- 1Departments of Cardiovascular Medicine, Regional Cardiocerebrovascular Center, Wonkwang University Hospital, Iksan, Republic of Korea
- 2Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, Republic of Korea
- 3Division of Cardiology, Department of Internal Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Republic of Korea
Background: The aim of this study was to evaluate the efficacy and safety of ticagrelor monotherapy in patients with small vessel disease compared with ticagrelor-based DAPT within the Ticagrelor Monotherapy after 3 Months in the Patients Treated with New Generation Sirolimus Eluting Stent for Acute Coronary Syndrome (TICO) trial population.
Methods: Reference vessel diameter ≤2.5 mm was considered as small vessel disease. We conducted a comparison of the incidence of target lesion failure (TLF) and Bleeding Academic Research Consortium (BARC) type 3 or 5 bleeding. TLF was defined as a composite of cardiac death, target lesion myocardial infarction, stent thrombosis, and target lesion revascularization.
Results: 652 patients among 3,056 TICO population (21.3%) had small vessel disease. Patients with small vessel disease showed a higher rate of TLF compared to those without small vessel disease (2.9% vs. 1.0%, log-rank p < 0.001). The presence of small vessel disease emerged as an independent predictor for 1-year TLF (HR 2.84, 95% CI 1.54–5.25), while it did not show a significant association with bleeding complications. The 12-month TLF rate was 1.6% for ticagrelor monotherapy after 3-month DAPT, and 4.2% for ticagrelor-based 12-month DAPT (p = 0.059) in patients with small vessel disease (HR 0.38, 95% CI 0.14–1.04, p for interaction = 0.261). The incidence of BARC type 3 or 5 bleeding rate 2.5% for ticagrelor monotherapy after 3-month DAPT, and 5.6% for ticagrelor-based 12-month DAPT (p = 0.052) in patients with small vessel disease (HR 0.44, 95% CI 0.19–1.01, p for interaction = 0.322). In the 3-month landmark analysis, ticagrelor monotherapy significantly reduced BARC type 3 or 5 bleeding in patients with small vessel disease (HR 0.09, 95% CI 0.01–0.69, log-rank p = 0.005) while demonstrating a similar incidence of TLF compared to ticagrelor based 12-month DAPT during the 3–12 months period.
Conclusions: There are no significant interactions between the antiplatelet strategy regarding the 12-month incidence of ischemic and bleeding complications. Ticagrelor monotherapy demonstrated a reduction in bleeding complications after a 3-month period of DAPT without increasing the rate of TLF, when compared to ticagrelor-based 12-month DAPT, specifically in patients with small vessel disease.
Clinical Trial Registration: www.ClinicalTrials.gov, identifier, NCT02494895.
Introduction
Despite the development of newer generation stents and techniques, small-vessel coronary artery disease continues to pose challenges for percutaneous coronary intervention (PCI) (1, 2). Small vessel disease is associated with a higher incidence of restenosis and stent thrombosis, leading to target lesion failure (TLF) (1–3). Several studies have aimed to identify the most effective device for patients with small vessel disease, yet the antiplatelet regimen for these patients remains poorly defined.
Ticagrelor Monotherapy after 3 Months in the Patients Treated with New Generation Sirolimus Eluting Stent for Acute Coronary Syndrome (TICO) trial demonstrated that ticagrelor monotherapy, following 3 months of dual antiplatelet therapy (DAPT), reduced the risk of bleeding without increasing the risk of ischemic complications when compared to 12 months of DAPT with aspirin and ticagrelor (4). Nevertheless, further studies are needed to assess specific subgroups that exhibit a high risk of ischemic or bleeding events. The objective of this study was to evaluate the efficacy and safety of ticagrelor monotherapy in patients with small vessel disease compared with ticagrelor-based DAPT within the TICO trial population.
Methods
Study population
This is a sub-study of the TICO randomized trial (clinicaltrials.gov identifier: NCT02494895), and the study design has been previously published (5). In summary, a total of 3,056 patients with acute coronary syndrome were randomly assigned to either ticagrelor monotherapy after a 3-month DPAT or ticagrelor-based 12-month DAPT with aspirin and ticagrelor. The patients underwent successful PCI with ultrathin bioresorbable polymer sirolimus-eluting stents (Orsiro, Biotronik AG, Berlin, Germany). Key exclusion criteria included patients with a history of hemorrhagic stroke, internal bleeding within the past 6 weeks, traumatic brain injury or brain surgery within the past 6 months, the need for oral anticoagulation therapy, and anemia (hemoglobin ≤8 g/dl). For this sub-study, the patients were categorized into those with small vessel disease and those with non-small vessel disease based on a reference vessel size of 2.5 mm.
Angiographic analysis
Angiographic analysis was conducted for all procedures, ensuring complete data collection. Quantitative coronary analysis (QCA) of the angiographic images was performed using offline software (CAAS system, Pie Medical Imaging, Maastricht, the Netherlands) at a central core laboratory (Cardiovascular Research Center, Seoul, Republic of Korea) by specialized technicians who were blinded to the contents and purpose of this study. The reference vessel diameter was determined as an average of the proximal and distal diameters. For the purpose of this study. small vessel coronary artery disease was defined as a minimal reference diameter ≤2.5 mm (6, 7).
Endpoint
The outcomes of the present study focused on evaluating ischemic and bleeding complications occurring within 12 months after PCI. The primary ischemic outcome was TLF, defined as a composite endpoint encompassing cardiac death, target vessel myocardial infarction, stent thrombosis, and target lesion revascularization. Additionally, all-cause death, ischemic stroke, and any revascularization were analyzed as additional ischemic complications in accordance with the criteria established by the Academic Research Consortium (8). Bleeding complications were assessed based on the Bleeding Academic Research Consortium (BARC) definition, specifically examining BARC type 3 or 5 bleeding events (9).
Statistical analyses
Baseline characteristics were compared between patients with small vessel disease and those with non-small vessel disease using independent t-test or the Mann–Whitney U test for continuous variables and using a χ2 test or Fisher’s exact test for categorical variables. Cumulative incidences of ischemic and bleeding complications were determined using Kaplan-Meier estimates and assessed through the log-rank test. Cox proportional hazard model was constructed to identify contributing factors to target vessel failure and bleeding complications, and the results were reported as hazard ratios (HR) with corresponding 95% confidence intervals (CI). The analysis adjusted for several covariates including age, gender, body mass index, hypertension, diabetes to assess the impact of small vessel disease and the antiplatelet strategy on TLF and bleeding complications. Treatment effects of the antiplatelet strategy were estimated based on the presence or absence of small vessel disease, with interaction terms included in the Cox proportional hazard model. A 3-month landmark analysis was performed, excluding patients who experienced ischemic or bleeding complications within this period, as all patients received the same treatment during the first 3 months. The statistical analyses were conducted on the intension-to-treat cohort using R version 4.0.2. (The R Foundation for Statistical Computing, Vienna, Austria). All p-values are two-sided, and statistical significance was defined as a p-value below 0.05.
Results
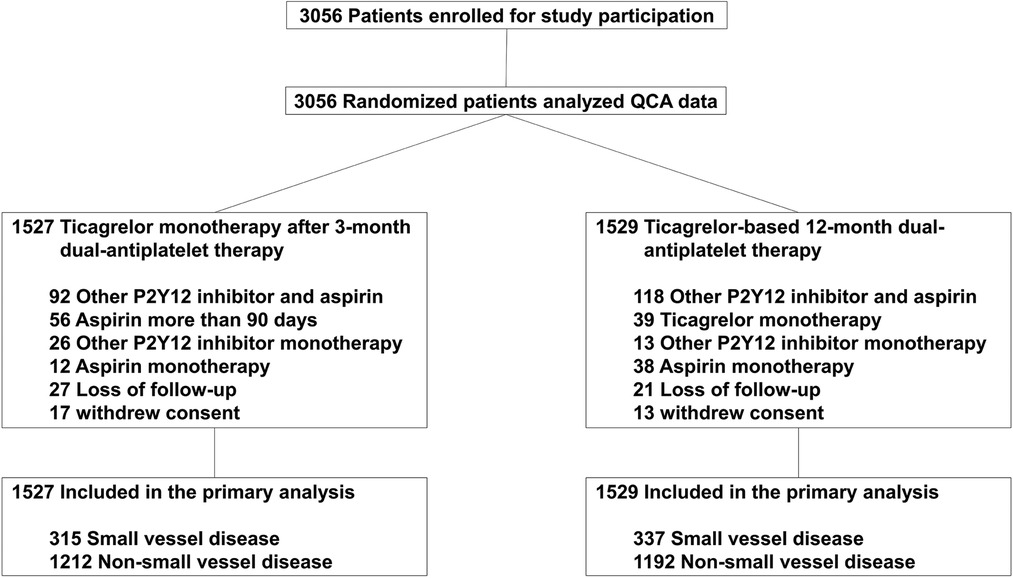
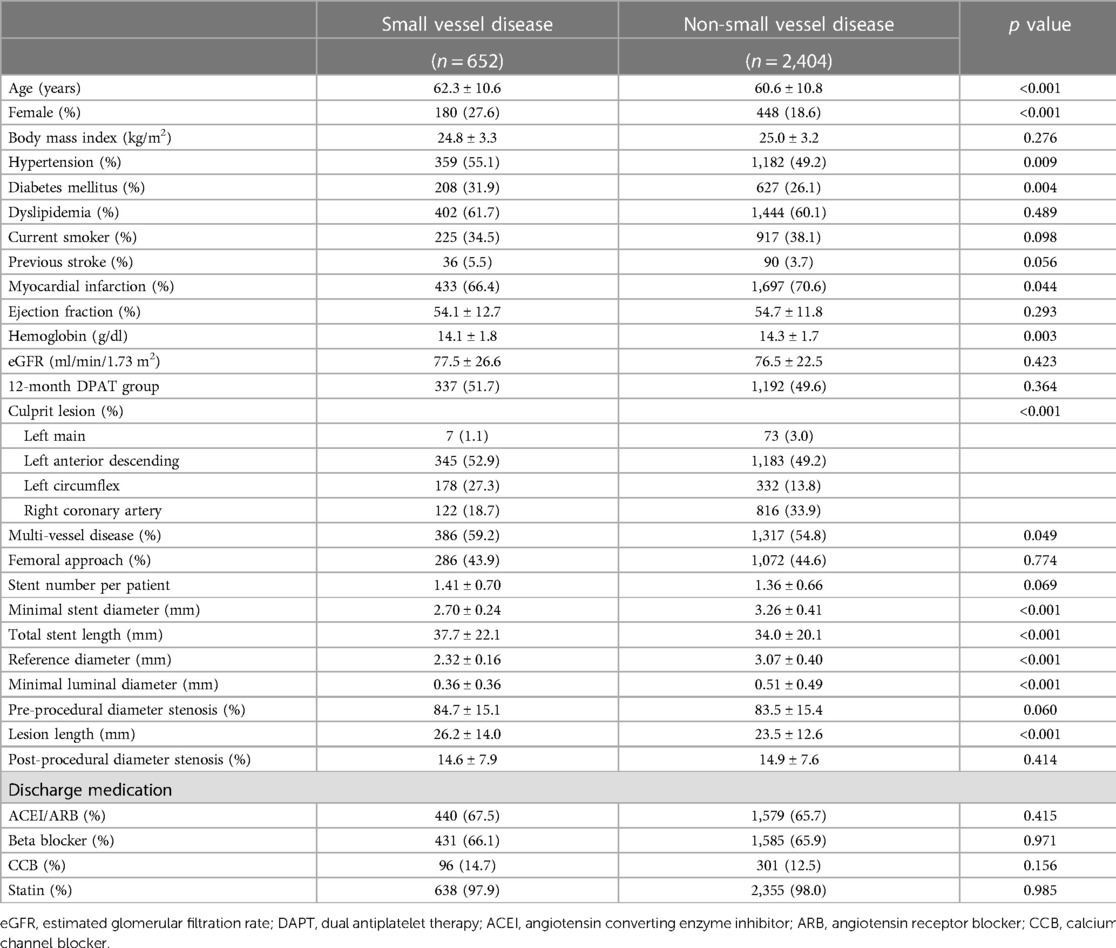
Among the 3,056 patients enrolled in the TICO trial, 652 patients (21.3%) had small vessel disease. The incidence of small vessel disease was similar between the two antiplatelet treatment strategies in the sub-analysis (Figure 1). Table 1 presented the baseline characteristics of the patients. Patients with small vessel disease were older, and had a higher incidence of female gender, hypertension, diabetes mellitus, and multi-vessel disease compared to those with non-small vessel disease. All patients received ultrathin bioresorbable polymer sirolimus-eluting stents (Orsiro stent) according to the study protocol. Smaller and longer stents were used in patients with small vessel disease.
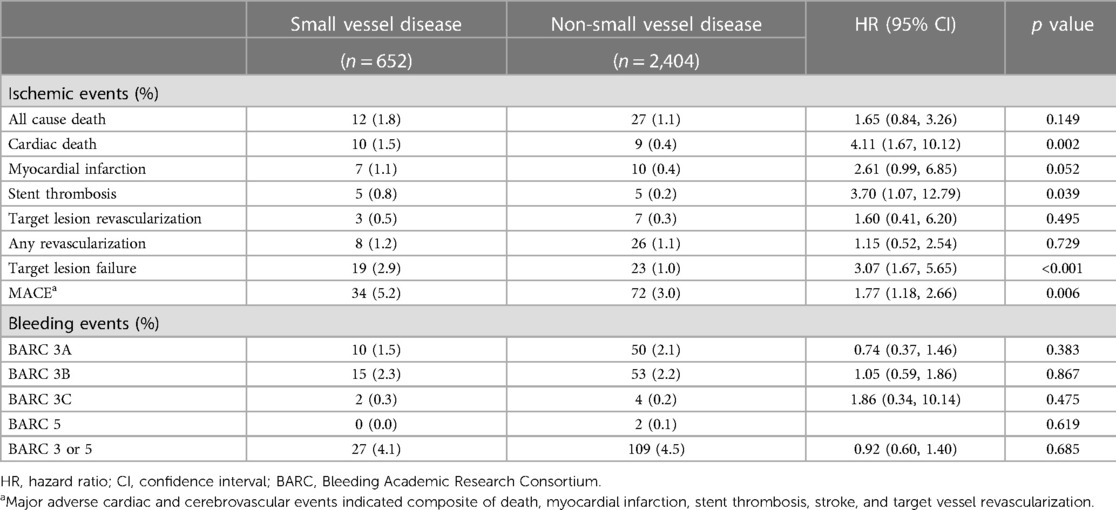
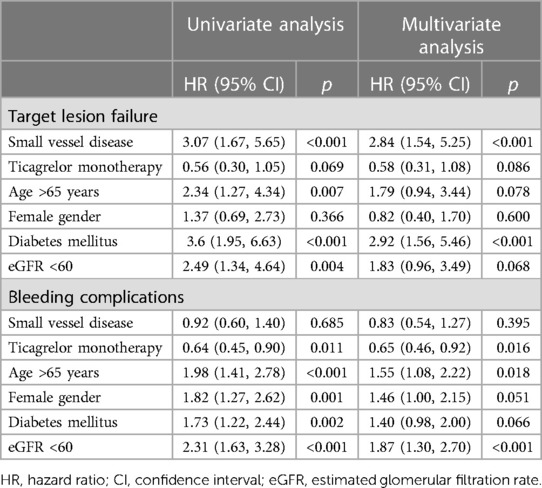
The patients with small vessel disease showed a higher TLF rate than those with non-small vessel disease (2.9% vs. 1.0%, log-rank p < 0.001) (Figure 2). Specifically, the patients with small vessel disease experienced a higher incidence of cardiac death and stent thrombosis (Table 2). In contrast, bleeding complications, including each component of the BARC definition and the total event, showed a similar incidence between the patients with small vessel disease and those without small vessel disease. The presence of small vessel disease was an independent predictor for 1-year TLF (HR 2.84, 95% CI 1.54–5.25) (Table 3). Regarding bleeding complications, age (HR 1.55, 95% CI 1.08–2.22), renal dysfunction (HR 1.87, 95% CI 1.30–2.70), and antiplatelet regimen (HR 0.65, 95% CI 0.46–0.92) were independent predictors, while small vessel disease was not.
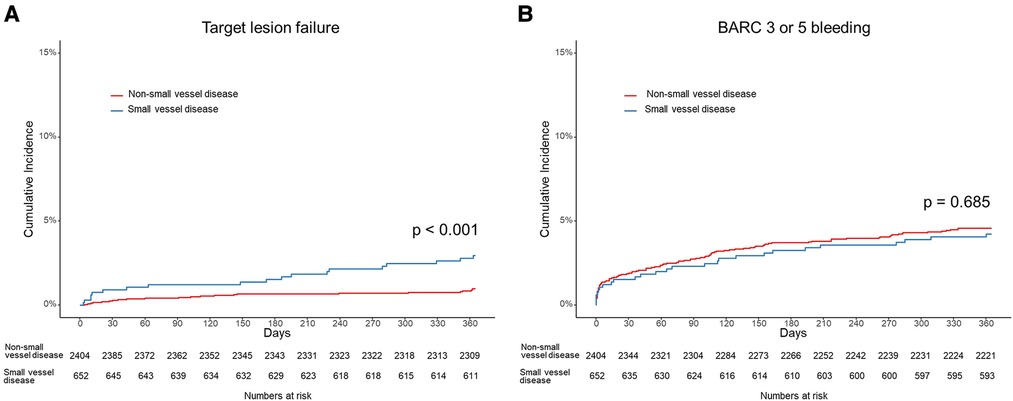
Figure 2. Cumulative incidence of (A) target lesion failure including cardiac death, target vessel myocardial infarction, stent thrombosis and target lesion revascularization, and (B) bleeding academic research consortium (BARC) definition 3 or 5 bleeding in patients with small vessel disease and those with non-small vessel disease.
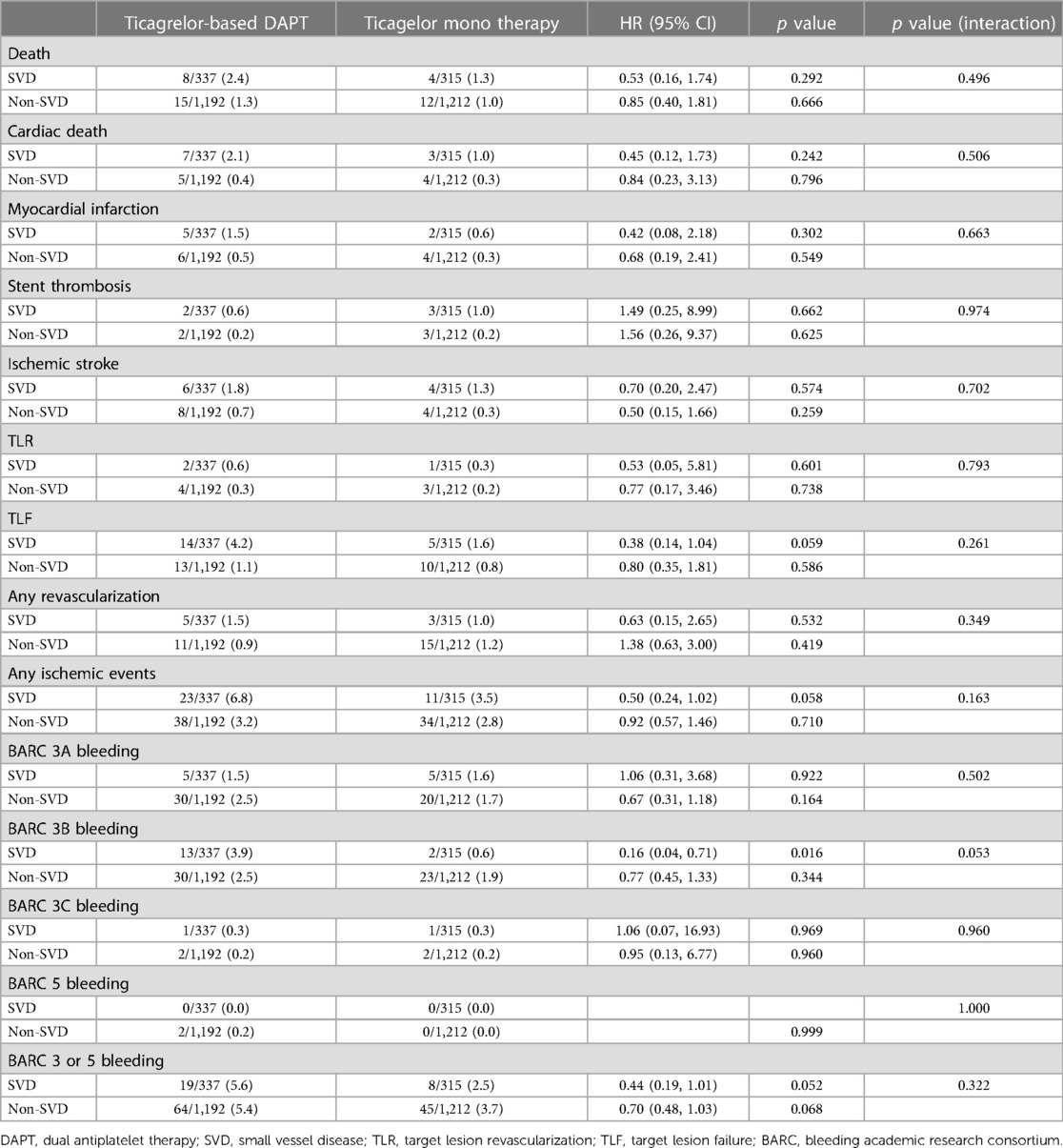
The antiplatelet strategy was not associated with ischemic and bleeding complications, regardless of the presence or absence of small vessel disease, after adjusting covariates (Table 4). Additionally, no significant group interactions were observed. In patients with small vessel disease, there are no significant difference in the incidence of ischemic and bleeding endpoints between the two treatment strategies. The TLF rate was 1.6% in the ticagrelor monotherapy after 3-month DAPT group and 4.2% in the ticagrelor-based 12-month DAPT group (p = 0.059) (HR 0.38, 95% CI 0.14–1.04, p for interaction = 0.261). The BARC 3 or 5 bleeding rate was 2.5% in the ticagrelor monotherapy after 3-month DAPT group and 5.6% in the ticagrelor-based 12-month DAPT group (p = 0.052) (HR 0.44, 95% CI 0.19–1.01, p for interaction = 0.322). Similar findings were observed in patients with non-small vessel disease.
The 3-month landmark analysis revealed that regardless of the presence or absence small vessel disease, there was a similar incidence of ischemic complications between the two treatment strategies during the 3–12 months period (Figures 3A,B). Between 3 and 12 months, the hazard ratio of TLF for ticagrelor monotherapy was 0.42 (95% CI 0.11–1.60) in patients with small vessel disease and 0.68 (95% CI 0.22–2.08) in patients with non-small vessel disease. However, ticagrelor monotherapy significantly reduced bleeding complications in the 3-month landmark analysis for both patients with small vessel disease (HR 0.09, 95% CI 0.01–0.69, log-rank p = 0.005) and those with non-small vessel disease (HR 0.40, 95% CI 0.20–0.77, log-rank p = 0.003) (Figures 3C,D).
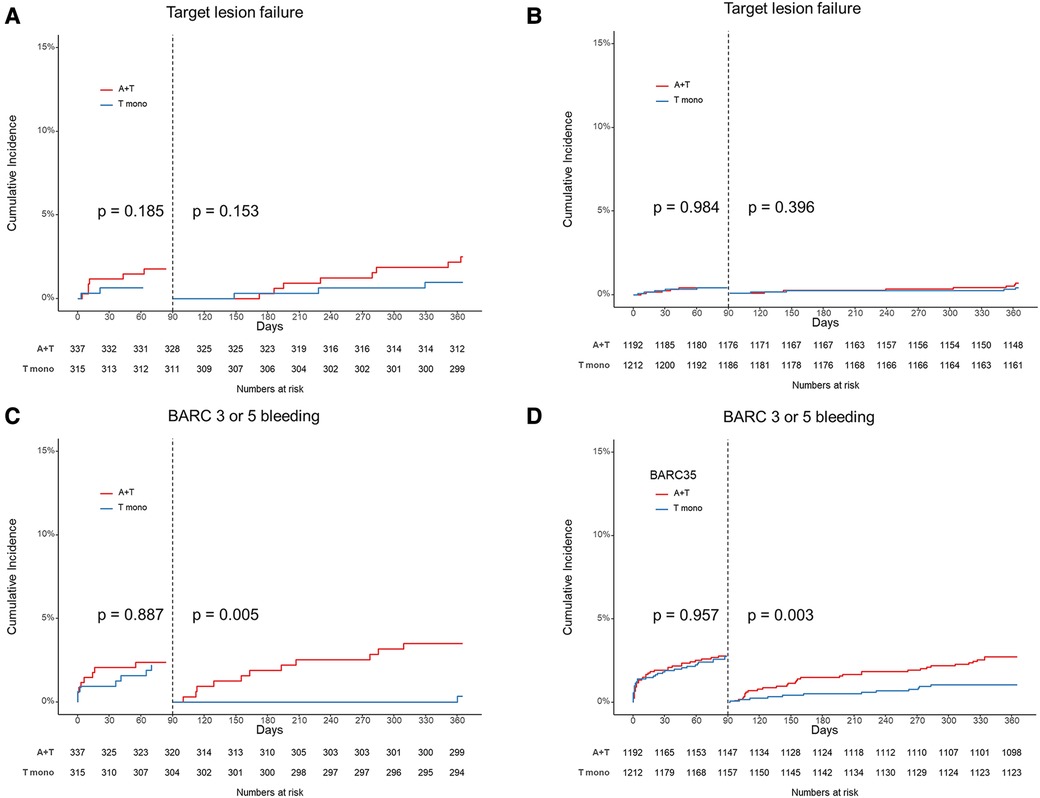
Figure 3. 3-month landmark analysis. Target lesion failure in patients with small vessel disease (A) and patients with non-small vessel disease (B). Bleeding Academic Research Consortium (BARC) definition 3 or 5 bleeding in patients with small vessel disease (C) and patients with non-small vessel disease (D). A + T indicated ticagrelor based 12-month dual antiplatelet therapy, and T mono indicated ticagrelor monotherapy after 3-month dual antiplatelet therapy.
Discussion
In this sub-analysis of the TICO trial, several key findings were observed: (1) small vessel disease was independently associated with a higher risk of 1-year TLF in patients treated with Orsiro stents, (2) ticagrelor-based 12-month DAPT was not significantly associated with a reduced TLF rate in patients with small vessel disease compared to ticagrelor monotherapy after 3 months of DAPT, (3) ticagrelor monotherapy after the 3-month DAPT was significantly associated with a reduced rate of BARC 3 or 5 bleeding in patients with small vessel disease without increasing the risk of ischemic events, and (4) no significant interactions were founded between the antiplatelet strategy and the presence or absence of small vessel disease regarding the occurrence of ischemic and bleeding complications.
Several definitions have been proposed for small vessel disease, including an angiographic reference vessel diameter of equal to or less than 2.75 mm or 2.5 mm (6, 7, 10). Despite variations in these definitions, small vessel disease has consistently been associated with an increased risk of adverse events, including restenosis and thrombosis (1, 3, 11, 12). However, newer generation drug-eluting stents with thinner struts and more biocompatible or biodegradable polymers have shown improved clinical outcomes compared to early generation stents. A subgroup analysis of the XIENCE V USA study, which included 2,853 patients treated with XIENCE V everolimus eluting-stent, found no significant difference in stent thrombosis (0.37% vs. 0.40%), cardiac death or myocardial infarction (4.5% vs. 5.1%), and target lesion revascularization (3.8% vs. 3.0%) at 1 year between small vessels (2.5 mm stent) and non-small vessels (>2.5 mm stent) (13). Similarly, pooled data analysis from the RESOLUTE global clinical program showed comparable 2-year clinical outcomes between small vessels (reference vessel diameter ≤2.5 mm) and non-small vessels (>2.5 mm) treated with Resolute zotarolimus-eluting stents (14). There was no significant difference in TLF rates between small vessels (10.1%) and non-small vessels (8.7%) at 2 years. The Orsiro stent, used in this study, has ultra-thin struts (60 μm for stent diameter ≤3.0 mm), therefore, has demonstrated better performance compared to other newer generation stents. An analysis of the BIOTRONIK—A Prospective Randomized Multicenter Study to Assess the Safety and Effectiveness of the Orsiro Sirolimus Eluting Coronary Stent System in the Treatment of Subjects With up to Three De Novo or Restenotic Coronary Artery Lesions (BIOFLOW) trials assessed the safety and effectiveness of the Orsiro stent system. It showed a significantly lower incidence of TLF (8.0% vs. 12.4%) and target vessel myocardial infarction (4.2% vs. 7.6%) in patients with small vessel disease (reference vessel diameter ≤2.75 mm) compared to everolimus-eluting stents (15). However, small vessel disease remains an important risk factor for ischemic complications even with the use of this ultra-thin strut stent, as demonstrated in our analysis.
Small coronary arteries have limited capacity to accommodate neointimal growth gollowing stent implantation, leading to a greater reduction in lumen diameter relative to the amount of neointimal thickening, compared to larger vessels (3, 7). Therefore, efforts should be made to achieve a larger stent diameter during PCI through the use of intracoronary imaging (16). It is also important to emphasize the significance of aggressive medical therapy, including the use of potent antiplatelet agents, in high risk patients such as small vessel disease. While potent antiplatelet therapy has shown a reduction in ischemic complications after PCI compared to the conventional P2Y12 inhibitor clopidogrel, direct comparisons in the context of high-risk features such as small vessel disease have been limited (17, 18). In our results, there are no significant interactions between the antiplatelet strategy regarding the 12-month incidence of ischemic complications. Although these results may be due to uncontrolled variables, it suggests that antiplatelet regimen itself may not play a critical role in prevention of TLF in patients under potent P2Y12 inhibitor such as ticagrelor.
To mitigate bleeding complications during DAPT, several studies have explored strategies to shorten the duration of DAPT. Among these studies, the TICO trial investigated a regimen where aspirin was discontinued while ticagrelor monotherapy continued in patients with acute coronary syndrome (4). The results demonstrated that ticagrelor monotherapy administered after 3-month of DAPT was significantly associated with a reduced risk of major bleeding without increasing the risk of major adverse cardio-cerebrovascular events compared to ticagrelor-based 12-month DAPT. This benefit was particularly observed in specific high-risk patient groups, such as those with ST-segment elevation myocardial infarction, diabetes, elderly patients, and obesity (19–22). Our study also demonstrated that ticagrelor monotherapy was not significantly associated with an increased incidence of ischemic events, including TLF and stent thrombosis, and it was not associated with and elevated bleeding risk in patients with small vessel disease. Several pieces of evidence have shown that aspirin does not provide additional platelet aggregation inhibition. In healthy volunteers, potent P2Y12 inhibitors reduce ADP and thromboxane A2 -mediated platelet aggregation, and this effect is minimally enhanced by aspirin (23). In post-PCI patients, ticagrelor monotherapy provided a similar platelet aggregation response to thrombin and thromboxane A2 receptor agonists compared to dual therapy with ticagrelor plus aspirin (24). Therefore, theoretically, ticagrelor could be employed as a single antiplatelet therapy following stent implantation.
This study has several limitations. First, it represents a sub-analysis and was not specifically designed to evaluate the performance of ticagrelor monotherapy in patients with small vessel disease. Therefore, our result should not be interpreted as superior to ticagrelor monotherapy compared to DAPT in patients with small vessel disease. Rather, it should be understood that aspirin withdrawal might be feasible under ticagrelor therapy in small vessel disease. Second, due to limitations in the study design, the sample size is not sufficient to draw definitive conclusions. Future investigations with larger sample sizes and a more rigorous study design are warranted. Third, the use of intracoronary imaging was not investigated in this study, as the original TICO study focused on comparing antiplatelet strategies. Lastly, caution should be exercised when applying the results of this study to non-Asian populations or patients who have received drug-eluting stents other than Orsiro stents, as the findings may not directly generalizable to these populations or devices.
In conclusion, our findings suggest that ticagrelor monotherapy, when compared with ticagrelor-based 12-month DAPT, can effectively reduce bleeding complications without increasing the risk of ischemic events in patients with small vessel disease after the 3-month DAPT period. Further research is warranted to validate and expand upon these findings in larger and more diverse patient populations.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Wonkwang University Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author contributions
KY and JC conceptualized and designed the study. DJ, B-KK, M-KH, and YJ contributed to data collection and organization. JC and DJ wrote the first draft of the manuscript. KY and SO were responsible for manuscript revisions and amendments. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Plitt A, Claessen BE, Sartori S, Baber U, Chandrasekhar J, Aquino M, et al. Impact of stent diameter on outcomes following percutaneous coronary intervention with second-generation drug-eluting stents: results from a large single-center registry. Catheter Cardiovasc Interv. (2020) 96:558–64. doi: 10.1002/ccd.28488
2. Wykrzykowska JJ, Serruys PW, Onuma Y, de Vries T, van Es GA, Buszman P, et al. Impact of vessel size on angiographic and clinical outcomes of revascularization with biolimus-eluting stent with biodegradable polymer and sirolimus-eluting stent with durable polymer the LEADERS trial substudy. JACC Cardiovasc Interv. (2009) 2:861–70. doi: 10.1016/j.jcin.2009.05.024
3. Biondi-Zoccai G, Moretti C, Abbate A, Sheiban I. Percutaneous coronary intervention for small vessel coronary artery disease. Cardiovasc Revasc Med. (2010) 11:189–98. doi: 10.1016/j.carrev.2009.04.007
4. Kim BK, Hong SJ, Cho YH, Yun KH, Kim YH, Suh Y, et al. Effect of ticagrelor monotherapy vs. ticagrelor with aspirin on major bleeding and cardiovascular events in patients with acute coronary syndrome: the TICO randomized clinical trial. JAMA. (2020) 323:2407–16. doi: 10.1001/jama.2020.7580
5. Kim C, Hong SJ, Shin DH, Kim BK, Ahn CM, Kim JS, et al. Randomized evaluation of ticagrelor monotherapy after 3-month dual-antiplatelet therapy in patients with acute coronary syndrome treated with new-generation sirolimus-eluting stents: TICO trial rationale and design. Am Heart J. (2019) 212:45–52. doi: 10.1016/j.ahj.2019.02.015
6. van der Heijden LC, Kok MM, Danse PW, Schramm AR, Hartmann M, Löwik MM, et al. Small-vessel treatment with contemporary newer-generation drug-eluting coronary stents in all-comers: insights from 2-year DUTCH PEERS (TWENTE II) randomized trial. Am Heart J. (2016) 176:28–35. doi: 10.1016/j.ahj.2016.02.020
7. Guedeney P, Claessen BE, Mehran R, Kandzari DE, Aquino M, Davis S, et al. Small-vessel PCI outcomes in men, women, and minorities following platinum chromium everolimus-eluting stents: insights from the pooled PLATINUM diversity and PROMUS element plus post-approval studies. Catheter Cardiovasc Interv. (2019) 94:82–90. doi: 10.1002/ccd.28071
8. Garcia-Garcia HM, McFadden EP, Farb A, Mehran R, Stone GW, Spertus J, et al. Standardized end point definitions for coronary intervention trials: the academic research consortium-2 consensus document. Circulation. (2018) 137:2635–50. doi: 10.1161/CIRCULATIONAHA.117.029289
9. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation. (2011) 123:2736–47. doi: 10.1161/CIRCULATIONAHA.110.009449
10. Akiyama T, Moussa I, Reimers B, Ferraro M, Kobayashi Y, Blengino S, et al. Angiographic and clinical outcome following coronary stenting of small vessels: a comparison with coronary stenting of large vessels. J Am Coll Cardiol. (1998) 32:1610–8. doi: 10.1016/s0735-1097(98)00444-6
11. Süselbeck T, Latsch A, Siri H, Gonska B, Poerner T, Pfleger S, et al. Role of vessel size as a predictor for the occurrence of in-stent restenosis in patients with diabetes mellitus. Am J Cardiol. (2001) 88:243–7. doi: 10.1016/s0002-9149(01)01633-2
12. Lee CW, Park SJ. Predictive factors for restenosis after drug-eluting stent implantation. Korean Circ J. (2007) 37:97–102. doi: 10.4070/kcj.2007.37.3.97
13. Hermiller JB, Rutledge DR, Mao VW, Zhao W, Wang J, Gruberg L, et al. Clinical outcomes in real-world patients with small vessel disease treated with XIENCE V® everolimus-eluting stents: one year results from the XIENCE V® USA condition of approval post-market study. Catheter Cardiovasc Interv. (2014) 84:7–16. doi: 10.1002/ccd.25325
14. Caputo R, Leon M, Serruys P, Neumann FJ, Yeung A, Windecker S, et al. Performance of the resolute zotarolimus-eluting stent in small vessels. Catheter Cardiovasc Interv. (2014) 84:17–23. doi: 10.1002/ccd.25485
15. Dan K, Garcia-Garcia HM, Kolm P, Windecker S, Saito S, Kandzari DE, et al. Comparison of ultrathin, bioresorbable-polymer sirolimus-eluting stents and thin, durable-polymer everolimus-eluting stents in calcified or small vessel lesions. Circ Cardiovasc Interv. (2020) 13:e009189. doi: 10.1161/CIRCINTERVENTIONS.120.009189
16. Lee JM, Choi KH, Song YB, Lee JY, Lee SJ, Lee SY, et al. Intravascular imaging-guided or angiography-guided Complex PCI. N Engl J Med. (2023) 388:1668–79. doi: 10.1056/NEJMoa2216607
17. Wiviott SD, Braunwald E, McCabe CH, Horvath I, Keltai M, Herrman JP, et al. Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON-TIMI 38 trial: a subanalysis of a randomised trial. Lancet. (2008) 371:1353–63. doi: 10.1016/S0140-6736(08)60422-5
18. James S, Angiolillo DJ, Cornel JH, Erlinge D, Husted S, Kontny F, et al. Ticagrelor vs. clopidogrel in patients with acute coronary syndromes and diabetes: a substudy from the PLATelet inhibition and patient outcomes (PLATO) trial. Eur Heart J. (2010) 31:3006–16. doi: 10.1093/eurheartj/ehq325
19. Lee SJ, Cho JY, Kim BK, Yun KH, Suh Y, Cho YH, et al. Ticagrelor monotherapy vs. ticagrelor with aspirin in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. (2021) 14:431–40. doi: 10.1016/j.jcin.2020.11.036
20. Yun KH, Cho JY, Lee SY, Rhee SJ, Kim BK, Hong MK, et al. Ischemic and bleeding events of ticagrelor monotherapy in Korean patients with and without diabetes Mellitus: insights from the TICO trial. Front Pharmacol. (2021) 11:620906. doi: 10.3389/fphar.2020.620906
21. Kim BG, Hong SJ, Kim BK, Lee SJ, Ahn CM, Shin DH, et al. Age-dependent effect of ticagrelor monotherapy versus ticagrelor with aspirin on major bleeding and cardiovascular events: a post hoc analysis of the TICO randomized trial. J Am Heart Assoc. (2021) 10:e022700. doi: 10.1161/JAHA.121.022700
22. Kim BG, Hong SJ, Kim BK, Lee YJ, Lee SJ, Ahn CM, et al. Body mass index affecting ticagrelor monotherapy vs. ticagrelor with aspirin in patients with acute coronary syndrome: a pre-specified sub-analysis of the TICO randomized trial. Front Cardiovasc Med. (2023) 10:1128834. doi: 10.3389/fcvm.2023.1128834
23. Armstrong PC, Leadbeater PD, Chan MV, Kirkby NS, Jakubowski JA, Mitchell JA, et al. In the presence of strong P2Y12 receptor blockade, aspirin provides little additional inhibition of platelet aggregation. J Thromb Haemost. (2011) 9:552–61. doi: 10.1111/j.1538-7836.2010.04160.x
24. Johnson TW, Baos S, Collett L, Hutchinson JL, Nkau M, Molina M, et al. Pharmacodynamic comparison of ticagrelor monotherapy versus ticagrelor and aspirin in patients after percutaneous coronary intervention: the TEMPLATE (ticagrelor monotherapy and platelet reactivity) randomized controlled trial. J Am Heart Assoc. (2020) 9:e016495. doi: 10.1161/JAHA.120.016495
Keywords: coronary artery disease, drug-eluting stent, dual anti-platelet therapy, ticagrelor, prognosis
Citation: Cho JY, Joo D, Yun KH, Kim B-K, Hong M-K, Jang Y and Oh SK (2023) Clinical implication of ticagrelor monotherapy in patients with small vessel coronary artery disease: results from the TICO randomized trial. Front. Cardiovasc. Med. 10:1237826. doi: 10.3389/fcvm.2023.1237826
Received: 10 June 2023; Accepted: 17 July 2023;
Published: 8 August 2023.
Edited by:
Mauro Chiarito, Humanitas University, ItalyReviewed by:
András Komócsi, University of Pécs, HungaryMohammed Faisaluddin, Rochester General Hospital, United States
© 2023 Cho, Joo, Yun, Kim, Hong, Jang and Oh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kyeong Ho Yun ZHJ5dW5raEBnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
 Jae Young Cho
Jae Young Cho Donghyeon Joo1,†
Donghyeon Joo1,† Kyeong Ho Yun
Kyeong Ho Yun Byeong-Keuk Kim
Byeong-Keuk Kim Myeong-Ki Hong
Myeong-Ki Hong