2012.10.015
18. Gensini GG. A more meaningful scoring system for determining the severity of coronary heart disease. Am J Cardiol. (1983) 51(3):606. doi: 10.1016/S0002-9149(83)80105-2
19. Greenwood JP, Ripley DP, Berry C, McCann GP, Plein S, Bucciarelli-Ducci C, et al. Effect of care guided by cardiovascular magnetic resonance, myocardial perfusion scintigraphy, or NICE guidelines on subsequent unnecessary angiography rates: the CE-MARC 2 randomized clinical trial. JAMA. (2016) 316(10):1051–60. doi: 10.1001/jama.2016.12680
20. Akboga MK, Yalcin R, Sahinarslan A, Yilmaz Demirtas C, Pasaoglu H, Abaci A. Increased serum YKL-40 level is associated with the presence and severity of metabolic syndrome. Anatol J Cardiol. (2016) 16(12):953–8.27443476
21. Pauley ME, Vinovskis C, MacDonald A, Baca M, Pyle L, Wadwa RP, et al. Triglyceride content of lipoprotein subclasses and kidney hemodynamic function and injury in adolescents with type 1 diabetes. J Diabetes Complications. (2023) 37(2):108384. doi: 10.1016/j.jdiacomp.2022.108384
22. Poznyak A, Grechko AV, Poggio P, Myasoedova VA, Alfieri V, Orekhov AN. The diabetes mellitus-atherosclerosis connection: the role of lipid and glucose metabolism and chronic inflammation. Int J Mol Sci. (2020) 21(5):1835. doi: 10.3390/ijms21051835
23. Ebrahim A, Mustafa AI, El-Shimi OS, Fathy MA. Serum YKL40: a novel potential link between inflammation and dyslipidemia in acne vulgaris. J Cosmet Dermatol. (2020) 19(5):1219–23. doi: 10.1111/jocd.13124
24. Di Rosa M, Malaguarnera L. Chitinase 3 like-1: an emerging molecule involved in diabetes and diabetic complications. Pathobiology. (2016) 83(5):228–42. doi: 10.1159/000444855
25. Zhang S, Sousa A, Lin M, Iwano A, Jain R, Ma B, et al. Role of chitinase 3-like 1 protein in the pathogenesis of hepatic insulin resistance in nonalcoholic fatty liver disease. Cells. (2021) 10(2):201. doi: 10.3390/cells10020201
26. Deng Y, Li G, Chang D, Su X. YKL-40 as a novel biomarker in cardio-metabolic disorders and inflammatory diseases. Clin Chim Acta. (2020) 511:40–6. doi: 10.1016/j.cca.2020.09.035
27. Huan W, Yandong L, Chao W, Sili Z, Jun B, Mingfang L, et al. YKL-40 aggravates early-stage atherosclerosis by inhibiting macrophage apoptosis in an aven-dependent way. Front Cell Dev Biol. (2021) 9:752773. doi: 10.3389/fcell.2021.752773
28. Rathcke CN, Vestergaard H. YKL-40, a new inflammatory marker with relation to insulin resistance and with a role in endothelial dysfunction and atherosclerosis. Inflamm Res. (2006) 55(6):221–7. doi: 10.1007/s00011-006-0076-y
29. Jankowska-Konsur A, Lyko M, Rubas K, Nowicka-Suszko D, Maj J, Szepietowski JC. Chitinase-3-like protein 1 (YKL-40): a new biomarker of inflammation in pyoderma gangrenosum. Acta Derm Venereol. (2022) 102:adv00646. doi: 10.2340/actadv.v101.978
30. Khashaba SA, Attwa E, Said N, Ahmed S, Khattab F. Serum YKL-40 and IL 17 in psoriasis: reliability as prognostic markers for disease severity and responsiveness to treatment. Dermatol Ther. (2021) 34(1):e14606. doi: 10.1111/dth.14606
31. Tang H, Sun Y, Shi Z, Huang H, Fang Z, Chen J, et al. YKL-40 induces IL-8 expression from bronchial epithelium via MAPK (JNK and ERK) and NF-kappaB pathways, causing bronchial smooth muscle proliferation and migration. J Immunol. (2013) 190(1):438–46. doi: 10.4049/jimmunol.1201827
32. Zhao T, Zeng J, Xu Y, Su Z, Chong Y, Ling T, et al. Chitinase-3 like-protein-1 promotes glioma progression via the NF-kappaB signaling pathway and tumor microenvironment reprogramming. Theranostics. (2022) 12(16):6989–7008. doi: 10.7150/thno.75069
33. Libby P. Inflammation in atherosclerosis-no longer a theory. Clin Chem. (2021) 67(1):131–42. doi: 10.1093/clinchem/hvaa275
34. Ridker PM, Rane M. Interleukin-6 signaling and anti-interleukin-6 therapeutics in cardiovascular disease. Circ Res. (2021) 128(11):1728–46. doi: 10.1161/CIRCRESAHA.121.319077
35. Arain F, Abraityte A, Bogdanova M, Solberg OG, Michelsen AE, Lekva T, et al. YKL-40 (chitinase-3-like protein 1) serum levels in aortic stenosis. Circ Heart Fail. (2020) 13(10):e006643. doi: 10.1161/CIRCHEARTFAILURE.119.006643
36. Wallentin L, Eriksson N, Olszowka M, Grammer TB, Hagstrom E, Held C, et al. Plasma proteins associated with cardiovascular death in patients with chronic coronary heart disease: a retrospective study. PLoS Med. (2021) 18(1):e1003513. doi: 10.1371/journal.pmed.1003513
37. Hobaus C, Tscharre M, Herz CT, Pesau G, Wrba T, Koppensteiner R, et al. YKL-40 levels increase with declining ankle-brachial index and are associated with long-term cardiovascular mortality in peripheral arterial disease patients. Atherosclerosis. (2018) 274:152–6. doi: 10.1016/j.atherosclerosis.2018.05.006
38. Tsantilas P, Lao S, Wu Z, Eberhard A, Winski G, Vaerst M, et al. Chitinase 3 like 1 is a regulator of smooth muscle cell physiology and atherosclerotic lesion stability. Cardiovasc Res. (2021) 117(14):2767–80. doi: 10.1093/cvr/cvab014
39. Zhang X, Liu L, Jiang N, Liu Y, Wang Q, Tang X, et al. Correlation of lipoprotein-associated phospholipase A2 and cerebral microbleeds in patients with acute ischaemic stroke. BMC Neurol. (2022) 22(1):482. doi: 10.1186/s12883-022-03000-w
40. Zhu Q, Xiao W, Bai Y, Ye P, Luo L, Gao P, et al. The prognostic value of the plasma N-terminal pro-brain natriuretic peptide level on all-cause death and major cardiovascular events in a community-based population. Clin Interv Aging. (2016) 11:245–53. doi: 10.2147/CIA.S98151
Keywords: coronary heart disease, chitinase-3-like protein 1, disease features, inflammatory cytokines, major adverse cardiovascular events
Citation: Song M, Zhang G, Shi H, Zhu E, Deng L and Shen H (2023) Serum YKL-40 in coronary heart disease: linkage with inflammatory cytokines, artery stenosis, and optimal cut-off value for estimating major adverse cardiovascular events. Front. Cardiovasc. Med. 10:1242339. doi: 10.3389/fcvm.2023.1242339
Received: 19 June 2023; Accepted: 22 September 2023;
Published: 31 October 2023.
Edited by:
Tommaso Gori, Johannes Gutenberg University Mainz, GermanyReviewed by:
Ümmügülsüm Can, Konya Education and Research Hospital, Türkiye© 2023 Song, Zhang, Shi, Zhu, Deng and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongtao Shen aHRzNzc5NTZAMTYzLmNvbQ== Li Deng ZGlsaWFvMDAzNzc0NzMxODNAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.