
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 06 November 2023
Sec. Cardiovascular Epidemiology and Prevention
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1236008
Aims: Recent studies have shown that mineralocorticoid receptor antagonists (MRAs) can decrease mortality in patients with heart failure; however, the application of MRAs in current clinical practice is limited because of adverse effects such as hyperkalemia that occur with treatment. Therefore, this meta-analysis used the number needed to treat (NNT) to assess the efficacy and safety of MRAs in patients with chronic heart failure.
Methods: We meta-analysed randomized controlled trials (RCTs) which contrasted the impacts of MRAs with placebo. As of March 2023, all articles are published in English. The primary outcome was major adverse cardiovascular events (MACE), and secondary outcomes included all-cause mortality, cardiovascular death, myocardial infarction (MI), stroke, and adverse events.
Results: We incorporated seven studies with a total of 9,056 patients, 4,512 of whom received MRAs and 4,544 of whom received a placebo, with a mean follow-up period of 2.1 years. MACE, all-cause mortality, and cardiovascular mortality were all reduced by MRAs, with corresponding numbers needed to treat for benefit (NNTB) of 37, 28, and 34; as well as no impact on MI or stroke. MRAs increased the incidence of hyperkalemia and gynecomastia, with the corresponding mean number needed to treat for harm (NNTH) of 18 and 52.
Conclusions: This study showed that enabling one patient with HF to avoid MACE required treating 37 patients with MRAs for 2.1 years. MRAs reduce MACE, all-cause mortality, and cardiovascular death; however, they increase the risk of hyperkalemia and gynecomastia.
One of the most prevalent public health issues of the 21st century is chronic heart failure (CHF) (1). Any structural or functional change, such as ventricular filling or blood ejection, is responsible for the onset and progression of heart failure (HF), a complex, clinically integrated disease (2). The prevalence of heart failure (HF) is increasing every year due to an ageing population and ongoing advancements in medical technology (3). Although much progress has been made with the management of patients suffering from HF, their prognosis remains unfavorable with high rates of hospitalization and mortality due to the devastating nature of HF (4). To further reduce mortality and morbidity and enhance HF patients' quality of life (QOL), new treatment strategies must be developed.
The role of Mineralocorticoid receptor (MR) is to maintain the Na+/K + balance in the nephron (5). It is mostly expressed in the heart (cardiomyocytes, fibroblasts, blood vessels) (6). Over-activation adversely affects the heart and kidneys, promoting inflammation and fibrosis as part of the pathogenesis of several cardiovascular and renal illnesses (7). In contrast, mineralocorticoid receptor antagonists (MRAs) can inhibit this receptor, thereby reducing the development of related diseases (8). MRAs can be classified as either steroidal MRAs (e.g., spironolactone, canrenone, eplerenone) or non-steroidal MRAs (e.g., finerenone). Canrenone is thought to be the main active metabolite of spironolactone (9). Eplerenone, on the other hand, is more selective, reducing non-targeted binding to progesterone or androgen receptors and therefore reducing the incidence of sexual adverse reactions, but is less binding to MR. A recently studied non-steroidal MRA, finerenone, however, binds MR in a different way to other steroidal MRAs, activates different gene pathways, and may attenuate aldosterone-induced hemodynamic and pro-fibrotic damage, thereby reducing some of the side effects seen in steroidal MRA treatment (10). MRAs have a proven performance history in reducing heart failure and have become one of the recommended modalities for the treatment of HF (11). Although the results of randomized controlled trials provide relatively reliable results, combining trials and meta-analyzing them may well provide stronger statistical power to demonstrate efficacy.
The Number Needed to Treat (NNT) represents a clinical efficacy standard that quantifies the effectiveness of pharmacological interventions within specific ranges, providing clarity of interpretation for physicians and patients (12). In addition, healthcare organizations can utilize this metric as a benefit-risk assessment tool to develop drug use strategies (13, 14). NNT values, in contrast to relative risk, are unaffected by sample size and, as a result, have no effect on p-values, which are used to reflect and explain clinical implications. Lower NNT values indicate greater effectiveness of the medication (15, 16).
Therefore, in this meta-analysis, we applied NNT to examine the risks and benefits associated with the treatment of people with chronic heart failure by using mineralocorticoid receptor antagonists.
We conducted a search of PubMed, Web of Science, the Cochrane Library, and ClinicalTrials.gov website, starting with the database until March 2023. MeSH terms were used for the search, and related terms include: heart failure, mineralocorticoid receptor antagonists, spironolactone, canrenone, eplerenone, finerenone, randomized controlled trials.
The following criteria must be met for inclusion: (1) randomized controlled trials (RCTs) (2) comparison with placebo (3) participants with chronic heart failure (4) use of MRAs (spironolactone, canrenone, eplerenone or finerenone) (5) adult patients (age 》18) (6) follow-up > 1 year (7) at least one outcome reported (8) sample size >100 cases.
Of the 2,601 articles identified, 7 met our inclusion criteria.
Two authors separately analyzed the risk of bias for the incorporated trials and extracted the required data for generalisation. Extract relevant information including subjects (number of participants, mean follow-up time, mean age at baseline), intervention (type of MRAs), control (placebo), and efficacy outcomes. The risk of bias was evaluated by applying a tool produced by the Cochrane Collaboration. Discrepancies, if any, were resolved by discussion.
We compared the outcomes of patients treated with MRAs with those not treated with MRAs. The primary outcome was major adverse cardiovascular events (MACE), including cardiovascular death, myocardial infarction (MI) and stroke. Secondary endpoints were all-cause mortality, cardiovascular death, MI, stroke, and adverse events. Adverse events include mainly hyperkalemia and gynecomastia.
It is more robust to use relative indices to calculate NNTs; therefore, we obtained pooled efficacy and NNTs by calculating risk ratios (RRs). The Mantel-Haenszel random effects model to measure and aggregate the RR was utilized, considering the proportion of each trial to the total. The I2 statistic was used to measure heterogeneity; an I2 of less than 25% was deemed minimal, a value between 25% and 50% was deemed moderate, and an I2 of greater than 50% was deemed large. The following formula was utilized when calculating NNT: NNT = 1/[(1-RR)CER], where CER is the range of 0 to 1 for the control (placebo) event rate (12). Supplementary Table S1 contains the CERs used to calculate the NNT values for each summary (17). To rationalize the interpretation of NNTs, NNTs are taken as integers. Assuming the 95% CI for NNTs crossed positive endlessness, this intended that there was no statistical significance. Due to differences in follow-up time, NNTs may be biased when compared between four distinct drug classes (spironolactone, canrenone, eplerenone, and finerenone) at a given outcome. Therefore, we normalized the follow-up time using the formula suggested by Sackett et al. (18). The formula is as follows. NNT:T × T ÷ S = NNT:S, (NNT:S is the rectified NNT, NNT:T is the real calculated to NNT, T is the real duration of follow-up and S is the normalized number of years). NNT typically decreases with increasing time since treatment began. In this equation, both the outcome incidence and the treatment effect of the drug are set to remain constant, despite changes in time.
In addition, we hypothesized that MACE might be associated with mean age, year of publication, mean follow-up time, percentage of male participants, and body mass index (BMI). To assess this hypothesis, a random effect univariate meta-regression was conducted. If the 2-tailed p-value was <0.05, it was regarded as statistically significant.
All analyses for the meta-analysis were calculated using Review Manager V.5.4.1 (RevMan), R software V.4.2.1, and Stata, V.16.0.
At the first time there were 3,222 records read. Among them, 3,215 were excluded because there were no associated results, the identical trial was published, lasted <1 year, had <100 patients, was not a randomized controlled trial, was not placebo-controlled, or was repeatedly published. Ultimately, there were seven trials enrolled in this meta-analysis (Figure 1) with a population of 9,056 patients and a mean follow-up of 2.1 years (range 1–3.7 years) for the included trials (19–25). Four trials (n = 5,660) compared spironolactone with placebo, one trial (n = 438) compared canrenone with placebo treatment, and two trials (n = 2,958) compared eplerenone with placebo treatment. Their overall characteristics are shown in Table 1. The majority from the study were men with a mean age of 68 years. The total quality of these studies was great. (Supplementary Figure S1).
In total, 7 studies evaluated the effect of MRAs on MACE in a total of 9,056 patients. Patients who were allocated to either the MRAs or the placebo group, NNT for MACE was 37 (95% CI, NNTB 22 to NNTB 130), that is, in order to avoid a single MACE, 37 patients needed to receive MRAs for 2.1 years (Figure 2). Low heterogeneity existed between trials (I2 = 22%) (Supplementary Figure S2).
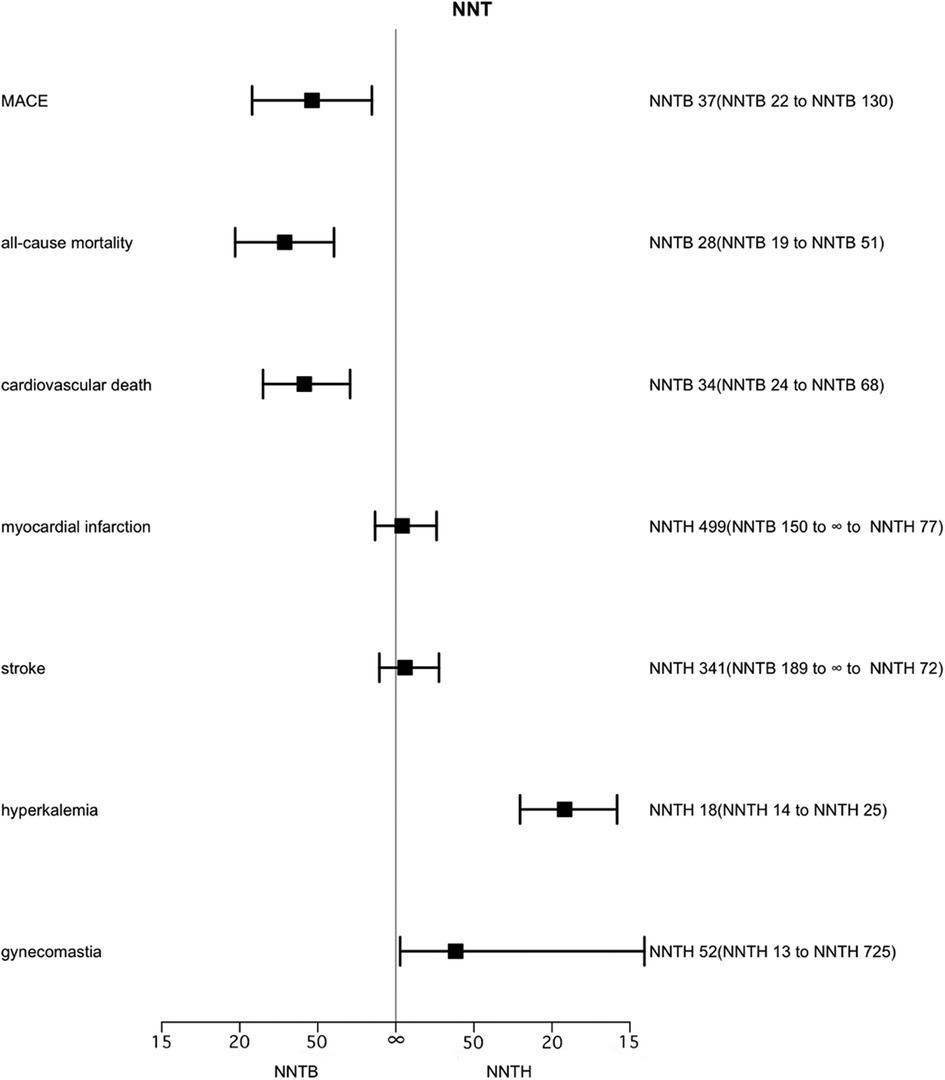
Figure 2. Effect of MRAs on major adverse cardiovascular events, all-cause mortality, cardiovascular death, myocardial infarction, stroke, hyperkalemia and gynecomastia over 2.1 years. Number needed to treat (NNT) with the corresponding confidence intervals (CIs). MACE, major adverse cardiovascular events; NNT, the number needed to treat; NNTB, number needed to treat to benefit; NNTH, number needed to treat to harm.
The role of MRA in MACE is shown in Figure 3. For a more visual comparison, we created cates plots by converting the NNT from the number of people treated needed to protect an outcome event to the number who were protected from an outcome event at 2.1 years of treating 1,000 people. Altogether 7 studies were enrolled, with a CER of 19.2% and an average of 27–28 beneficiaries out of 1,000 patients treated with MRAs.
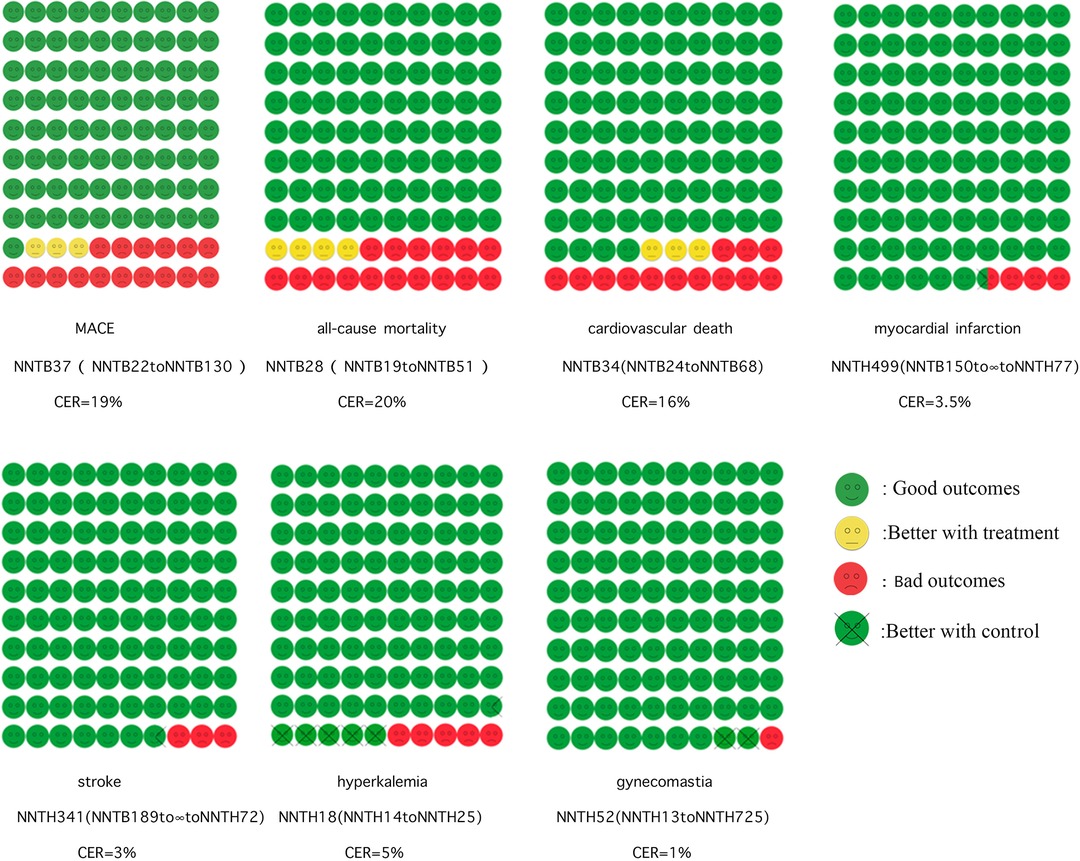
Figure 3. Cates plot. Shows the effect of MRAs on major adverse cardiovascular events, all-cause mortality, cardiovascular death, myocardial infarction, stroke, and hyperkalemia. The 100 smiley faces represent 1,000 participants treated with MRAs. Yellow faces indicate that no outcome event occurred if treated with MRAs. Green faces mean no outcome event occurred even without treatment with MRAs. Red faces mean that the outcome event will occur even if treated with MRAs. Crossed green faces indicate that the patient did not reach the outcome event with the control group.
Seven studies assessed the effect of MRAs on all-cause mortality in 9,056 patients. MRAs significantly reduced all-cause mortality (RR 0.82, 95% CI 0.74–0.90), with low heterogeneity (I2 = 9%) (Supplementary Figure S3). Among patients randomized to the MRAs and placebo groups, 28 patients had to take MRAs for 2.1 years to avoid one all-cause mortality (95% CI, NNTB 19 to NNTB 51). When translated into the number of adverse events preventable by treating 1,000 people, treatment with MRA resulted in 36–37 avoided deaths.
Six trials involving 8,634 patients reported the effect of MRAs on cardiovascular mortality in patients. The effect of MRAs on cardiovascular death was statistically significant (RR 0.80, 95% CI 0.71–0.90), with low heterogeneity (I2 = 15%) (Supplementary Figure S4). In patients randomized to the MRAs and placebo groups, the NNT for cardiovascular death was 34 (95% CI, NNTB 24 to NNTB 68) and 34 patients had to be used with MRAs for 2.1 years to have one cardiovascular death averted. Alternatively, treating 1,000 patients with MRA for 2.1 years could benefit 29–30 people.
The analysis of MI included 4 studies (5,751 patients). The corresponding NNTH for MRAs was 499 (95% CI, NNTB 150 to ∞ to NNTH 77). No significant differences were found in MI between MRAs and controls (RR 1.06, 95% CI 0.80–1.39), with no heterogeneity (I2 = 0%, p = 0.69) (Supplementary Figure S5).
Four examinations (5,774 patients) evaluated the impact of MRAs on the event of a stroke. The role of MRAs in stroke occurrence was not statistically significantly different (RR 1.10, 95% CI 0.82–1.47), with no heterogeneity (I2 = 0%, p = 0.53) (Supplementary Figure S6). The corresponding NNTH is 341 (95% CI, NNTB 189 to ∞ to NNTH 72).
Seven trials have reported data on MRAs for hyperkalemia, including 9,056 patients. Hyperkalemia was more common with MRAs (RR 2.06, 95% CI 1.78–2.39) than without MRAs treatment, with no heterogeneity (I2 = 0%) (Supplementary Figure S7), NNTH 18 (95% CI, NNTH 14 to NNTH 25).
Seven trials including 9,048 patients published the effectiveness of treatment with MRAs on improving gynecological inflammation in this population of patients. The outcomes were like those for hyperkalemia, with an NNTH of 52 (95% CI, NNTH 13 to NNTH 725) for gynecomastia in patients randomized to MRAs and placebo treatment. There was a serious level of heterogeneity (I2 = 83%). When comparing MRA drug types in groups, spironolactone was found to produce more gynecomastia [RR 7.48 (4.42–12.68)] compared to eplerenone [RR 0.72 (0.32–1.61)] (Supplementary Figure S8).
There was no proof that the noticed impact of MRA on MACE contrasted across preliminary subgroups characterized by baseline characteristics, for example, type of MRA, mean age, year of publication, mean follow-up time, level of male members, and BMI (Supplementary Table S2). Examination of the funnel plot for every result aside from gynecomastia showed no critical unevenness (Supplementary Figure S9), as has been demonstrated by the Egger test (Supplementary Figure S10).
This paper assesses the effectiveness and safety of MRA in patients with HF through the calculation of NNT. Seven studies compared MRAs with placebo in patients with HF and provided better help with the practical application of these medications through the calculation of NNT. MRAs reduced MACE, all-cause mortality and cardiovascular death, with NNTs of 37, 28 and 34 for MRAs preventing one case over 2.1 years of treatment, respectively. MRAs increase the risk of both hyperkalaemia and gynaecomastia, with 18 and 52 patients with HF being treated for one patient to develop these complications, respectively, over 2.1 years of treatment. These results may might assume a significant part in the medical decision-making of doctors and the development and implementation of related policies.
The action of MRAs in preventing aldosterone through competitive association with mineralocorticoid receptors is proving to be an effective complementary drug to ACEi for patients with HF, thus making MRAs one of the four classes of drugs used in the treatment of heart failure (26). MRAs also reduce NP levels and LA volume in patients with HF and have mild diuretic properties, the addition of MRAs can help with diuresis in addition to the significant cardiovascular benefits in patients with HF (27). In patients with HFrEF and NYHA class II to IV symptoms, MRA (spironolactone or eplerenone) is recommended to reduce morbidity and mortality if eGFR > 30 ml/min/1.73 m2 and serum potassium < 5.0 mEq/L. MRA treatment still has a high economic value compared to SGLT2i. In contrast, the benefits of MRA for HFrEF cover a wide range of etiologies and disease severity. Use in an outpatient or hospital environment is very common. Although the addition of SGLT2i to MRA therapy reduces the risk of MACE, the fact that different drugs do not have the same targets of action and therapeutic pathways does not mean that MRA therapy is ineffective (28). The role of MRA in reducing all-cause mortality compared to SGLT2i is also well studied.
This study found that the utilization of MRAs was related to a significant decrease in adverse cardiovascular outcomes, as opposed to some studies (25, 29). This may be related to the baseline risk of the selected group of people for the trial, or it may be related to the choice and dose of the drug. The binding ability of spironolactone to MR receptors is similar to that of aldosterone, but binding to the receptor inhibits its activity, resulting in an antagonistic effect. The combination of spironolactone with mineralocorticoid receptors is unstable due to the lower ability of sulfhydryl groups to form hydrogen bonds than hydroxyl groups. In contrast, non-steroidal MRAs have more selective and stable antagonistic effects due to their unique mechanism of action, and their application is more favorable with relatively fewer side effects (30). Although some studies suggest that non-steroidal MRAs (finerenone) may be as effective as steroidal MRAs in patients with HF with fewer side effects (31, 32), because the number and duration of studies of non-steroidal MRAs in cardiovascular settings are currently low, no studies of non-steroidal MRAs were included as part of search studies. Therefore, the reliability of this section needs to be improved and needs to be validated by a more definitive assessment of the results.
Studies have shown that MRAs increase the incidence of hyperkalemia, which is in agreement with previous studies (33, 34). This also limits the use of MRAs in the clinic, especially when administered externally to patients with concomitant renal dysfunction (35). Recent data have shown statistically significant clinical benefits of MRA treatment in reducing mortality in both CHF patients. This suggests that although MRA increases the incidence of hyperkalemia, it appears to protect patients from death more than patients with hyperkalemia with similar baseline characteristics and without MRA (36). And although discontinuation after the development of hyperkalemia is related to a reduced risk of repetitive hyperkalemia, the danger of mortality or cardiovascular events is higher, so the need to discontinue MRA therapy should be critically assessed (37).
In addition, we found that MRAs also produce anti-androgen-like effects, with steroidal MRAs being more likely to cause gynecological inflammation, probably because spironolactone in steroidal MRAs is less selective for mineralocorticoid receptors and also binds to sex hormones (38). These side effects make it limited in practical application. It is important to mention here that experiments on non-steroidal MRAs did not show evidence of gynecological inflammation. However, given the relatively few numbers of experiments incorporated into the analysis, the findings may not be sufficient to draw firm conclusions. More trials on non-steroidal MRAs ought to be analyzed later on to produce more statistically significant results. Therefore, when selecting drugs, we should also regulate their use from an individual perspective and consider the basic characteristics of the patient to maintain a balance between benefits and risks.
This paper is clear and unambiguous compared to other meta-analyses analyzing MRA drugs, mainly compared to placebo and in the form of NNT. The main strengths of this study are that it considered the effect of CER and that NNT expresses efficacy through a combination of baseline risk and treatment risk reduction, providing an advantage over RR. NNT is more useful than relative risk because it can tell doctors and patients more concretely, through numbers, how much effort must be put into preventing outcomes. We standardized the results for trials with different follow-up times to make the results comparable. Finally, the included trials were all placebo-controlled, reflecting the actual efficacy of the drug and ensuring that the results derived herein are not interfered with by other drugs.
There are some limitations to this paper. Firstly, the meta-analysis is an analysis of data from seven trials, not an individually based analysis of data from all those patients included in the trial. Consequently, we were unable to dependably analyze the relationship of certain variables with the noticed endpoints. Second, NNT is firmly connected with baseline risk, but the baseline risk varies among trials. According to the guidelines, previous trials have typically excluded patients with serum potassium levels >5.0 mmol/L and eGFR <30 ml/min, but due to the higher risk of patients, it is not clear that these individuals may benefit more from MRA if the hyperkalemia can be controlled.
In conclusion, this study showed that prevention of MACE in one patient with HF required treating 37 patients with HF with MRAs for 2.1 years. MRAs reduced MACE, all-cause mortality, and cardiovascular death, but no effect of MRAs on myocardial infarction and stroke was observed. MRA leads to an increment in the incidence of hyperkalaemia and gynaecomastia, with corresponding NNTs of 18 and 52.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
CG: investigation, validation, visualization, formal analysis, and manuscript drafting. YM: investigation, validation, visualization, and formal analysis. SQ: formal analysis, writing – review, and editing. KS, HW and ZZ: investigation. QT: supervision, conceptualization, and methodology. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1236008/full#supplementary-material
1. Deedwania P, Acharya T. Cardiovascular protection with anti-hyperglycemic agents. Am J Cardiovasc Drugs. (2019) 19(3):249–57. doi: 10.1007/s40256-019-00325-9
2. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 Aha/Acc/Hfsa guideline for the management of heart failure: executive summary: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2022) 79(17):1757–80. doi: 10.1016/j.jacc.2021.12.011
3. Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail. (2020) 22(8):1342–56. doi: 10.1002/ejhf.1858
4. Ide T, Kaku H, Matsushima S, Tohyama T, Enzan N, Funakoshi K, et al. Clinical characteristics and outcomes of hospitalized patients with heart failure from the large-scale Japanese registry of acute decompensated heart failure (jroadhf). Circ J. (2021) 85(9):1438. doi: 10.1253/circj.CJ-20-0947
5. Lv RL, Xu LL, Che L, Liu S, Wang YA, Dong BZ. Cardiovascular-renal protective effect and molecular mechanism of finerenone in type 2 diabetic mellitus. Front Endocrinol. (2023) 14:1125693. doi: 10.3389/fendo.2023.1125693
6. Barrera-Chimal J, Bonnard B, Jaisser F. Roles of mineralocorticoid receptors in cardiovascular and cardiorenal diseases. Annu Rev Physiol. (2022) 84:585–610. doi: 10.1146/annurev-physiol-060821-013950
7. Kolkhof P, Nowack C, Eitner F. Nonsteroidal antagonists of the mineralocorticoid receptor. Curr Opin Nephrol Hy. (2015) 24(5):417–24. doi: 10.1097/Mnh.0000000000000147
8. Lerma E, White WB, Bakris G. Effectiveness of nonsteroidal mineralocorticoid receptor antagonists in patients with diabetic kidney disease. Postgrad Med. (2023) 135(3):224–33. doi: 10.1080/00325481.2022.2060598
9. Kolkhof P, Barfacker L. 30 years of the mineralocorticoid receptor: mineralocorticoid receptor antagonists: 60 years of research and development. J Endocrinol. (2017) 234(1):T125–T40. doi: 10.1530/JOE-16-0600
10. Lytvyn Y, Godoy LC, Scholtes RA, van Raalte DH, Cherney DZ. Mineralocorticoid antagonism and diabetic kidney disease. Curr Diab Rep. (2019) 19(1):4. doi: 10.1007/s11892-019-1123-8
11. Zannad F, Stough WG, Rossignol P, Bauersachs J, McMurray JJV, Swedberg K, et al. Mineralocorticoid receptor antagonists for heart failure with reduced ejection fraction: integrating evidence into clinical practice. Eur Heart J. (2012) 33(22):2782–U18. doi: 10.1093/eurheartj/ehs257
12. Mendes D, Alves C, Batel-Marques F. Number needed to treat (nnt) in clinical literature: an appraisal. BMC Med. (2017) 15(1):112. doi: 10.1186/s12916-017-0875-8
13. Mendes D, Alves C, Batel-Marques F. Number needed to harm in the post-marketing safety evaluation: results for rosiglitazone and pioglitazone. Pharmacoepidemiol Drug Saf. (2015) 24(12):1259–70. doi: 10.1002/pds.3874
14. Mendes D, Alves C, Marques FB. Testing the usefulness of the number needed to treat to be harmed (nnth) in benefit-risk evaluations: case study with medicines withdrawn from the European market due to safety reasons. Expert Opin Drug Saf. (2016) 15(10):1301–12. doi: 10.1080/14740338.2016.1217989
15. Citrome L. Relative vs. Absolute measures of benefit and risk: What's the difference? Acta Psychiatr Scand. (2010) 121(2):94–102. doi: 10.1111/j.1600-0447.2009.01449.x
16. Citrome L, Ketter TA. When does a difference make a difference? Interpretation of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Int J Clin Pract. (2013) 67(5):407–11. doi: 10.1111/ijcp.12142
17. Veroniki AA, Bender R, Glasziou P, Straus SE, Tricco AC. The number needed to treat in pairwise and network meta-analysis and its graphical representation. J Clin Epidemiol. (2019) 111:11–22. doi: 10.1016/j.jclinepi.2019.03.007
18. Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. N Engl J Med. (1988) 318(26):1728–33. doi: 10.1056/NEJM198806303182605
19. Vizzardi E, Nodari S, Caretta G, D'Aloia A, Pezzali N, Faden G, et al. Effects of spironolactone on long-term mortality and morbidity in patients with heart failure and mild or no symptoms. Am J Med Sci. (2014) 347(4):271–6. doi: 10.1097/MAJ.0b013e31829dd6b1
20. Tsutsui H, Ito H, Kitakaze M, Komuro I, Murohara T, Izumi T, et al. Double-blind, randomized, placebo-controlled trial evaluating the efficacy and safety of eplerenone in Japanese patients with chronic heart failure (J-emphasis-hf). Circ J. (2017) 82(1):148–58. doi: 10.1253/circj.CJ-17-0323
21. Boccanelli A, Mureddu GF, Cacciatore G, Clemenza F, Di Lenarda A, Gavazzi A, et al. Anti-remodelling effect of canrenone in patients with mild chronic heart failure (area in-chf study): final results. Eur J Heart Fail. (2009) 11(1):68–76. doi: 10.1093/eurjhf/hfn015
22. Preiss D, van Veldhuisen DJ, Sattar N, Krum H, Swedberg K, Shi H, et al. Eplerenone and new-onset diabetes in patients with mild heart failure: results from the eplerenone in mild patients hospitalization and survival study in heart failure (emphasis-hf). Eur J Heart Fail. (2012) 14(8):909–15. doi: 10.1093/eurjhf/hfs067
23. Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the aldo-dhf randomized controlled trial. JAMA. (2013) 309(8):781–91. doi: 10.1001/jama.2013.905
24. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized aldactone evaluation study investigators. N Engl J Med. (1999) 341(10):709–17. doi: 10.1056/NEJM199909023411001
25. Dalzell JR. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. (2014) 371(2):179. doi: 10.1056/NEJMc1405715
26. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, et al. Esc guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European society of cardiology. Developed in collaboration with the heart failure association (hfa) of the esc. Eur J Heart Fail. (2012) 14(8):803–69. doi: 10.1093/eurjhf/hfs105
27. Kagami K, Obokata M, Harada T, Saito Y, Naito A, Sorimachi H, et al. Effects of mineralocorticoid receptor antagonists in early-stage heart failure with preserved ejection fraction. CJC Open. (2023) 5(5):380–91. doi: 10.1016/j.cjco.2023.03.001
28. Malgie J, Clephas PRD, Brunner-La Rocca HP, de Boer RA, Brugts JJ. Guideline-directed medical therapy for HFrEF: sequencing strategies and barriers for life-saving drug therapy. Heart Fail Rev. (2023) 28(5):1221–34. doi: 10.1007/s10741-023-10325-2
29. Berbenetz NM, Mrkobrada M. Mineralocorticoid receptor antagonists for heart failure: systematic review and meta-analysis. BMC Cardiovasc Disord. (2016) 16(1):246. doi: 10.1186/s12872-016-0425-x
30. Chen Q, Zhao D, Sun J, Lu C. Aldosterone blockade in acute myocardial infarction: a systematic review and meta-analysis. Cardiovasc Ther. (2021) 2021:1710731. doi: 10.1155/2021/1710731
31. Pitt B, Kober L, Ponikowski P, Gheorghiade M, Filippatos G, Krum H, et al. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist bay 94–8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: a randomized, double-blind trial. Eur Heart J. (2013) 34(31):2453–63. doi: 10.1093/eurheartj/eht187
32. Filippatos G, Anker SD, Bohm M, Gheorghiade M, Kober L, Krum H, et al. A randomized controlled study of finerenone vs. Eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur Heart J. (2016) 37(27):2105–14. doi: 10.1093/eurheartj/ehw132
33. Henrysson J, Thunstrom E, Chen X, Fu M, Basic C. Hyperkalaemia as a cause of undertreatment with mineralocorticoid receptor antagonists in heart failure. ESC Heart Fail. (2023) 10(1):66–79. doi: 10.1002/ehf2.14137
34. Trevisan M, de Deco P, Xu H, Evans M, Lindholm B, Bellocco R, et al. Incidence, predictors and clinical management of hyperkalaemia in new users of mineralocorticoid receptor antagonists. Eur J Heart Fail. (2018) 20(8):1217–26. doi: 10.1002/ejhf.1199
35. Pandey AK, Bhatt DL, Cosentino F, Marx N, Rotstein O, Pitt B, et al. Non-steroidal mineralocorticoid receptor antagonists in cardiorenal disease. Eur Heart J. (2022) 43(31):2931–45. doi: 10.1093/eurheartj/ehac299
36. Jonsson Holmdahl A, Wessberg G, Norberg H, Soderstrom A, Valham F, Bergdahl E, et al. Motives, frequency, predictors and outcomes of mra discontinuation in a real-world heart failure population. Open Heart. (2022) 9(2):e002022. doi: 10.1136/openhrt-2022-002022
37. Trevisan M, Fu EL, Xu Y, Savarese G, Dekker FW, Lund LH, et al. Stopping mineralocorticoid receptor antagonists after hyperkalaemia: trial emulation in data from routine care. Eur J Heart Fail. (2021) 23(10):1698–707. doi: 10.1002/ejhf.2287
Keywords: mineralocorticoid receptor antagonists, chronic heart failure (CHF), cardiovascular—history, number needed to treat (NNT), meta-analysis
Citation: Geng C, Mao Y-C, Qi S-f, Song K, Wang H-F, Zhang Z-y and Tian Q-B (2023) Mineralocorticoid receptor antagonists for chronic heart failure: a meta-analysis focusing on the number needed to treat. Front. Cardiovasc. Med. 10:1236008. doi: 10.3389/fcvm.2023.1236008
Received: 7 June 2023; Accepted: 19 October 2023;
Published: 6 November 2023.
Edited by:
Gen-Min Lin, Hualien Armed Forces General Hospital, TaiwanReviewed by:
Bertram Pitt, University of Michigan, United States© 2023 Geng, Mao, Qi, Song, Wang, Zhang and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qing-Bao Tian dHFiMTk4MEBoZWJtdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.