- Division of Cardiology, Department of Internal Medicine, Yonsei University College of Medicine, Yonsei University Health System, Seoul, Republic of Korea
Background: The comparative efficacy, saftey, and heart rate variability (HRV) parameters after pulmonary vein isolation using cryoballoon (Cryo-PVI), high-power short-duration (HPSD-PVI), and conventional radiofrequency ablation (conventional-PVI) for atrial fibrillation (AF) is unclear.
Materials and methods: In this propensity score-weighted, retrospective analysis of a single-center cohort, we analyzed 3,395 patients (26.2% female, 74.5% paroxysmal AF) who underwent AF catheter ablation without an empirical left atrial ablation. Procedural factors, recurrence rates, complication rates, and the post-procedural HRV parameters were compared across the Cryo-PVI (n = 625), HPSD-PVI (n = 748), and conventional-PVI (n = 2,022) groups.
Results: Despite the shortest procedural time in the Cryo-PVI group (74 min for Cryo-PVI vs. 104 min for HPSD-PVI vs. 153 min for conventional-PVI, p < 0.001), the major complication (p = 0.906) and clinical recurrence rates were similar across the three ablation groups (weighted log-rank, p = 0.824). However, the Cryo-PVI group was associated with a significantly lower risk of recurrent AF in patients with paroxysmal AF [weighted hazard ratio (WHR) 0.57, 95% confidence interval (CI) 0.37–0.86], whereas it was associated with a higher risk of recurrent AF in patients with persistent AF (WHR 1.41, 95% CI 1.06–1.89, p for interaction of <0.001) compared with the conventional-PVI group. In the subgroup analysis for the HRV, the Cryo-PVI group had the highest low-frequency-to-high-frequency ratio at 1-year post-procedure, whereas the HPSD-PVI group had the lowest low-frequency-to-high-frequency ratio at 1-year post-procedure (p < 0.001).
Conclusions: The Cryo-PVI group had better rhythm outcomes in patients with paroxysmal AF but worse rhythm outcomes in patients with persistent AF and a higher long-term post-procedural sympathetic nervous activity and sympatho-vagal balance compared with the conventional-PVI group.
Introduction
Atrial fibrillation (AF) is a growing global health burden that is associated with substantial morbidity and mortality (1). Rhythm control for AF improves cardiovascular outcomes (2, 3), and the need for AF catheter ablation (AFCA) is continuously increasing. However, long-term maintenance of sinus rhythm after conventional pulmonary vein isolation (conventional-PVI) has been unsatisfactory (4) and has been limited by frequent pulmonary vein (PV) reconnections due to tissue edema and non-transmural lesion formation (5, 6). Also, conventional-PVI is technically complicated, and the operator’s skills and experiences are important for an efficient and safe procedure. Therefore, alternative ablation technologies have gained interest to improve clinical rhythm outcomes, shorten procedural times, and reduce procedure-related complications in patients with AF.
Recently, high-power short-duration pulmonary vein isolation (HPSD-PVI) has gained interest as an alternative ablation strategy that uses resistive heating with better transmural lesion formation (7, 8). Kottmaier et al. (9) reported that HPSD-PVI (70 W for 5–7 s) improves rhythm outcomes compared with conventional-PVI in patients with paroxysmal AF (PAF). Another promising technology, cryoballoon pulmonary vein isolation (Cryo-PVI), freezes and ablates large volumes of PV ostium at a single shot with less catheter manipulation and is less sensitive to the operator's experiences compared with conventional-PVI. Cryo-PVI showed comparable rhythm outcomes with conventional-PVI among patients with PAF, and a rather low PV reconnection rate has been reported (10–12).
However, studies that directly compare the three ablation modalities (conventional-PVI, HPSD-PVI, and Cryo-PVI) are limited. In this propensity score-weighted study, we compared the efficacy and safety outcomes along with changes in heart rate variability (HRV) parameters after Cryo-PVI, HPSD-PVI, and conventional-PVI in patients with AF.
Materials and methods
Study population
The study protocol was approved by the Institutional Review Board of the Yonsei University Health System. It adhered to the principles of the Declaration of Helsinki. A written informed consent was obtained from each patient in the Yonsei AF ablation cohort. We included 3,395 participants who underwent de novo AFCA without an empirical left atrial (LA) ablation (Figure 1). The exclusion criteria of this study were identified, namely, (1) prior history of AF ablation, (2) AF with rheumatic valvular disease, (3) prior cardiac surgery, and (4) extra-PV LA ablation. The participants were categorized into three groups according to the AFCA modality: Cryo-PVI (n = 625), HPSD-PVI (n = 748), and conventional-PVI (n = 2,022).
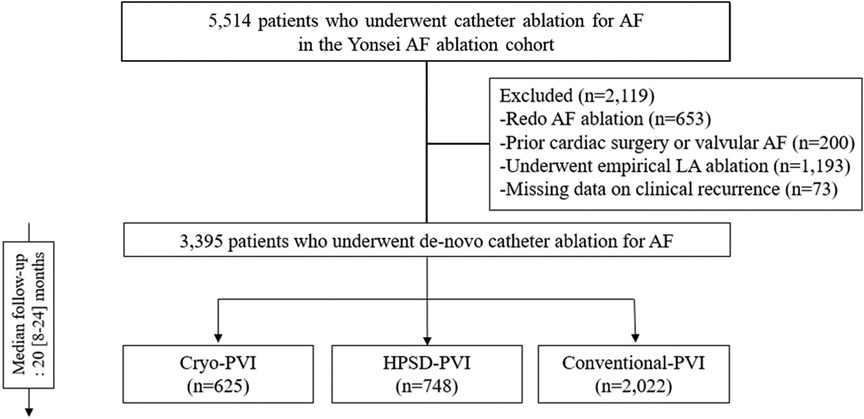
Figure 1. Study flow chart. AAD, anti-arrhythmic drugs; AF, atrial fibrillation; AFCA, atrial fibrillation catheter ablation; ASD, absolute standardized difference; CPVI, circumferential pulmonary vein isolation; E/e’, ratio of early diastolic mitral inflow velocity to early diastolic mitral annulus velocity; HPSD, high-power short-duration; IPTW, inverse probability of treatment weighting; LA, left atrium; LV, left ventricle; LVEDD, left ventricular end diastolic dimension; PVI, pulmonary vein isolation; SVC-RA, superior pulmonary vein-right atrial.
Echocardiographic evaluation
All participants underwent pre-procedural transthoracic echocardiography. The LA volume index (LAVI), LV end-diastolic dimension (LVEDD), LV ejection fraction, and peak trans-mitral inflow velocity to tissue Doppler echocardiography of the peak septal mitral annular velocity (E/e') were assessed in accordance with the guidelines published by the American Society of Echocardiography (13).
Electrophysiological mapping and AFCA
Prucka CardioLab Electrophysiology system (General Electric Medical Systems, Inc., Milwaukee, WI, USA) was used for intracardiac electrogram recordings. After a trans-septal puncture, a multi-view pulmonary venogram was obtained, and 350–400 s of activated clotting time was maintained via intravenous heparin injection. A 3D electro-anatomical mapping system (NavX; Abbott or CARTO; Biosense Webster) merged with 3D spiral CT was used to guide the AFCA in all patients. The luminal esophageal temperature was monitored and maintained below 38.4°C for the HPSD-PVI or conventional-PVI and 15°C for the Cryo-PVI. The esophageal temperature in most of the conventional-PVI group was not monitored because routine esophageal temperature monitoring started in 2019.
For the HPSD-PVI, we used an open irrigated-tip deflectable catheter (FlexAbility; Abbott Inc.). Radiofrequency (RF) power of 50–60 W for 7–15 s and 50 W for <10 s was used for the anterior and posterior area of the LA with a target temperature of 45°C. For the conventional-PVI, we used an open irrigated, 3.5-mm-tip deflectable catheter (Celsius and Smart-Touch; Johnson & Johnson, Inc., Diamond Bar, CA, USA; Coolflex and FlexAbility, Abbott Inc., Minnetonka, MN, USA). RF power of 30–35 and 20–25 W was used for the anterior and posterior area of the LA, respectively. Ablation of the cavotricuspid isthmus (CTI) was performed in most of the patients in the HPSD-PVI and conventional-PVI groups except for those with AV conduction disease. All patients underwent CPVI that encircled the right and left PVs.
For the Cryo-PVI group, a 28-mm cryoballoon (Arctic Front Advance, Medtronic) with a 12 Fr deflectable trans-septal sheath (FlexCath Advance, Medtronic) was used with a multipolar spiral catheter (Achieve, 20 mm; Medtronic). Optimal PV ostium and cryoballoon contact (PV occlusion) was confirmed using a contrast medium. Each PV ostium was frozen for 4 min. The protocol for cryoballoon dosing was modified from the time-to-isolation-based ICE-T (Individualized Cryoballoon Energy Pulmonary Vein Isolation Guided by Real-Time Pulmonary Vein Recordings) protocol (14). The freezing was discontinued, and the catheter was repositioned when the time to isolation exceeded 90 s to achieve better PV occlusion.
Post-ablation management and follow-up
Telemetry monitoring was used in all patients before discharge. Except for those who had extra-PV triggers, symptomatic frequent premature atrial complexes (PACs), non-sustained atrial tachycardia (AT), or AF, all patients were discharged without anti-arrhythmic drugs (AADs). Patients were regularly followed up at the outpatient clinic (1, 3, 6, and 12 months from discharge and every 6 months thereafter) or whenever they experienced signs and symptoms suggestive of arrhythmia recurrence. A 12-lead ECG was obtained at every outpatient clinic visit with regular 24-h Holter recordings (3 and 6 months from discharge and every 6 months thereafter) in accordance with the guidelines issued by the 2017 Heart Rhythm Society/European Heart Rhythm Association/European Cardiac Arrhythmia Society Expert Consensus Statement (15). An 24-h Holter monitoring or event monitor recordings were performed in patients who had signs or symptoms of arrhythmia recurrence. All 24-h Holter recordings were analyzed and verified by an independent researcher.
AF recurrence was defined as any ECG documentation of AF or AT of at least 30 s. Early recurrence was defined as AF recurrence of ≤3 months post-procedure, whereas a clinical recurrence was defined as AF recurrence of >3 months post-procedure.
Holter monitor recordings and the heart rate variability analysis
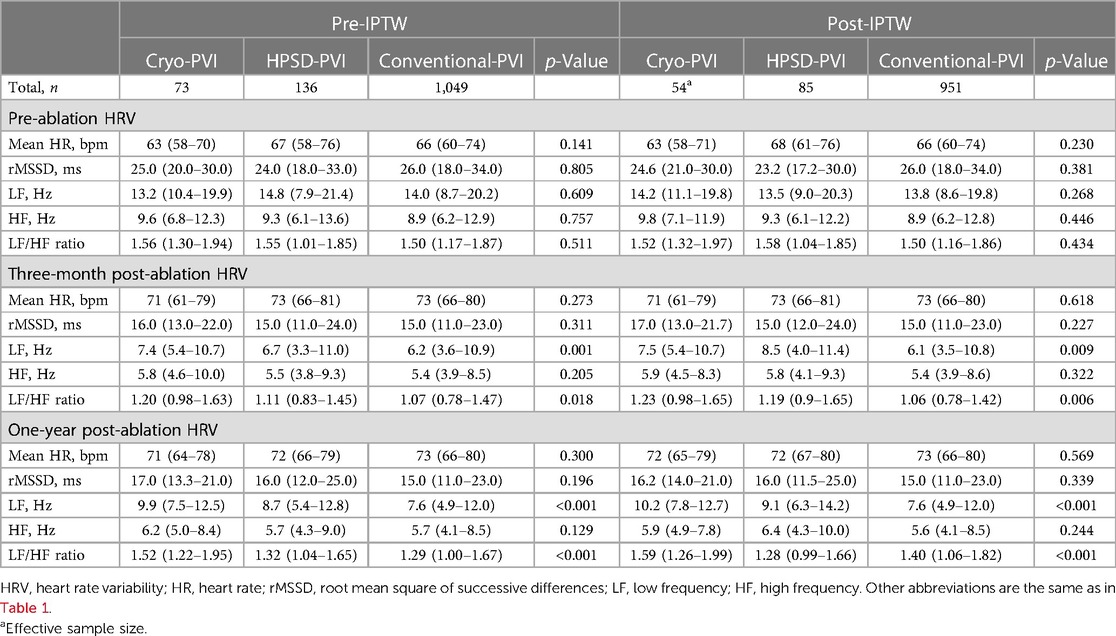
The HRV parameters were obtained from the 24-h Holter monitor recordings with a GE Marquette MARS 8000 Holter analyzer (General Electric Medical System, Inc.). Premature ventricular complexes, PACs, electrical artifacts, or low-quality recordings were excluded from the analysis. All 24-h Holter recordings were reviewed by an experienced operator. We were not able to obtain HRV parameters from all participants, and a total of 73 of the Cryo-PVI group, 136 of the HPSD-PVI group, and 1,049 of the conventional-PVI group had serial long-term HRV parameter follow-up data at pre-ablation and 3-month and 1-year post-ablation.
As a sensitivity analysis, we provided the HRV results for a total of 1,529, 1,809, 1,464, and 637 individuals who had available HRV data (but without complete serial follow-up data) at pre-ablation and 3-month, 1-year, and 2-year post-ablation periods, respectively. The time-domain HRV parameters analyzed were mean heart rate (HR) and root mean square of the differences between successive NN intervals (rMSSD). The frequency-domain HRV parameters analyzed were low-frequency component (LF; 0.040 Hz–0.150 Hz), high-frequency component (HF; 0.150 Hz–0.400 Hz), and the LF/HF ratio. In brief, the mean HR is a surrogate of the HRV, and rMSSD represents the beat-to-beat variance in the heart rate in which low mean HR and high rMSSD indicate an increased HRV (16). The HF and LF component represents parasympathetic and sympathetic nervous activity, and the LF/HF ratio represents sympatho-vagal balance (17).
Statistical analysis
Categorical variables were reported as numbers (percentages) and were compared using the Chi-square or Fisher's exact test. Continuous variables with normal distribution were reported as mean ± standard deviation and non-normal distribution as medians (interquartile range), in which normality was tested using the Shapiro–Wilk or Kolmogorov–Smirnov test. Continuous variables were compared with using the ANOVA test (normal distribution) or the Kruskal–Wallis test (non-normal distribution).
Among the echocardiographic parameters, 121 missing values for E/e' (10 in Cryo-PVI, 15 in HPSD-PVI, 96 in conventional-PVI), 65 for LAVI (eight in Cryo-PVI, 12 in HPSD-PVI, 45 in conventional-PVI), and 75 for LVEDD (seven in Cryo-PVI, 14 in HPSD-PVI, 54 in conventional-PVI) in our study sample were reported. We used multiple imputation by chain equation using mice package in R statistics to generate 50 matrices of imputed dataset. After then, for an unbiased comparison between the AFCA groups, we used an inverse probability of treatment weighting (IPTW) approach. The propensity score, which is the probability of receiving treatment, was estimated using a multinomial logistic regression based on sociodemographic factors, medical and medication history, and echocardiographic parameters (variables are presented in Table 1). We examined the balance between the AFCA groups using the absolute standardized differences of all covariates with a threshold of 0.1 to indicate an imbalance. The weights were truncated at the 1st and 99th percentiles to avoid extreme weights.
We conducted a weighted Kaplan–Meier analysis to compare the clinical recurrence rates across the three AFCA groups. In addition, the predictors associated with clinical recurrence were assessed using Cox proportional hazard models.
As the AF type and SVC-RA ablation (among the covariates) were not sufficiently balanced after IPTW (Table 1), the weighted Cox proportional hazard model was additionally adjusted for those covariates (doubly robust IPTW model). The proportional hazard assumption was not violated as examined by Schoenfeld residual plots (18). We performed multiple subgroup analyses using the weighted Cox proportional hazards regression analysis by the pre-specified baseline covariates to detect any potential interaction with clinical recurrence according to the AFCA modality. For the subgroup analysis, a BMI of ≥25 kg/m2 was considered obese according to the Asian guidelines on the definition of obesity (19). An LAVI of >34 mL/m2 was considered enlarged LA according to the guidelines published by the American Society of Echocardiography (13).
All analyses were performed using R statistics, version 4.0.2 software (R Foundation for Statistical Computing), and a two-sided p-value of <0.05 was considered statistically significant.
Sensitivity analysis
Several sensitivity analyses were conducted in this study. First, because the study sample included patients who underwent conventional-PVI in earlier years compared with Cryo-PVI (started 2019) or HPSD-PVI (started 2018), we repeated the main analysis after excluding patients who underwent conventional-PVI before the year at which first Cryo-PVI or HPSD-PVI was performed. Second, because the study sample included patients who underwent conventional-PVI with the Celsius catheter, the non-contact force electrode catheter, we repeated the main analysis after excluding patients (0.8%, 27 of 3,395) to reduce the risk of potential confounding. Third, we analyzed the risk of clinical recurrence according to 3-month and 1-year post-ablation HRV. The risk of clinical recurrence according to 1-year post-ablation HRV was calculated only for AF recurrences that occurred after 1 year, whereas that according to 3-month post-ablation HRV included all AF recurrences. The weighted hazard ratios per one-standard deviation increase in each of the HRV parameters were assessed with the same doubly robust IPTW model.
Results
Baseline characteristics and procedural outcomes
Overall, the study participants had a mean age of 60 (52–67) years, 889 (26.2%) were female, and 2,528 (74.5%) had PAF. The baseline characteristics of the study participants according to the AFCA modality are presented in Table 1. After IPTW, the baseline covariates were well-balanced across the three ablation groups (Table 1) except for AF type and SVC-RA ablation (Supplementary Figures S1, S2).
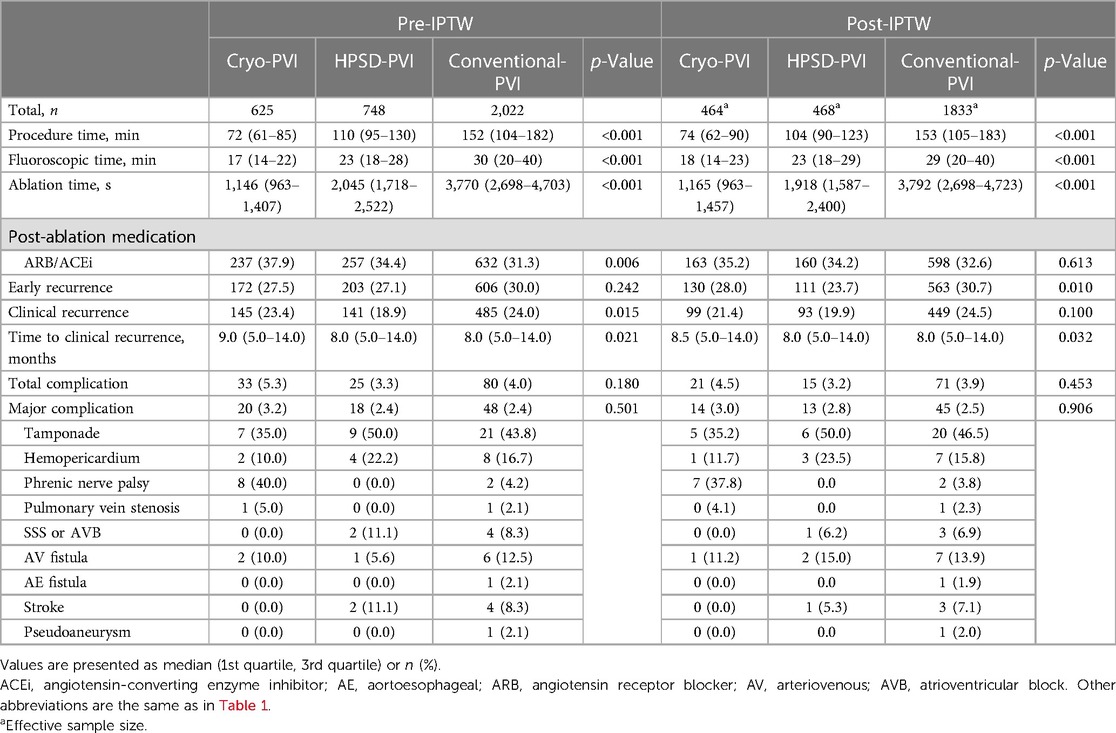
The ablation characteristics are presented in Table 2. The procedural time was shortest in the Cryo-PVI group (p < 0.001 for three groups, Table 2), and early recurrence was lowest in the HPSD-PVI group (p = 0.010 for three groups, Table 2). The median time to recurrence across the three AFCA groups was 8.5 months for Cryo-PVI and 8 months for HPSD-PVI and conventional-PVI groups (p = 0.032 for three groups, Table 2).
The total and major complication rates did not significantly differ across the three ablation groups (p = 0.906 for major complications, Table 2). Specifically, among the cardiac tamponade, three from conventional-PVI and one from HPSD-PVI required pericardial window formation, whereas others were managed with pericardiocentesis. All the phrenic nerve palsies after Cryo-PVI were transient, and all the strokes in the conventional-PVI did not result in a clinically meaningful sequelae. One pulmonary vein stenosis from conventional-PVI required a percutaneous stent, and one SSS/AVB from conventional-PVI required a pacemaker implantation.
Overall rhythm outcome
Clinical recurrences within 24 months after AFCA were analyzed in this study because of differences in rhythm follow-up duration across the three ablation groups. For a median of 20 (8–24) months, the overall clinical recurrence rate was similar across the three ablation groups (weighted log-rank, p = 0.824 for the three ablation groups, Figure 2A). In the weighted Cox regression analysis, the overall risk of clinical recurrence in the Cryo-PVI or HPSD-PVI group was similar compared with the conventional-PVI group (Supplementary Table S2).
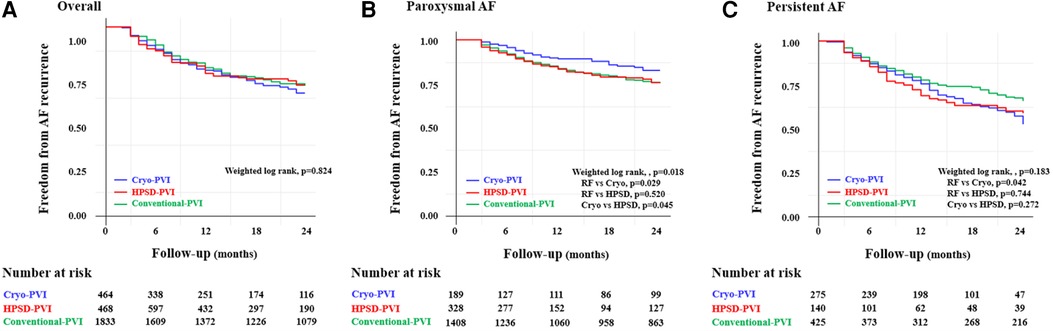
Figure 2. Freedom from clinical recurrence according to the AFCA modality among (A) overall, (B) paroxysmal AF, and (C) persistent AF patients. PaF, paroxysmal atrial fibrillation; PeAF, persistent atrial fibrillation; AAD, anti-arrhythmic drugs; AF, atrial fibrillation; AFCA, atrial fibrillation catheter ablation; ASD, absolute standardized difference; CPVI, circumferential pulmonary vein isolation; E/e’, ratio of early diastolic mitral inflow velocity to early diastolic mitral annulus velocity; HPSD, high-power short-duration; IPTW, inverse probability of treatment weighting; LA, left atrium; LV, left ventricle; LVEDD, left ventricular end diastolic dimension; PVI, pulmonary vein isolation; SVC-RA, superior pulmonary vein-right atrial.
Subgroup analysis
Among patients with PAF, the Cryo-PVI group had a lower rate of clinical recurrence compared with the conventional-PVI group (weighted log-rank, p = 0.018 for the three groups, p = 0.029 for the Cryo-PVI vs. conventional-PVI group, Figure 2B). Among patients with persistent AF (PeAF), no significant difference in the rate of clinical recurrence across the three ablation groups (weighted log-rank, p = 0.183, Figure 2C) was found. However, the rate of clinical recurrence in the Cryo-PVI group was significantly worse than the rate of clinical recurrence in the conventional-PVI group (weighted log-rank, p = 0.042, Figure 2C).
In the subgroup analysis for the weighted hazard ratio, the risk of clinical recurrence in the Cryo-PVI group was independently associated with AF type compared with the conventional-PVI group (Figure 3A). The risk of clinical recurrence in the Cryo-PVI group was significantly lower among patients with PAF [weighted hazard ratio (WHR) 0.57, 95% CI 0.37–0.86, p = 0.008, Figure 3A], whereas it higher among patients with PeAF (WHR 1.41, 95% CI 1.06–1.89, p = 0.019, Figure 3A) compared with the conventional-PVI group (p for interaction of <0.001, Figure 3A). In addition, a significant interaction with clinical recurrence according to LAVI in the conventional-PVI and HPSD-PVI groups was identified compared with the Cryo-PVI group (LAVI; p for interaction of 0.040 for the conventional-PVI group and 0.030 for the HPSD-PVI group, Figure 3A,B, respectively). In contrast, no significant interaction with clinical recurrence across the specified subgroups in the HPSD-PVI group was noted compared with the conventional-PVI group (Figure 3C).
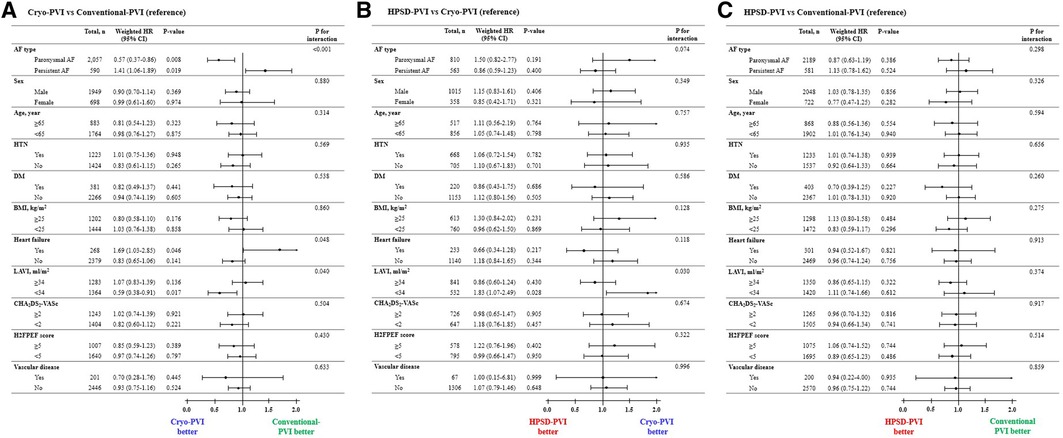
Figure 3. Subgroup analysis of the risk of clinical recurrence according to the AFCA modality: (A) Cryo-PVI versus Conventional-PVI, (B) HPSD-PVI versus Cryo-PVI, and (C) HPSD-PVI versus Conventional-PVI. AAD, anti-arrhythmic drugs; AF, atrial fibrillation; AFCA, atrial fibrillation catheter ablation; ASD, absolute standardized difference; CPVI, circumferential pulmonary vein isolation; E/e’, ratio of early diastolic mitral inflow velocity to early diastolic mitral annulus velocity; HPSD, high-power short-duration; IPTW, inverse probability of treatment weighting; LA, left atrium; LV, left ventricle; LVEDD, left ventricular end diastolic dimension; PVI, pulmonary vein isolation; SVC-RA, superior pulmonary vein-right atrial. aWeighted HR was additionally adjusted for AF type and SVC-RA ablation.
The sensitivity analyses after excluding patients who underwent conventional-PVI before the first Cryo-PVI or HPSD-PVI (Supplementary Figure S3) and excluding patients who underwent conventional-PVI with the Celsius catheter (Supplementary Figure S4) showed similar findings with the main analysis representing better rhythm outcomes after Cryo-PVI in patients with PAF and worse rhythm outcomes in patients with PeAF.
Changes in the HRV parameters after AFCA
The pre-ablation HRV parameters were not significantly different across the three ablation groups (Table 3). However, the post-ablation LF/HF ratio revealed significant differences that persisted for up to 1 year of follow-up. At 1-year post-procedure, the LF/HF ratio was highest in the Cryo-PVI group and lowest in the HPSD-PVI group (p < 0.001, Table 3). Similar results were found in the sensitivity analysis among those without full serial HRV follow-up data (Supplementary Table S2).
The results for risk of clinical recurrence according to post-ablation HRV parameters are provided in Supplementary Table S3. Among the HRV parameters, high LF and increased LF (delta LF) after AFCA were associated with an increased risk of clinical recurrence.
Discussion
Major findings
In this propensity score-weighted, retrospective analysis of a single-center cohort, we compared the efficacy and safety outcomes along with cardiac autonomic nervous activity among AF patients who underwent Cryo-PVI, HPSD-PVI, or conventional-PVI. Similar rhythm outcomes and complication rates among the three ablation groups were reported. However, Cryo-PVI was associated with better rhythm outcomes in patients with PAF but worse rhythm outcomes inpatients with PeAF compared with conventional-PVI. In addition, Cryo-PVI was associated with better rhythm outcomes among patients with an LAVI of <34 mL/m2 compared with HPSD-PVI and conventional-PVI. We found significantly shorter ablation and procedure times but higher cardiac sympathetic nervous activity over long-term periods in the Cryo-PVI group compared with the HPSD-PVI or conventional-PVI group.
AF types and the PVI methodology
Although randomized trials on the efficacy of Cryo-PVI vs. conventional-PVI have reported similar AF recurrences in PAF (20, 21), several studies consistently suggested fewer PV reconnections among patients who underwent Cryo-PVI (22, 23). However, the results of these randomized studies were based on a small number of patients that did not compare the rhythm outcomes among patients with PeAF (24, 25). In terms of PeAF, several case-only comparison studies have reported similar AF recurrences between the Cryo-PVI and conventional-PVI (26–28), and one study group reported that Cryo-PVI had a worse 1-year outcome than conventional-PVI (29).
Our study suggests that patients with PAF may benefit more from Cryo-PVI than from conventional-PVI in contrast to patients with PeAF. The reason for this is not clear, but PV ostium remodeling (diameter and shape) in patients with PeAF might have resulted in an imperfect cryoballoon tissue contact, or the cryothermal energy transfer could have been affected by the degree of tissue fibrosis (30–32). This possibility is supported by better rhythm outcomes among patients with an LAVI of <34 mL/m2 in the Cryo-PVI group compared with the HPSD-PVI or conventional-PVI group.
Martino et al. (33) reported that stepwise additional ablation including endocardial and epicardial LA ablation after PVI showed favorable rhythm outcomes in the patients with non-paroxysmal AF. Because the current study excluded patients who underwent extra-PV LA ablation and showed differing results in rhythm outcomes according to the AF type, future research on the effect of additional LA ablation after both Cryo-PVI and RF-PVI in patients with persistent AF might benefit.
Cardiac autonomic nervous activity after AF ablation
The cardiac autonomic nervous system is reported to initiate and maintain AF, and autonomic neural modulation via a circumferential PVI and SVC-RA ablation is reported to reduce AF recurrence (34–36). The post-procedural HRV analysis in this study suggests that Cryo-PVI is associated with a higher cardiac sympathetic nervous activity and sympatho-vagal balance compared with HPSD-PVI or conventional-PVI. Cryo-PVI may generate large tissue damage due to a larger balloon–tissue contact area compared with conventional-PVI that may result in sympathetic/parasympathetic nerve sprouting that leads to an overall higher post-procedural sympathetic nervous activity and sympatho-vagal balance, especially among patients with PeAF (37, 38). HPSD-PVI had the lowest LF/HF ratio at 1-year post-procedure, suggesting an optimal cardiac autonomic neural modulation but equivalent long-term rhythm outcome.
The clinical implications of post-AFCA HRVs have been described in previous studies. Kang et al. (39) reported that reduced LF/HF ratio after 3 months of AFCA was associated with poor rhythm outcomes. Yu et al. (40) reported that high sinus heart rate after AFCA was associated with decreased LF and better rhythm outcomes. In this study, higher 3-month and 1-year post-ablation sympathetic nervous activity (LF) was associated with an increased risk of clinical recurrence, and patients who underwent Cryo-PVI had higher sympathetic nervous activity and higher sympatho-vagal balance at post-ablation periods. These findings showed that the patients who had increased HRV parameters such as LF and LF/HF ratio after Cryo-PVI, representing high sympathetic activity and sympatho-vagal balance, might be at high risk for clinical recurrence.
Future technologies in AF ablation
Cryo-PVI ablates a large volume of PV ostium at a single shot that involves less catheter manipulation and shorter procedural time compared with RF-PVI. Although Cryo-PVI is associated with higher radiation exposure initially to identify proper PV occlusion using radiation and contrast injection, intracardiac echocardiography-guided zero-fluoroscopy Cryo-PVI showed comparable rhythm outcomes in a recent clinical trial (41). In contrast, RF-PVI ablates PV ostium in a point-to-point manner and requires more catheter manipulation which depends on the operator's skills and experiences. Steerable sheaths help the operators improve contact and stability of the ablation catheter, leading to achieve efficient lesion formation for AF ablation. Recently, with the advent of visualizable steerable sheaths, RF-PVI is associated with minimal radiation exposure (42, 43), and zero-fluoroscopy RF-PVI (44) showed equal efficacy and safety compared with traditional procedures (45). Therefore, future direction for AF ablation using cryoballoon and radiofrequency catheter possibly studies better rhythm outcomes, lower complication rates, reduced procedure time, and also reduced radiation exposure.
Limitations
This study has several potential limitations. First, this was a single-center study from a tertiary hospital, and the study sample may not represent the general AF patients, in which the results of this study need careful interpretation and further multi-center studies would benefit. However, data from a single center are still valuable because the AFCA procedure as well as the rhythm follow-up protocols were largely consistent. Second, the HRV analysis in this study should be interpreted with caution because not all patients had available post-procedural HRV parameters. However, our results are one of the largest single-center, long-term pre- and post-ablation HRV analysis (46) that might provide a reasonable estimation. In addition, the complementary analysis (Supplementary Table S2) that included all patients who underwent HRV at pre- and post-ablation at 3 months, 1 year, and 2 years might provide an additional insight.
Third, despite appropriate efforts to perform unbiased comparisons between the AFCA groups using propensity score-weighted models, a residual confounding from other potential covariates such as AF duration or AF burden, which were not accounted for in this study, remains. In addition, because of the retrospective, observational nature of the study, a causal relationship cannot be drawn, and further randomized trials are needed.
Fourth, this study included patients who received right atrial ablation, and a slight difference in the number of right atrial ablations performed among the three ablation groups was found. Although we performed the doubly robust IPTW model to reduce confounding, concerns for residual confounding remains, and the results of this study requires careful interpretation.
Conclusions
Cryo-PVI, HPSD-PVI, and conventional-PVI had similar rhythm outcomes and complication rates in patients with AF. However, Cryo-PVI was associated with better rhythm outcomes in patients with PAF but worse rhythm outcomes in patients with PeAF compared with conventional-PVI. Cryo-PVI had significantly shorter procedure times but had higher long-term post-procedural sympathetic nervous activity. AF type might be useful information for the selection of AFCA modality for better rhythm outcomes in patients with AF.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of the Yonsei University Health System. The patients/participants provided their written informed consent to participate in this study.
Author contributions
J-WP contributed to the conception and design of the work and critical revision of the manuscript. HP, J-WP, DK, and H-NP contributed to the conception and design of the work, interpretation of the data, and drafting of the manuscript. HY, T-HK, J-SU, and BJ contributed to the acquisition and analysis of the data. M-HL, CH, and H-NP contributed to the conception and design of the work and revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a grant [HI21C0011] from the Ministry of Health and Welfare and the Korea Medical Device Development Fund [project number 1711174471; RS-2022-00141473] funded by the Ministry of Science and ICT; Ministry of Trade, Industry, and Energy; Ministry of Health & Welfare; and Ministry of Food and Drug Safety of the Korean government.
Acknowledgments
The authors would like to thank John Martin for his linguistic assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1238363/full#supplementary-material
References
1. Kornej J, Borschel CS, Benjamin EJ, Schnabel RB. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ Res. (2020) 127(1):4–20. doi: 10.1161/CIRCRESAHA.120.316340
2. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. (2020) 383(14):1305–16. doi: 10.1056/NEJMoa2019422
3. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. (2021) 42(S):373–98. doi: 10.1093/eurheartj/ehaa612
4. Parameswaran R, Al-Kaisey AM, Kalman JM. Catheter ablation for atrial fibrillation: current indications and evolving technologies. Nat Rev Cardiol. (2021) 18(3):210–25. doi: 10.1038/s41569-020-00451-x
5. Macle L, Khairy P, Weerasooriya R, Novak P, Verma A, Willems S, et al. Adenosine-guided pulmonary vein isolation for the treatment of paroxysmal atrial fibrillation: an international, multicentre, randomised superiority trial. Lancet. (2015) 386(9994):672–9. doi: 10.1016/S0140-6736(15)60026-5
6. Mulder MJ, Kemme MJB, Allaart CP. Radiofrequency ablation to achieve durable pulmonary vein isolation. Europace. (2022) 24(6):874–6. doi: 10.1093/europace/euab279
7. Park JW, Yang SY, Kim M, Yu HT, Kim TH, Uhm JS, et al. Efficacy and safety of high-power short-duration radiofrequency catheter ablation of atrial fibrillation. Front Cardiovasc Med. (2021) 8:709585. doi: 10.3389/fcvm.2021.709585
8. Pak HN, Park JW, Yang SY, Kim TH, Uhm JS, Joung B, et al. Cryoballoon versus high-power, short-duration radiofrequency ablation for pulmonary vein isolation in patients with paroxysmal atrial fibrillation: a single-center, prospective, randomized study. Circ Arrhythm Electrophysiol. (2021) 14(9):e010040. doi: 10.1161/CIRCEP.121.010040
9. Kottmaier M, Popa M, Bourier F, Reents T, Cifuentes J, Semmler V, et al. Safety and outcome of very high-power short-duration ablation using 70 W for pulmonary vein isolation in patients with paroxysmal atrial fibrillation. Europace. (2020) 22(3):388–93. doi: 10.1093/europace/euz342
10. Park JW, Yu HT, Kim TH, Uhm JS, Joung B, Lee MH, et al. Mechanisms of long-term recurrence 3 years after catheter ablation of atrial fibrillation. JACC Clin Electrophysiol. (2020) 6(8):999–1007. doi: 10.1016/j.jacep.2020.04.035
11. Kuck KH, Brugada J, Schluter M, Braegelmann KM, Kueffer FJ, Chun KRJ, et al. The FIRE AND ICE trial: what we know, what we can still learn, and what we need to address in the future. J Am Heart Assoc. (2018) 7(24):e010777. doi: 10.1161/JAHA.118.010777
12. Sorensen SK, Johannessen A, Worck R, Hansen ML, Hansen J. Radiofrequency versus cryoballoon catheter ablation for paroxysmal atrial fibrillation: durability of pulmonary vein isolation and effect on atrial fibrillation burden: the RACE-AF randomized controlled trial. Circ Arrhythm Electrophysiol. (2021) 14(5):e009573. doi: 10.1161/CIRCEP.120.009573
13. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2016) 29(4):277–14. doi: 10.1016/j.echo.2016.01.011
14. Chun KR, Stich M, Furnkranz A, Bordignon S, Perrotta L, Dugo D, et al. Individualized cryoballoon energy pulmonary vein isolation guided by real-time pulmonary vein recordings, the randomized ICE-T trial. Heart Rhythm. (2017) 14(4):495–500. doi: 10.1016/j.hrthm.2016.12.014
15. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. (2017) 14(10):e275–44. doi: 10.1016/j.hrthm.2017.05.012
16. Monfredi O, Lyashkov AE, Johnsen AB, Inada S, Schneider H, Wang R, et al. Biophysical characterization of the underappreciated and important relationship between heart rate variability and heart rate. Hypertension. (2014) 64(6):1334–43. doi: 10.1161/HYPERTENSIONAHA.114.03782
17. Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. (2017) 5:258. doi: 10.3389/fpubh.2017.00258
18. Grant S, Chen YQ, May S. Performance of goodness-of-fit tests for the cox proportional hazards model with time-varying covariates. Lifetime Data Anal. (2014) 20(3):355–68. doi: 10.1007/s10985-013-9277-1
19. Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. (2004) 363(9403):157–63. doi: 10.1016/S0140-6736(03)15268-3
20. Kuck KH, Brugada J, Furnkranz A, Metzner A, Ouyang F, Chun KR, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. (2016) 374(23):2235–45. doi: 10.1056/NEJMoa1602014
21. Andrade JG, Champagne J, Dubuc M, Deyell MW, Verma A, Macle L, et al. Cryoballoon or radiofrequency ablation for atrial fibrillation assessed by continuous monitoring: a randomized clinical trial. Circulation. (2019) 140(22):1779–88. doi: 10.1161/CIRCULATIONAHA.119.042622
22. Glowniak A, Tarkowski A, Fic P, Wojewoda K, Wojcik J, Wysokinski A. Second-generation cryoballoon ablation for recurrent atrial fibrillation after an index procedure with radiofrequency versus cryo: different pulmonary vein reconnection patterns but similar long-term outcome-results of a multicenter analysis. J Cardiovasc Electrophysiol. (2019) 30(7):1005–12. doi: 10.1111/jce.13938
23. Kuck KH, Albenque JP, Chun KJ, Furnkranz A, Busch M, Elvan A, et al. Repeat ablation for atrial fibrillation recurrence post cryoballoon or radiofrequency ablation in the FIRE AND ICE Trial. Circ Arrhythm Electrophysiol. (2019) 12(6):e007247. doi: 10.1161/CIRCEP.119.007247
24. Hunter RJ, Baker V, Finlay MC, Duncan ER, Lovell MJ, Tayebjee MH, et al. Point-by-point radiofrequency ablation versus the cryoballoon or a novel combined approach: a randomized trial comparing 3 methods of pulmonary vein isolation for paroxysmal atrial fibrillation (the cryo versus RF trial). J Cardiovasc Electrophysiol. (2015) 26(12):1307–14. doi: 10.1111/jce.12846
25. Providencia R, Defaye P, Lambiase PD, Pavin D, Cebron JP, Halimi F, et al. Results from a multicentre comparison of cryoballoon vs. radiofrequency ablation for paroxysmal atrial fibrillation: is cryoablation more reproducible? Europace. (2017) 19(1):48–57. doi: 10.1093/europace/euw080
26. Straube F, Hartl S, Dorwarth U, Wankerl M, Bunz B, Ebersberger U, et al. Cryoballoon ablation for persistent atrial fibrillation—Large single-center experience. J Cardiol. (2016) 68(6):492–97. doi: 10.1016/j.jjcc.2016.02.007
27. Tondo C, Iacopino S, Pieragnoli P, Molon G, Verlato R, Curnis A, et al. Pulmonary vein isolation cryoablation for patients with persistent and long-standing persistent atrial fibrillation: clinical outcomes from the real-world multicenter observational project. Heart Rhythm. (2018) 15(3):363–8. doi: 10.1016/j.hrthm.2017.10.038
28. Sawhney V, Schilling RJ, Providencia R, Cadd M, Perera D, Chatha S, et al. Cryoablation for persistent and longstanding persistent atrial fibrillation: results from a multicentre European registry. Europace. (2020) 22(3):375–81. doi: 10.1093/europace/euz313
29. Choi JH, Park SJ, Park KM, Kim JS, On YK. Efficacy and safety of cryoballoon pulmonary vein isolation for paroxysmal and persistent atrial fibrillation: A comparison with radiofrequency ablation. PLos One. (2022) 17(7):e0265482. doi: 10.1371/journal.pone.0265482
30. Mugnai G, Cecchini F, Stroker E, Paparella G, Iacopino S, Sieira J, et al. Pulmonary vein size is associated with reconnection following cryoballoon ablation of atrial fibrillation. J Interv Card Electrophysiol. (2022) 65(3):717–24. doi: 10.1007/s10840-022-01330-w
31. Schmidt M, Dorwarth U, Straube F, Daccarett M, Rieber J, Wankerl M, et al. Cryoballoon in AF ablation: impact of PV ovality on AF recurrence. Int J Cardiol. (2013) 167(1):114–20. doi: 10.1016/j.ijcard.2011.12.017
32. Terasawa M, Chierchia GB, Al Housari M, Bala G, Cosyns B, Droogmans S, et al. Predictors of late pulmonary vein reconnection in patients with arrhythmia recurrence after cryoballoon ablation-per vein analysis including cardiac computed tomography-based anatomic factors. Eur Heart J Cardiovasc Imaging. (2023) 24(7):972–81. doi: 10.1093/ehjci/jeac255
33. De Martino G, Compagnucci P, Mancusi C, Vassallo E, Calvanese C, Della Ratta G, et al. Stepwise endo-/epicardial catheter ablation for atrial fibrillation: the mediterranea approach. J Cardiovasc Electrophysiol. (2021) 32(8):2107–15. doi: 10.1111/jce.15151
34. Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res. (2014) 114(9):1500–15. doi: 10.1161/CIRCRESAHA.114.303772
35. Pappone C, Santinelli V, Manguso F, Vicedomini G, Gugliotta F, Augello G, et al. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. (2004) 109(3):327–34. doi: 10.1161/01.cir.0000112641.16340.c7
36. Jin MN, Lim B, Yu HT, Kim TH, Uhm JS, Joung B, et al. Long-term outcome of additional superior vena cava to septal linear ablation in catheter ablation of atrial fibrillation. J Am Heart Assoc. (2019) 8(22):e013985. doi: 10.1161/JAHA.119.013985
37. Okuyama Y, Pak HN, Miyauchi Y, Liu YB, Chou CC, Hayashi H, et al. Nerve sprouting induced by radiofrequency catheter ablation in dogs. Heart Rhythm. (2004) 1(6):712–7. doi: 10.1016/j.hrthm.2004.09.012
38. Shen MJ, Choi EK, Tan AY, Lin SF, Fishbein MC, Chen LS, et al. Neural mechanisms of atrial arrhythmias. Nat Rev Cardiol. (2011) 9(1):30–9. doi: 10.1038/nrcardio.2011.139
39. Kang KW, Kim TH, Park J, Uhm JS, Joung B, Hwang C, et al. Long-term changes in heart rate variability after radiofrequency catheter ablation for atrial fibrillation: 1-year follow-up study with irrigation tip catheter. J Cardiovasc Electrophysiol. (2014) 25(7):693–700. doi: 10.1111/jce.12398
40. Yu HT, Kim TH, Uhm JS, Kim JY, Joung B, Lee MH, et al. Prognosis of high sinus heart rate after catheter ablation for atrial fibrillation. Europace. (2017) 19(7):1132–39. doi: 10.1093/europace/euw142
41. Ahn J, Shin DG, Han SJ, Lim HE. Safety and efficacy of intracardiac echocardiography-guided zero-fluoroscopic cryoballoon ablation for atrial fibrillation: a prospective randomized controlled trial. Europace. (2023) 25(5):1–8. doi: 10.1093/europace/euad086
42. Fitzpatrick N, Mittal A, Galvin J, Jauvert G, Keaney J, Keelan E, et al. The impact of steerable sheath visualization during catheter ablation for atrial fibrillation. Europace. (2023) 25(4):1345–51. doi: 10.1093/europace/euad029
43. Janosi K, Debreceni D, Janosa B, Bocz B, Simor T, Kupo P. Visualizable vs. standard, non-visualizable steerable sheath for pulmonary vein isolation procedures: randomized, single-centre trial. Front Cardiovasc Med. (2022) 9:1033755. doi: 10.3389/fcvm.2022.1033755
44. Bulava A, Hanis J, Eisenberger M. Catheter ablation of atrial fibrillation using zero-fluoroscopy technique: a randomized trial. Pacing Clin Electrophysiol. (2015) 38(7):797–806. doi: 10.1111/pace.12634
45. Debreceni D, Janosi K, Bocz B, Turcsan M, Lukacs R, Simor T, et al. Zero fluoroscopy catheter ablation for atrial fibrillation: a systematic review and meta-analysis. Front Cardiovasc Med. (2023) 10:1178783. doi: 10.3389/fcvm.2023.1178783
Keywords: atrial fibrillation, catheter ablation, cryoballoon ablation, high-power short-duration ablation, heart rate variability
Citation: Park H, Park J-W, Kim D, Yu HT, Kim T-H, Uhm J-S, Joung B, Lee M-H, Hwang C and Pak H-N (2023) Comparison of pulmonary vein isolation using cryoballoon, high-power short-duration, and conventional radiofrequency ablation for atrial fibrillation: a propensity score-weighted study. Front. Cardiovasc. Med. 10:1238363. doi: 10.3389/fcvm.2023.1238363
Received: 11 June 2023; Accepted: 18 September 2023;
Published: 9 October 2023.
Edited by:
Teresa Strisciuglio, University of Naples Federico II, ItalyReviewed by:
Paolo Compagnucci, Marche Polytechnic University, ItalyPeter Kupo, University of Pécs, Hungary
Marco Valerio Mariani, Sapienza University of Rome, Italy
© 2023 Park, Park, Kim, Yu, Kim, Uhm, Joung, Lee, Hwang and Pak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Je-Wook Park dGhlYnJhaW4yMDA1QHl1aHMuYWM=
 Hanjin Park
Hanjin Park Je-Wook Park
Je-Wook Park Daehoon Kim
Daehoon Kim Hee Tae Yu
Hee Tae Yu Tae-Hoon Kim
Tae-Hoon Kim Boyoung Joung
Boyoung Joung Hui-Nam Pak
Hui-Nam Pak