
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cardiovasc. Med. , 28 November 2023
Sec. Cardiovascular Surgery
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1239019
This article is part of the Research Topic Case Reports in Heart Surgery: 2023 View all 15 articles
Introduction: Left atrial dissection is a rare event, typically associated with cardiac manipulation. We report the first case of a left atrial dissection caused by parasitic infectious endocarditis, which required the use of patch repair for the damaged mitral annulus and valve.
Case Presentation: To treat heart failure in a 43-year-old man with left atrial dissection, we performed a patch repair of the mitral annulus and valve using autologous pericardium.
Conclusion: We encourage novel surgery for complicated infectious endocarditis.
Left atrial dissection (LatD) is an extremely rare condition characterized by a false blood-filled cavity or lumen extending from the mitral annulus (MA) to the left atrium (LA) (1, 2). It is often linked to cardiac manipulation, myocardial infarction (MI) and blunt cardiac trauma (1, 2). Spontaneous LatD are associated with conditions such as cardiac amyloidosis, severe mitral annular calcification, and infectious endocarditis (IE) in some studies (1, 2). The majority of IE cases are caused by bacterial or fungal infestation (1–3). Here, we present a unique LatD case caused by parasitic infestation of heart, along with the first report using pericardial patch repair for posterior mitral annulus in a LatD associated with IE (1–4). To prevent reinfection, we utilized autologous pericardial patch to repair MA and mitral valve (MV).
A 43-year-old Chinese man with no prior history of cardiac surgery or trauma complained diarrhea, general fatigue, and lower extremities edema for three months. Laboratory test revealed an absolute eosinophil count of 5.69 cells × 109/L with 37.8% eosinophils. Transthoracic echocardiogram (TTE) showed a 5.4 × 6.0 cm mass containing thrombus in the posterior wall of the left atrium (LA), causing functional mitral insufficiency and MV obstruction. In addition, there was a suspicious endocardial orifice beneath the root of the posterior mitral leaflet (PML) on the left ventricle (LV). Enhanced Computed tomography (CT) revealed the contrast filling the mass cavity, indicating intramural hematoma. (Figure 1) Left atrial dissection caused by parasitic infestation was suspected. Based on the patient's hemodynamic collapse, emergency surgery was necessary.

Figure 1. TTE showed a mass containing thrombus in the LA and a suspicious endocardial orifice beneath the root of the PML on the LV. CT revealed contrast filling the mass cavity.
The surgical procedures were recorded in Supplementary Material Video 1. By removing the thrombus and debris from the false cavity and excising the dissected endocardium, the surgeon found an orifice on the left ventricle beneath the root of the PML, which was confirmed as the entry of the LatD. Direct incision was performed in the P2, and the posterior MA was cut through to reach the orifice. An autologous pericardial patch with an ellipse shape was employed to reconstruct the defect of the LV and MA. After reaching the proposed neo-annulus line, the rest of the ellipse was used to augment the PML. An annuloplasty was performed with another pericardial band (refer to Figure 2). The reconstructed MV performed well under TEE after weaning from the cardiopulmonary bypass.
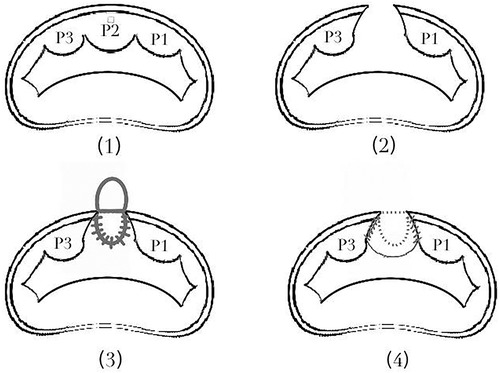
Figure 2. The procedure of patch repair for defect of LV, MV and MA. (2) To reach the orifice, we direct incised the P2, and cut through the posterior MA. (3) An ellipse-shape autologous pericardial patch was employed to reconstruct the defect of the LV and MA. (4) After reaching the proposed neo-annulus line, the rest of the ellipse was used to augment the PML.
Although the specimen produced no findings, the eosinophil count and the percentage of eosinophils were sharply decreased after surgery. The stool examination revealed the ova of Clonorchis Sinensis, Metagonimus yokogawai and Echinostoma (refer to Figure 3), confirming a LatD associated with parasitic infestation of heart. After completing anti-parasitic treatment, the patient was discharged and remained healthy during the 2-year follow-up period (5) (refer to Table 1).
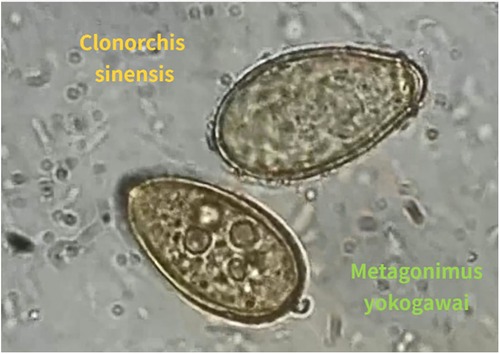
Figure 3. The ova of clonorchis sinensis (orange) and metagonimus yokogawai (green) in the stool examination.
Left atrial dissection can result from various cardiac interventions, including both surgical procedures (primarily MV surgery) and catheter-based interventions (1, 2). Cases of LatD resulting from MI and cardiac trauma have also been recorded (1, 2). Fukuhara et al. reported only 7 cases of spontaneous LatD, one of which was related to IE without cardiac manipulation (1). With another case of LatD with IE in other report, both patients were caused by bacterial infection and died before emergency surgery could be performed (1–3). This might be the first report of LatD resulting from parasitic infestation of heart and survived after surgery.
Transesophageal echocardiography (TEE) is the preferred method for diagnosing LatD, but its efficacy and diagnostic value are still limited (1). Echocardiogram often shows a left atrial mass or a tamponade, particularly when there is thrombus attached (1). Visualizing the entry of the dissection or communication between the inflow and the LA can be challenging (1). A TTE was performed in the emergency room on our patient and revealed an ambiguous left atrial mass with regurgitation flow under the PML, which could have been misdiagnosed as a leaflet perforation. Enhanced CT imaging is also limited in identifying precisely when the thrombus filled in the false cavity.
Hemodynamic instability is mainly secondary to obstruction of MV inflow or pulmonary vein orifice and eventually becomes congestive heart failure and low-output syndrome, making conservative management impractical. Surgery was necessary for our patient (2).
To achieve successful repair, three main keys are the following: (a.) to gain adequate evacuation of the hematoma; (b.) obliteration of the false lumen; and (c.) addressing the entry point, if identified. If the communication is not repaired, the dissection may recur under persistent pressurized inflow (1, 2). Therefore, we debride thrombus and resect the dissected endocardium. To close the orifice in the LV, a posterior annulus cutting was necessary. However, the tissue of LA and MA is often delicate and should be preserved whenever possible. Encouraged by Masashi's successful application of the technique for patch repair of mitral annulus calcification, we proceeded with posterior MA cutting and patch repair (6). We chose the autologous pericardium as patch to prevent artificial graft reinfection.
By introducing this rare parasitic infestation of LatD and its surgical details, we aim to encourage novel surgery for complicated infective endocarditis.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
This study was approved by the Institutional Review Board (IRB). Informed written consent was obtained from the patient. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
PC drafted the manuscript and drew the figure. YT clipped the video. XL revised the manuscript. QM supervised the report. All authors contributed to the article and approved the submitted version.
This work was supported by the National High Level Hospital Clinical Research Funding Grant No. 2022-PUMCH-B-104.
I would like to thank QM and XL who taught me every aspect of mitral valve and annulus repair as well as mitral annuloplasty, and YT for the video editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1239019/full#supplementary-material
LatD, left atrial dissection; LA, left atrial; MI, myocardial infarction; IE, infectious endocarditis; MA, mitral annulus; MV, mitral valve; LV, left ventricle; PML, posterior mitral leaflet.
1. Fukuhara S, Dimitrova KR, Geller CM, Hoffman DM, Tranbaugh RF. Left atrial dissection: an almost unknown entity. Interact Cardiovasc Thorac Surg. (2015) 20(1):96–100. doi: 10.1093/icvts/ivu317
2. Gallego P, Oliver JM, Gonzalez A, Dominguez FJ, Sanchez-Recalde A, Mesa JM. Left atrial dissection: pathogenesis, clinical course, and transesophageal echocardiographic recognition. J Am Soc Echocardiogr. (2001) 14(8):813–20. doi: 10.1067/mje.2001.113366
3. Saad M, Isbitan A, Roushdy A, Shamoon F. Left atrial wall dissection: a rare sequela of native-valve endocarditis. Tex Heart Inst J. (2015) 42(2):178–80. doi: 10.14503/THIJ-13-3989
4. Cordero Lorenzana ML, López Pérez JM, Merayo Macías E, Gulías López JM, Paz Rodríguez J. Disección auricular izquierda y endocarditis infecciosa. Rev Esp Cardiol. (1998) 51:402–3. doi: 10.1016/S0300-8932(98)74765-7
5. Li L, Liu X, Zhou B, Zhang S, Wang G, Ma G, et al. Multiple food-borne trematodiases with profound systemic involvement: a case report and literature review. BMC Infect Dis. (2019) 19(1):526. doi: 10.1186/s12879-019-4140-y
Keywords: left atrial dissection, parasite, infectious endocarditis, patch repair of mitral annulus and valve, patch repair for left atrial dissection
Citation: Chu P, Tang Y, Liu X and Miao Q (2023) Case Report: Pericardial patch repair of mitral annulus and mitral valve for a left atrial dissection caused by parasitic infective endocarditis. Front. Cardiovasc. Med. 10:1239019. doi: 10.3389/fcvm.2023.1239019
Received: 12 June 2023; Accepted: 25 October 2023;
Published: 28 November 2023.
Edited by:
Giuseppe Gatti, Azienda Sanitaria Universitaria Giuliano Isontina, ItalyReviewed by:
Palleti Rajashekar, All India Institute of Medical Sciences, India© 2023 Chu, Tang, Liu and Miao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Miao bWlhb3FpcHVtY0Bob3RtYWlsLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.