
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 08 September 2023
Sec. General Cardiovascular Medicine
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1232327
This article is part of the Research Topic Challenges and Future Perspectives of Transcatheter Valve Interventions View all 7 articles
 Isabel Mattig1,2,3,4
Isabel Mattig1,2,3,4 Fabian Barbieri2,5
Fabian Barbieri2,5 Mario Kasner2,5
Mario Kasner2,5 Elena Romero Dorta1,2
Elena Romero Dorta1,2 Anna Lisa Heinrich-Schüler1,2
Anna Lisa Heinrich-Schüler1,2 Miry Zhu2,5
Miry Zhu2,5 Karl Stangl1,2,3
Karl Stangl1,2,3 Ulf Landmesser2,5
Ulf Landmesser2,5 Markus Reinthaler2,5,6
Markus Reinthaler2,5,6 Henryk Dreger2,3,7*
Henryk Dreger2,3,7*
Background: In recent years, new interventional therapies for tricuspid regurgitation (TR) demonstrated their effectiveness in reducing TR severity and improving symptoms. Currently, tricuspid transcatheter edge-to-edge repair (T-TEER) and percutaneous annuloplasty are the most widely used techniques in Europe. In this retrospective study, we compared procedural characteristics and learning curves of both TR devices in a real-world cohort.
Material and methods: Eligible patients with severe to torrential TR underwent either percutaneous annuloplasty or T-TEER as recommended by the local heart team. Patients with combined mitral and tricuspid interventions were excluded from the analysis. The study focused on procedural characteristics, TR reduction and learning curves.
Results: A total of 122 patients underwent either percutaneous annuloplasty (n = 64) or T-TEER (n = 58) with a technical and device success rate of 98% and 97%, respectively. Reasons for technical failure included right coronary artery (RCA) dissection prior to percutaneous annuloplasty, and two single leaflet device attachments (SLDA) during T-TEER implantation. The mean improvement of TR severity was 2.4 ± 0.8 degrees after T-TEER and 2.5 ± 0.8 after percutaneous annuloplasty. T-TEER procedures were shorter in terms of both procedure time and radiation exposure, while percutaneous annuloplasty, although taking longer, showed a significant reduction in procedure time over the course of the analysed period.
Conclusion: In summary, both interventional therapies reduce TR severity by approximately two degrees when used in the appropriate anatomy. The learning curve for annuloplasty group showed a significant decrease of procedure times.
Significant tricuspid regurgitation (TR) is a prevalent valvular heart disease that is observed in 4% of patients over 75 years of age (1). TR is mostly secondary due to left heart pathologies, atrial fibrillation, or pulmonary hypertension (1). Even mild to moderate TR leads to a significant increase in morbidity and mortality (2). Recommended surgical repair or replacement in severe symptomatic TR is associated with a high intra-hospital mortality rate of 8%–10% and is rarely performed (3, 4). In recent years, new interventional therapies have been developed to address this clinical need (5). These therapies comprise edge-to-edge repair, annuloplasty devices as well as orthotopic and heterotopic valve implantations (6, 7). Transcatheter edge-to-edge repair (T-TEER) and percutaneous annuloplasty are currently the only approved and consequently most commonly used interventional therapies for tricuspid regurgitation in Europe (5–10). The European Society of Cardiology (ESC) and European Association for Cardiothoracic Surgery (EACTS) suggest interventional TR therapy in inoperable patients (3).
The T-TEER approach primarily diminishes TR by clipping the leaflets, while tricuspid annuloplasty reduces the annular circumference. Consequently, both devices are implanted in varying TR morphologies (5, 7). T-TEER is favored in patients with an anteroseptal jet and a small to moderate gap between the two target leaflets (≤7 mm), preferably in a trileaflet morphology (5, 7). In contrast, best results after annuloplasty are expected in patients with predominantly annular dilatation, none or mild tethering, a central jet and a suitable landing zone to insert the anchors, such as a sufficient distance to the right coronary artery (RCA) (5, 7). Both techniques can be used in case of cardiac implantable electronic devices (CIEDs) (7). However, a superior post-interventional outcome is expected without any right ventricular lead (7). Precondition for a successful procedure is a favorable transesophageal echocardiography (TOE) window or the availability of intracardiac ultrasound (5, 7).
A significant number of patients present with anatomies amenable by either T-TEER or annuloplasty. Therefore, other considerations such as procedure time and potential complications can play a role in the decision-making process for the appropriate device. In addition, as interventional TR repair is still a novel therapy, starting centers might be interested in expected learning curves for both approaches. Thus, the aim of present study was to provide a comparison of procedural characteristics and learning curves of the most widely used TR devices, i.e., percutaneous annuloplasty and T-TEER, in a real-world cohort.
The retrospective multi-centre study compared percutaneous annuloplasty and T-TEER of the tricuspid valve in a real-world cohort. Patients with an age ≥18 years, high surgical risk and symptomatic severe to torrential TR of different pathologies and despite optimal medical therapy underwent either Cardioband® implantation (Edwards Lifesciences, Irvine, CA, USA) or T-TEER (TriClip®, Abbott, Chicago, Illinois, USA, or PASCAL®, Edwards Lifesciences, Irvine, California, USA) from 2019 to 2022. Patients with combined procedures of the tricuspid and mitral valve were excluded from the analysis. In case of two sequential TR procedures, only the first intervention was evaluated. The study was approved by the institutional ethics committee of the Charité – Universitätsmedizin Berlin, Germany (EA1/005/23).
Interventional therapy of TR was recommended by local heart team consisting of interventional cardiologists, cardiovascular surgeons, anesthetists, heart failure specialists, and imaging experts. The appropriate device was selected based on individual tricuspid anatomy and function according to current recommendations (5, 7). Patients with a systolic pulmonary artery pressure ≥75 mmHg measured by right heart catheterization did not undergo an interventional therapy. Evaluation of tricuspid regurgitation and its interventional therapy comprised an echocardiographic assessment, right and left heart catheterization as well as computed tomography in line with the guidelines of the ESC and EACTS (3). Echocardiography was performed according to the standards of the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI) using a GE healthcare Vivid E9 or E95 with a M5S(c) 1.5–4.5 MHz or 4Vc-D 3D/4D Phased Array 1.4–5.2 MHz transducer (GE Vingmed, Horton, Norway) (11, 12). TR was quantified by assessment of the effective regurgitant orifice area (EROA) and regurgitant volume (RegVol) using the proximal isovelocity surface area (PISA) method, vena contracta measurements, as well as hepatic vein reflux. TR severity was graded into five categories as proposed by Hahn et al. (13). TOE or intracardiac ultrasound was used for guidance of the interventional therapy. All procedures were performed as described previously under general anesthesia (8, 10, 14).
Outcome parameters were the following procedural characteristics: technical and device success and complications based on the Mitral Valve Academic Research Consortium (MVARC) criteria (15) as well as TR reduction and learning curves, i.e., changes of outcome and procedural characteristics over time. Technical and device success were combined and defined as successful implantation of the device and retrieval of the delivery system without conversion to open heart surgery or other interventional therapy as well as TR reduction of at least one grade at the time of leaving the operating room (adapted from MVARC criteria). TR grade was quantified at the end of the procedure and at discharge.
SPSS Statistics version 28 for Windows (IBM Corporation, New York, NY, USA) was used for data analysis. Categorical and ordinal variables were listed in percentages and were analysed by chi-squared test or Mann-Whitney U-test, respectively. Continuous variables were presented as median with 25th and 75th percentile or mean with standard deviation depending on their skewness and uniform per variable for a better inter- and intragroup comparison. Continuous variables were assessed by t-test (normally distributed parameters) and Wilcoxon-test or Mann-Whitney U-test (not normally distributed parameters). The impact of TR reduction on right ventricular function and learning curves were evaluated by linear regression analysis. TR improvement (difference between TR grade after intervention and at baseline) was assumed to be a continuous variable. A p-value of <0.05 was considered statistically significant.
A total of 122 patients with severe to torrential TR underwent either percutaneous annuloplasty or T-TEER in two centres between 2019 and 2022. Baseline characteristics of the study cohort are listed in Table 1. Patients undergoing percutaneous annuloplasty or T-TEER had a median age of 81 and 82 years, respectively. Overall, 80% of patients reported a New York Heart Association Class (NYHA class) of III or IV, and 31% had a history of CIED implantation including one patient with a leadless pacemaker in the annuloplasty group. In detail, 25 patients (43%) in the T-TEER group and 12 patients (19%) in the annuloplasty group had a right ventricular lead crossing the tricuspid valve at the time of the intervention (p = 0.003). There was no significant difference in leading TR pathology between annuloplasty or T-TEER group (p = 0.329, Figure 1). Echocardiographic characteristics and pulmonary artery pressures did not differ significantly between the two groups except for tenting height at baseline (Tables 1–3). While the mean left ventricular function was mildly reduced in the T-TEER group, the mean right ventricular function was within a normal range in both cohorts. The mean right ventricular diameter and atrial area were dilated in both groups and patients who underwent annuloplasty had more often a torrential TR at baseline. One patient each from the annuloplasty and T-TEER group received a second intervention due to TR deterioration after 1 year and after 4 months, respectively. Only the first procedures were included in our analysis, as stated before.
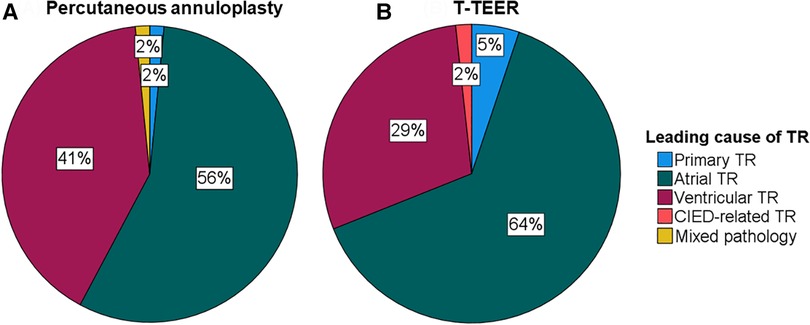
Figure 1. Leading cause of TR of patients undergoing percutaneous annuloplasty (A) or T-TEER (B). CIED, cardiac implantable electronic device; T-TEER, transcatheter edge-to-edge repair; TR, tricuspid regurgitation.
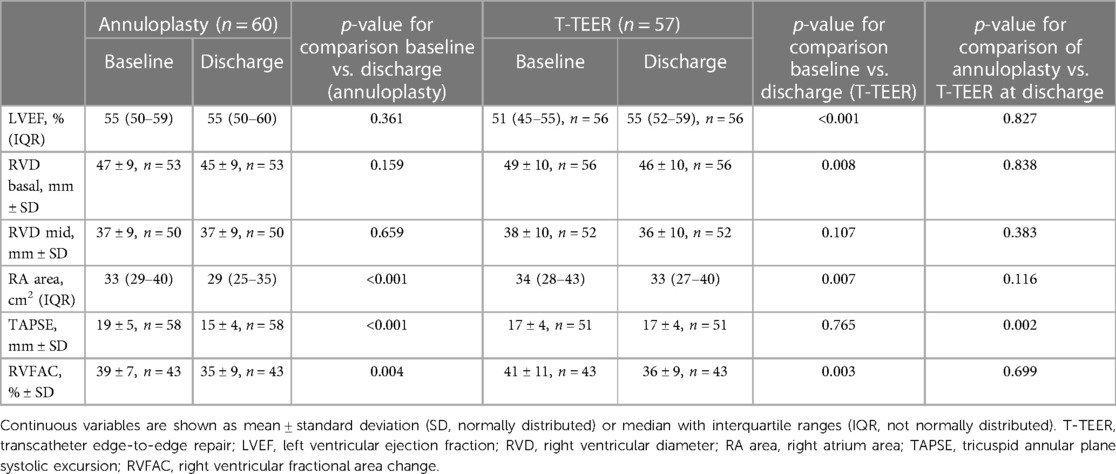
Table 3. Echocardiographic characteristics of patients with complete measurements at baseline and discharge.
Technical and device success was achieved in 97% of patients in the T-TEER group and 98% of patients in the annuloplasty group (p = 0.502). Reasons for device failure were two single leaflet device attachments (SLDA) after T-TEER. Two additional SLDA occurred during the intra-hospital stay. One patient with planned Cardioband® implantation developed cardiac arrest due to iatrogenic coronary dissection after engagement of the right coronary artery, necessitating cardiopulmonary resuscitation. Therefore, the Cardioband® implantation was aborted. We did not include this case in the further analysis of procedural characteristic and durable repair. None of the patients died during the procedure. One patient of the annuloplasty group suffered from a major vascular access complication, a dissection of the left iliac artery due to pronounced kinking, treated by stent implantation. The implantation of the Cardioband® was performed during a second procedure without further complications.
Procedure time and duration of radiation exposure were significantly shorter in the T-TEER group compared to the annuloplasty group (T-TEER: 102 [73–122] min and 10 [7–15] min vs. annuloplasty: 165 [143–236] min and 61 [44–74] min, p < 0.001; Figures 2, 3). On average, 1.9 ± 0.6 devices per patient were implanted in the T-TEER group, mostly into the anteroseptal, followed by the posteroseptal commissure. Annuloplasty devices comprised the sizes C to F. On average, 121 ± 62 ml of contrast were used for Cardioband® implantation compared to none for T-TEER. Annuloplasty patients presented significantly more often a new or progressive but hemodynamically not relevant pericardial effusion compared to patients after T-TEER. Moreover, four cases of transient bradycardia and three incidents of right coronary artery injuries requiring intervention occurred during percutaneous annuloplasty. Detailed procedural complications are listed in Table 4.
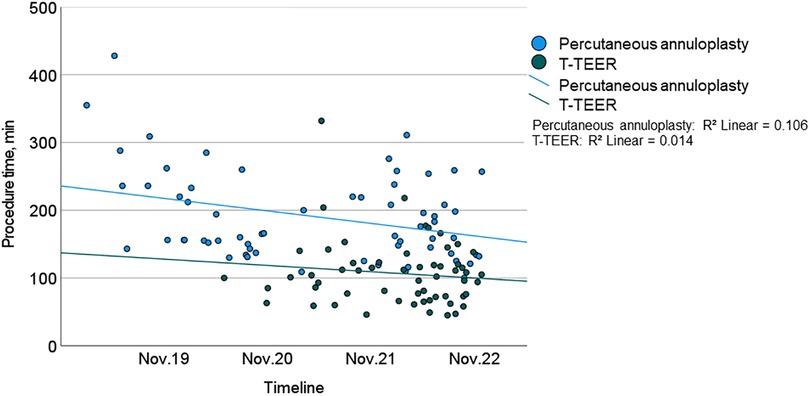
Figure 2. Procedure time of T-TEER (green) and percutaneous annuloplasty (blue) including trend lines during the study from 2019 to 2022. T-TEER, transcatheter edge-to-edge repair.
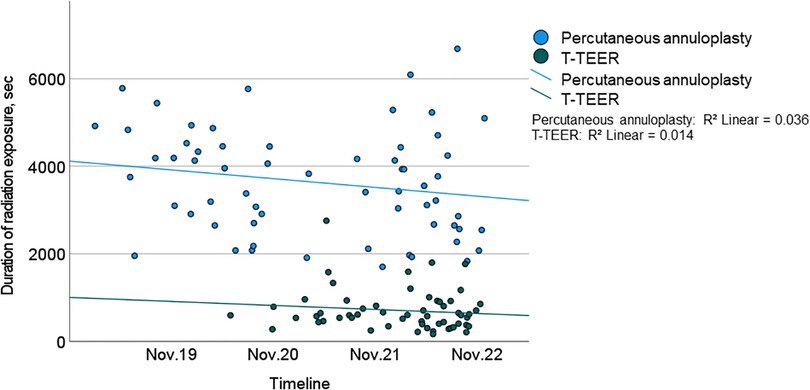
Figure 3. Duration of radiation exposure of T-TEER (green) and percutaneous annuloplasty (blue) including trend lines over the course of the study (2019–2022). T-TEER, transcatheter edge-to-edge repair.
TR was reduced at least one grade in 100% of patients, with 91% of T-TEER patients and 80% of annuloplasty patients achieving a TR grade ≤2 post-intervention. The mean reduction was 2.4 ± 0.8 grades after T-TEER and 2.5 ± 0.8 grades after annuloplasty (Figure 4). Moreover, we observed a significant decrease in annulus diameter in both groups after the procedure, which was more pronounced after annuloplasty (Figure 5). Grading of TR severity by transthoracic echocardiography at discharge was consistent with the intraprocedural assessment at the end of the intervention in 69% of patients in the T-TEER group and in 64% of patients in the annuloplasty group (p = 0.650 for comparison of both groups).
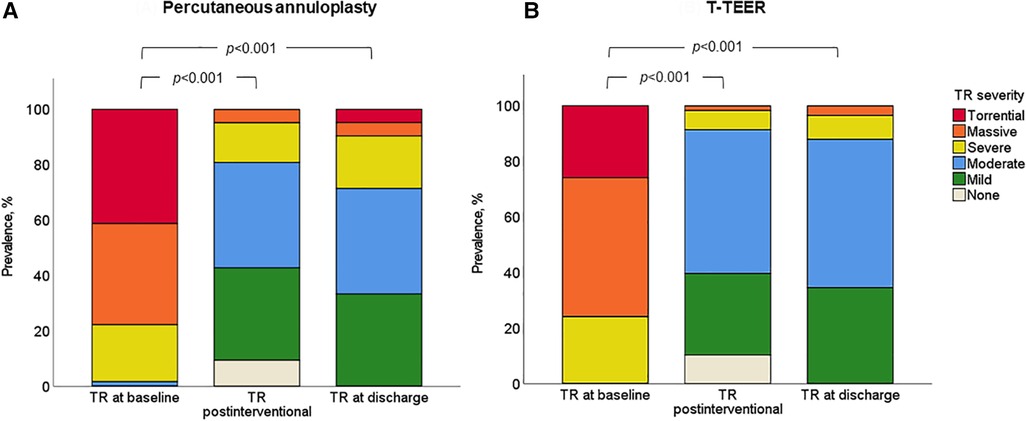
Figure 4. Severity of TR in the annuloplasty (A) and T-TEER group (B) at baseline, postinterventional and at discharge (p < 0.001 for comparison of baseline and postinterventional as well as baseline and discharge in both groups). T-TEER, transcatheter edge-to-edge repair; TR, tricuspid regurgitation.
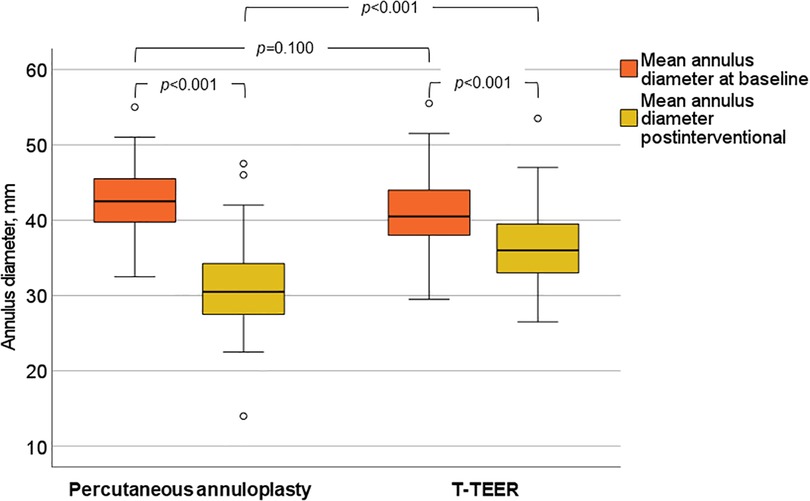
Figure 5. Mean annulus diameter of the tricuspid valve before and after annuloplasty and T-TEER. T-TEER, transcatheter edge-to-edge repair.
In both groups, we observed a significant decrease of right ventricular function as assessed by right ventricular fractional area change (RVFAC) and a significant reduction of right atrial area, while tricuspid annular plane systolic excursion (TAPSE) was only reduced after annuloplasty (Table 3). Moreover, we detected a significant impact of TR reduction on RVFAC after T-TEER, but not after annuloplasty (linear regression analysis for T-TEER: F-value 7.7, p = 0.008, regression coefficient 46.1, p < 0.001).
The procedure time of annuloplasty showed a significant reduction throughout the study period. There was neither a relevant effect of a learning curve on TR reduction in both groups nor on procedure time in the T-TEER group.
The present study compares procedural characteristics and learning curves of percutaneous annuloplasty and T-TEER in a real-world setting. Both interventional therapies reduced TR severity by approximately two degrees. Technical and device success was achieved in 97% of patients in the T-TEER and 98% of patients in the annuloplasty group. Complications during percutaneous annuloplasty comprised intermittent bradycardia and injuries of the right coronary artery. SLDA occurred in 7% of patients after edge-to-edge repair. Overall, however, the number of clinically relevant complications was low.
TR reduction of at least one grade in 100% of patients in our study cohort is comparable to results of previous T-TEER and annuloplasty trials (8, 14, 16, 17). Device selection was based on tricuspid anatomy and function assessed by echocardiography, computed tomography, and right heart catheterization (5, 7, 8, 16, 17). Figure 6 illustrates our algorithm for device selection, which aligns with similar recommendations published in the literature (5). Although percutaneous annuloplasty is primarily applied in annulus dilatation in atrial TR, TR etiologies did not differ significantly between both groups and annuloplasty as well as T-TEER resulted in a significantly reduced tricuspid annular diameter. Interestingly, the algorithm is not well reflected in the baseline measurements which in fact showed a greater tenting height in the annuloplasty group and a wider coaptation gap in the T-TEER group. The reason for this apparent discrepancy is that a substantial number of cases (n = 10) could not be treated with the preferred device—either due to RCA proximity (for annuloplasty) or due to gap size (for T-TEER). This resulted in patients with large gaps (often associated with larger tenting height) being treated by annuloplasty especially in the early phase of the study period. Similarly, patients with suitable valve anatomy for annuloplasty had to undergo T-TEER when RCA proximity made annuloplasty not feasible. With the availability of larger edge-to-edge devices, e.g., TriClip G4 and PASCAL Ace, we observed a reduction of tenting height in the annuloplasty group and an increase of baseline gap size in T-TEER patients. In both groups, patients had right ventricular leads. Lurz et al. already reported comparable efficacy of T-TEER in patients with and without right ventricular leads (7, 18). However, TR improvement was more pronounced in patients with a commissural or central lead compared to a lead in contact to the leaflet body (18). In line with the results by Lurz et al., we also observe no significant difference in TR reduction in patients with or without right ventricular leads in both groups. We observed a decrease in right ventricular function, as measured by TAPSE, after interventional therapy, possibly driven by reduction of regurgitation volume in both groups and the annuloplasty device itself. Therefore, we recommend further follow-up visits including assessment of functional status, medical therapy, and echocardiography. This is necessary to prevent the potential oversight of clinical deterioration associated with an afterload mismatch and the decrease in right ventricular function. At baseline, the majority of our patients were either receiving diuretic treatment or undergoing dialysis in addition to heart failure therapy (Table 1). It is advisable to adjust diuretic therapy depending on venous congestion during the follow-up visits.
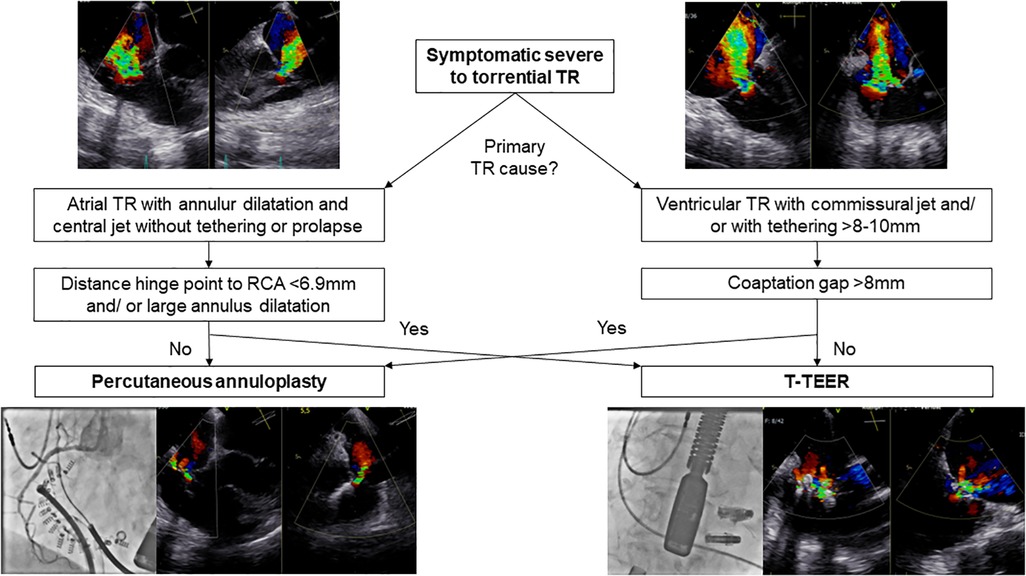
Figure 6. Flow chart for selecting the appropriate device in different TR anatomies. RCA, right coronary artery; T-TEER, transcatheter edge-to-edge repair; TR, tricuspid regurgitation.
Intermittent bradycardia occurred only in the annuloplasty group, most likely due to mechanical compression on the conduction system in the region of Koch's triangle (19). While the observed bradycardia was usually self-limiting and of short duration, operators should be aware of this possible complication occurring during placement of the last anchors. Compression or injury of the right coronary artery (RCA) requiring percutaneous coronary intervention after anchor placement (5% of our patients) was mostly caused by severe proximity of the RCA to the tricuspid annulus. In addition, we found new or progressive pericardial effusions in 27% of patients after percutaneous annuloplasty, which were most likely due to pericardial irritation resulting from anchor insertion. The pericardial effusions were usually constant until discharge and had no clinical relevance.
In the T-TEER cohort, four SLDA occurred during the procedure (n = 2) and up to discharge (n = 2) between the posterior and septal (n = 2) as well as the anterior and septal leaflets (n = 2). All T-TEER patients with SLDA presented a massive TR with degenerative and short leaflets as well as a larger tricuspid annulus diameter at baseline (45 [44–49] mm) compared to those without SLDA (40 [38–44] mm). However, the tenting height and coaptation gaps were comparable between the two groups, measuring approximately 4 ± 3 mm (n = 4) and ranging from 2 to 5 mm (n = 3) in the SLDA group, respectively. All patients underwent a re-intervention (T-TEER) resulting in a moderate (n = 1), severe (n = 2) or massive TR (n = 1) at discharge. Based on these findings, we propose that the combination of shortened and degenerative leaflets, along with excessive annular dilation, may represent potential risk factors for the development of SLDAs in T-TEER. SLDA is a well-known complication in tricuspid and mitral edge-to-edge repair (20–22). Known risk factors comprise a big coaptation gap between both target leaflets, a severe tethering resulting in a small leaflet insertion and a high mechanical stress on the target leaflet caused by the devices as well as the leaflet tissue itself (20, 22, 23). Current strategies to treat residual TR include stabilization of the device by implanting another device or, in case of no available landing zone, the implantation of a cava valve (24, 25).
We observed a significant learning curve only in the annuloplasty group in terms of procedural duration. Specifically, the first studies of Cardioband® implantation showed a mean procedure time of 4.2 h (8), which was significantly longer than the mean procedure time of 2.8 h in our cohort. This suggests that further development of annuloplasty devices, the use of intracardiac ultrasound and increasing experience of interventional cardiologists may enable further reduction in procedure times. However, it is likely that percutaneous annuloplasty will continue to take longer than T-TEER, as it involves significantly more procedural steps. In the T-TEER group, the duration of procedures also tended to get shorter during the study period, but this did not reach statistical significance. There was no relevant change in the number of implanted devices over time. No further improvements in terms of a learning curve were observed during the study period, in particular no improvement in TR reduction in either group.
In summary, the following aspects might be considered when selecting patients for TR procedure beyond anatomical characteristics: The longer procedure time of percutaneous annuloplasty results in longer duration of general anesthesia and mechanical ventilation, which might increase complications such as pneumonia due to microaspiration and prolonged ventilation as well as delirium in older patients. The implantation requires the use of contrast, which should be taken into account in patients with renal failure including patients with cardiorenal syndrome. However, more severe TRs were treated successfully with annuloplasty compared to T-TEER. T-TEER might be challenging in patients with a big coaptation gap leading to an increased risk of SLDA. Nevertheless, percutaneous annuloplasty and T-TEER resulted in a comparable TR reduction in our study cohort. Long-term data for both devices revealed a durable TR improvement, which was associated with symptoms relief and reduction of mortality and heart failure hospitalizations (19, 26–28). However, prospective, randomized trials are needed to better understand pros and cons of both devices and its use in different TR etiologies.
The present study utilized a retrospective design, and patients were not randomized to specific treatment options. Device selection was based on the TR anatomy. As a result, baseline characteristics showed differences in three aspects: presence of CIEDs, bilirubin levels, and peripheral artery disease. These differences reflect real-world data. Therefore, the present study serves as a hypothesis-generating investigation for further randomized-controlled trials.
To conclude, both T-TEER and percutaneous annuloplasty reduce TR severity by approximately two degrees when used in the appropriate anatomy without clinically significant complications. The learning curve of the annuloplasty group presented a significant decrease of procedure time, which might be a relevant aspect for starting centres.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the ethics committee of the Charité – Universitätsmedizin Berlin, Berlin, Germany (EA1/005/23). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
IM, HD, FB and MR contributed to conception and design of the study. IM, ER, FB, AH-S, and MZ organized the database. IM and HD performed the statistical analysis. IM and HD wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Abbott, Chicago, Illinois, USA.
IM is participant in the BIH Charité Clinician Scientist Program funded by the Charité – Universitätsmedizin Berlin and the Berlin Institute of Health at Charité (BIH).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Topilsky Y, Maltais S, Medina Inojosa J, Oguz D, Michelena H, Maalouf J, et al. Burden of tricuspid regurgitation in patients diagnosed in the community setting. JACC Cardiovasc Imaging. (2019) 12(3):433–42. doi: 10.1016/j.jcmg.2018.06.014
2. Offen S, Playford D, Strange G, Stewart S, Celermajer DS. Adverse prognostic impact of even mild or moderate tricuspid regurgitation: insights from the national echocardiography database of Australia. J Am Soc Echocardiogr. (2022) 35(8):810–7. doi: 10.1016/j.echo.2022.04.003
3. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. (2022) 43(7):561–632. doi: 10.1093/eurheartj/ehab395
4. Kilic A, Saha-Chaudhuri P, Rankin JS, Conte JV. Trends and outcomes of tricuspid valve surgery in North America: an analysis of more than 50,000 patients from the society of thoracic surgeons database. Ann Thorac Surg. (2013) 96(5):1546–52; discussion 52. doi: 10.1016/j.athoracsur.2013.06.031
5. Praz F, Muraru D, Kreidel F, Lurz P, Hahn RT, Delgado V, et al. Transcatheter treatment for tricuspid valve disease. EuroIntervention. (2021) 17(10):791–808. doi: 10.4244/EIJ-D-21-00695
6. Taramasso M, Hahn RT, Alessandrini H, Latib A, Attinger-Toller A, Braun D, et al. The international multicenter TriValve registry: which patients are undergoing transcatheter tricuspid repair? JACC Cardiovasc Interv. (2017) 10(19):1982–90. doi: 10.1016/j.jcin.2017.08.011
7. Russo G, Taramasso M, Pedicino D, Gennari M, Gavazzoni M, Pozzoli A, et al. Challenges and future perspectives of transcatheter tricuspid valve interventions: adopt old strategies or adapt to new opportunities? Eur J Heart Fail. (2022) 24(3):442–54. doi: 10.1002/ejhf.2398
8. Nickenig G, Weber M, Schueler R, Hausleiter J, Nabauer M, von Bardeleben RS, et al. 6-Month outcomes of tricuspid valve reconstruction for patients with severe tricuspid regurgitation. J Am Coll Cardiol. (2019) 73(15):1905–15. doi: 10.1016/j.jacc.2019.01.062
9. Lurz P, Stephan von Bardeleben R, Weber M, Sitges M, Sorajja P, Hausleiter J, et al. Transcatheter edge-to-edge repair for treatment of tricuspid regurgitation. J Am Coll Cardiol. (2021) 77(3):229–39. doi: 10.1016/j.jacc.2020.11.038
10. Kodali S, Hahn RT, Eleid MF, Kipperman R, Smith R, Lim DS, et al. Feasibility study of the transcatheter valve repair system for severe tricuspid regurgitation. J Am Coll Cardiol. (2021) 77(4):345–56. doi: 10.1016/j.jacc.2020.11.047
11. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. (2015) 16(3):233–70. doi: 10.1093/ehjci/jev014
12. Lancellotti P, Pibarot P, Chambers J, La Canna G, Pepi M, Dulgheru R, et al. Multi-modality imaging assessment of native valvular regurgitation: an EACVI and ESC council of valvular heart disease position paper. Eur Heart J Cardiovasc Imaging. (2022) 23(5):e171–232. doi: 10.1093/ehjci/jeab253
13. Hahn RT, Zamorano JL. The need for a new tricuspid regurgitation grading scheme. Eur Heart J Cardiovasc Imaging. (2017) 18(12):1342–3. doi: 10.1093/ehjci/jex139
14. Sorajja P, Whisenant B, Hamid N, Naik H, Makkar R, Tadros P, et al. Transcatheter repair for patients with tricuspid regurgitation. N Engl J Med. (2023) 389(9):865–6. doi: 10.1056/NEJMc2306962
15. Stone GW, Adams DH, Abraham WT, Kappetein AP, Genereux P, Vranckx P, et al. Clinical trial design principles and endpoint definitions for transcatheter mitral valve repair and replacement: part 2: endpoint definitions: a consensus document from the mitral valve academic research consortium. J Am Coll Cardiol. (2015) 66(3):308–21. doi: 10.1016/j.jacc.2015.05.049
16. Nickenig G, Weber M, Lurz P, von Bardeleben RS, Sitges M, Sorajja P, et al. Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. Lancet. (2019) 394(10213):2002–11. doi: 10.1016/S0140-6736(19)32600-5
17. Kitamura M, Fam NP, Braun D, Ruf T, Sugiura A, Narang A, et al. 12-Month outcomes of transcatheter tricuspid valve repair with the PASCAL system for severe tricuspid regurgitation. Catheter Cardiovasc Interv. (2021) 97(6):1281–9. doi: 10.1002/ccd.29583
18. Lurz J, Rommel KP, Unterhuber M, Besler C, Noack T, Borger M, et al. Safety and efficacy of transcatheter edge-to-edge repair of the tricuspid valve in patients with cardiac implantable electronic device leads. JACC Cardiovasc Interv. (2019) 12(20):2114–6. doi: 10.1016/j.jcin.2019.05.034
19. Pardo Sanz A, Gomez JLZ, Tahoces LS, Ruiz JMM, Martin AG, Gomez AG, et al. Long-term outcomes of percutaneous tricuspid annuloplasty with cardioband device. Eur Heart J Cardiovasc Imaging. (2022) 23(7):979–88. doi: 10.1093/ehjci/jeac079
20. Fam NP, Braun D, von Bardeleben RS, Nabauer M, Ruf T, Connelly KA, et al. Compassionate use of the PASCAL transcatheter valve repair system for severe tricuspid regurgitation: a multicenter, observational, first-in-human experience. JACC Cardiovasc Interv. (2019) 12(24):2488–95. doi: 10.1016/j.jcin.2019.09.046
21. Zhang Y, Kogler W, Sattiraju S. Single leaflet device attachment. BMJ Case Rep. (2022) 15(2):e247747. doi: 10.1136/bcr-2021-247747
22. Kitamura M, Besler C, Lurz P, Noack T. Bail-out edge-to-edge mitral repair for an acute single leaflet device attachment: a case report. Eur Heart J Case Rep. (2021) 5(4):ytab147. doi: 10.1093/ehjcr/ytab147
23. Braun D, Rommel KP, Orban M, Karam N, Brinkmann I, Besler C, et al. Acute and short-term results of transcatheter edge-to-edge repair for severe tricuspid regurgitation using the MitraClip XTR system. JACC Cardiovasc Interv. (2019) 12(6):604–5. doi: 10.1016/j.jcin.2018.11.028
24. Rroku A, Barbieri F, Landmesser U, Skurk C, Kasner M, Reinthaler M. Transcatheter caval valve implantation for tricuspid regurgitation after single leaflet device attachment. JACC Case Rep. (2022) 4(8):481–5. doi: 10.1016/j.jaccas.2022.02.014
25. So CY, Tam KC, Lam YY, Lee AP. Single leaflet device attachment complicating percutaneous edge-to-edge repair of the tricuspid valve using the MitraClip. J Invasive Cardiol. (2018) 30(9):E93–E4. PMID: 30158327
26. Taramasso M, Alessandrini H, Latib A, Asami M, Attinger-Toller A, Biasco L, et al. Outcomes after current transcatheter tricuspid valve intervention: mid-term results from the international TriValve registry. JACC Cardiovasc Interv. (2019) 12(2):155–65. doi: 10.1016/j.jcin.2018.10.022
27. Mehr M, Taramasso M, Besler C, Ruf T, Connelly KA, Weber M, et al. 1-Year outcomes after edge-to-edge valve repair for symptomatic tricuspid regurgitation: results from the TriValve registry. JACC Cardiovasc Interv. (2019) 12(15):1451–61. doi: 10.1016/j.jcin.2019.04.019
Keywords: tricuspid regurgitation, percutaneous annuloplasty, transcatheter edge-to-edge repair, procedural characteristics, transcatheter valve intervention
Citation: Mattig I, Barbieri F, Kasner M, Romero Dorta E, Heinrich-Schüler AL, Zhu M, Stangl K, Landmesser U, Reinthaler M and Dreger H (2023) Comparison of procedural characteristics of percutaneous annuloplasty and edge-to-edge repair for the treatment of severe tricuspid regurgitation. Front. Cardiovasc. Med. 10:1232327. doi: 10.3389/fcvm.2023.1232327
Received: 31 May 2023; Accepted: 28 August 2023;
Published: 8 September 2023.
Edited by:
Junjie Xiao, Shanghai University, ChinaReviewed by:
Fabian Islas, Complutense University of Madrid, Spain© 2023 Mattig, Barbieri, Kasner, Romero Dorta, Heinrich-Schüler, Zhu, Stangl, Landmesser, Reinthaler and Dreger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Henryk Dreger aGVucnlrLmRyZWdlckBkaHpjLWNoYXJpdGUuZGU=
Abbreviations ASE, American Society of Echocardiography; CIED, cardiac implantable electronic device; EACTS, European Association for Cardiothoracic Surgery; EACVI, European Association of Cardiovascular Imaging; EROA, effective regurgitant orifice area; ESC, European Society of Cardiology; MVARC, Mitral Valve Academic Research Consortium; PISA, proximal isovelocity surface area (PISA) method; RCA, right coronary artery; RegVol, regurgitant volume; SLDA, single leaflet device attachments; TOE, transesophageal echocardiography; TR, tricuspid regurgitation; T-TEER, tricuspid transcatheter edge-to-edge repair.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.