
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 07 September 2023
Sec. Cardiovascular Metabolism
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1230051
This article is part of the Research Topic The Interplay Between Oxidative Stress, Immune Cells and Inflammation in Cardiovascular Diseases View all 6 articles
 Joanna Sulicka-Grodzicka1,2
Joanna Sulicka-Grodzicka1,2 Piotr Szczepaniak3,4
Piotr Szczepaniak3,4 Ewelina Jozefczuk3,4
Ewelina Jozefczuk3,4 Karol Urbanski3
Karol Urbanski3 Mateusz Siedlinski3,4
Mateusz Siedlinski3,4 Łukasz Niewiara5
Łukasz Niewiara5 Bartłomiej Guzik5
Bartłomiej Guzik5 Grzegorz Filip6
Grzegorz Filip6 Bogusław Kapelak6
Bogusław Kapelak6 Karol Wierzbicki6
Karol Wierzbicki6 Mariusz Korkosz1
Mariusz Korkosz1 Tomasz J. Guzik3,4,7
Tomasz J. Guzik3,4,7 Tomasz P. Mikolajczyk2,3,4*
Tomasz P. Mikolajczyk2,3,4*
Background: Systemic inflammation may cause endothelial activation, mediate local inflammation, and accelerate progression of atherosclerosis. We examined whether the levels of circulating inflammatory cytokines reflect local vascular inflammation and oxidative stress in two types of human arteries.
Methods: Human internal mammary artery (IMA) was obtained in 69 patients undergoing coronary artery bypass graft (CABG) surgery and left anterior descending (LAD) artery was obtained in 17 patients undergoing heart transplantation (HTx). Plasma levels of tumor necrosis factor α (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β) were measured using ELISA, high-sensitivity C-reactive protein (hs-CRP) was measured using Luminex, and mRNA expression of proinflammatory cytokines in the vascular tissues was assessed. Furthermore, formation of superoxide anion was measured in segments of IMA using 5 uM lucigenin-dependent chemiluminescence. Vascular reactivity was measured using tissue organ bath system.
Results: TNF-α, IL-6 and IL-1β mRNAs were expressed in all studied IMA and LAD segments. Plasma levels of inflammatory cytokines did not correlate with vascular cytokine mRNA expression neither in IMA nor in LAD. Plasma TNF-α and IL-6 correlated with hs-CRP level in CABG group. Hs-CRP also correlated with TNF-α in HTx group. Neither vascular TNF-α, IL-6 and IL-1β mRNA expression, nor systemic levels of either TNF-α, IL-6 and IL-1β were correlated with superoxide generation in IMAs. Interestingly, circulating IL-1β negatively correlated with maximal relaxation of the internal mammary artery (r = −0.37, p = 0.004). At the same time the mRNA expression of studied inflammatory cytokines were positively associated with each other in both IMA and LAD. The positive correlations were observed between circulating levels of IL-6 and TNF-α in CABG cohort and IL-6 and IL-1β in HTx cohort.
Conclusions: This study shows that peripheral inflammatory cytokine measurements may not reflect local vascular inflammation or oxidative stress in patients with advanced cardiovascular disease (CVD). Circulating pro-inflammatory cytokines generally correlated positively with each other, similarly their mRNA correlated in the arterial wall, however, these levels were not correlated between the studied compartments.
Atherosclerosis is known as a chronic inflammatory disease (1). Endothelial dysfunction, characterized by oxidative stress and increased expression of adhesion molecules and chemokines as well as reduced expression of nitric oxide, is regarded as a pivotal stage in the initiation of atherogenesis (2). Oxidation inherently interacts with inflammation in the pathogenesis of endothelial dysfunction. Leukocyte adherence and infiltration into the vessel wall (3) has been shown to stimulate vascular reactive oxygen species (ROS) production by both infiltrating immune cells and activated vascular cells (4, 5). Increased superoxide production is a critical step in endothelial dysfunction (4, 6–9). At the same time low grade inflammation is a hallmark of cardiovascular disease (CVD). Recent clinical trials including CANTOS [Canakinumab Anti-inflammatory Thrombosis Outcome Trial (10)], as well as COLCOT (Colchicine Cardiovascular Outcomes Trial) and LoDoCo2 (low-dose colchicine trial) (11, 12) have demonstrated proof of principle of potential targeting of the inflammatory mechanisms in cardiovascular risk. These are supported by mechanistic evidence in murine and human studies linking systemic inflammation to local events in the vessel wall (13).
Inflammation can be a cause and a consequence of heart failure (HF), and it contributes to disease pathogenesis and progression. The hemodynamic stress associated with HF induces inflammation by eliciting the release of proinflammatory cytokines by cardiomyocytes and cardiac fibroblasts, including TNF-α, IL-6, IL-1β as well as angiotensin II, and myostatin. HF also causes mitochondrial dysfunction, reactive oxygen species generation, and leads to activation of the NLRP3 inflammasome, with subsequent production of proinflammatory cytokines (14–16). The proinflammatory cytokines IL-1β and TNF-α may induce systolic and diastolic dysfunction, TNF-α also stimulates cardiac remodeling (17). Additionally, comorbidities that commonly coexist with HF including diabetes, obesity, and chronic kidney disease are associated with chronic low-grade inflammation, which may have detrimental effects on cardiac structure and function (18). A recently published analysis of the CANTOS trial found that, anti-IL-1β therapy was associated with a significant reduction in HF hospitalizations and a reduction in the composite of HF hospitalizations and all-cause death, in participants who had a reduction in hs-CRP to <2 mg/L compared with placebo (19).
In light of these relationships, it becomes essential to identify biomarkers that could reflect vascular inflammation as well as related pathogenetic mechanisms of vascular dysfunction (20–22). While increased levels of circulating biomarkers such as IL-6 as well as hs-CRP independently predict the risk of cardiovascular diseases (23, 24), it remains unclear to what extent they represent local processes occurring in the vessel wall.
Accordingly, as there are regional differences in arterial vessels in susceptibility to atherosclerosis, and different pathological pathways might be involved in different vessels, yet some systemic markers of inflammation have been reported to be associated with increased risk of CVD. We sought to investigate whether levels of circulating inflammatory cytokines (TNF-α, IL-6 and IL-1β) reflect the local vascular expression of these inflammatory molecules in different types of blood vessels (internal mammary artery and left anterior descending artery) of patients with advanced cardiovascular diseases [coronary artery disease patients undergoing coronary artery bypass graft (CABG) surgery, and patients with end-stage heart failure undergoing heart transplantation]. We also investigated whether oxidative stress is associated with inflammation in atherosclerosis resistant artery (internal mammary artery) in patients with coronary artery disease (CAD). We chose these blood vessels as a model as they represent different aspects of the spectrum of susceptibility to atherosclerosis. While human internal mammary arteries do not develop atherosclerosis in spite of overt endothelial dysfunction, coronary arteries are an essential site of atherosclerosis development (25).
The study population consisted of 69 patients undergoing coronary artery bypass graft (CABG) surgery and 17 patients undergoing heart transplantation (HTx). Exclusion criteria included active infections, inflammatory, neoplastic, or hepatic disorders (26). Anthropometric measurements were recorded, and body mass index (BMI) was calculated. Blood pressure measurements were performed using validated devices. Each measurement was performed twice with the participant in a seated position, after at least 5 min of rest. Hypertension was defined as blood pressure ≥140/90 mmHg or taking antihypertensive medications. Smoking status was defined as past-smokers, those who had stopped cigarette smoking for ≥6 months prior to surgery, or current smokers. Dilated cardiomyopathy (DCM) diagnosis was based on morphology criteria when elevated left ventricular end-diastolic diameter (LVEDd) and systolic dysfunction were reported in transthoracic echocardiography. Ischemic heart disease (IHD) diagnosis was established either based on the presence of significant coronary lesions in recent coronary angiography, any history of prior coronary revascularization, or a history of myocardial infarction. Hypertrophic cardiomyopathy (HCM) and arrhythmogenic right ventricular cardiomyopathy (ARVCM) diagnoses were reported only when they were explicitly present in prior medical history. Otherwise, when a systolic dysfunction was present in echocardiography, but no specific criteria for the aforementioned cardiomyopathies were met, the patients were identified as idiopathic cardiomyopathy. Written informed consent was obtained from all patients. The study was approved by local Bioethics Committee of the Jagiellonian University (KBET124/B/2012 and Approval No. 122.6120.46.2017 and 1072.6120.162.2019).
Tissue samples of internal mammary artery (IMA) were obtained during CABG surgery using the no-touch technique prior to surgical distension, as previously described (26). Tissue samples of the left anterior descending coronary artery (LAD) were obtained during heart transplantation. All samples were received from the Department of Cardiovascular Surgery and Transplantology of the John Paul II Hospital in Krakow (Poland). IMA and LAD samples were immediately transferred to ice-cold Hank's Balanced Salt Solution (Gibco; Thermo Fisher Scientific) buffer and were maintained at 4°C (27). The surrounding tissues were separated from the vessels using microsurgical instruments and a microscope.
The isolated blood vessels were stored in a RNA-later stabilisation solution (Ambion, Thermo Fisher Scientific) until RNA isolation. The tissue was homogenised for 20 min in TRI reagent solution (Thermo Fischer Scientific) using the TissueLyser LT bead mill (Qiagen, USA). Total RNA was isolated from the samples using the Direct-zol RNA Miniprep kit (Zymo Research) according to the manufacturer's protocol. Reverse transcription was performed using 500 ng of RNA using the High Capacity cDNA reverse transcription kit (Applied Biosystems, USA). Real-time PCR reactions were performed on the 7900HT instrument (Applied Biosystems, USA) using commercially available TaqMan assays for IL-1β (Hs01555410_m1), IL-6 (Hs00174131_m1), TNF (Hs00174128_m1). Data were normalized to levels of Eukaryotic Translation Elongation Factor 2 (EEF2) (Hs00157330_m1) mRNA, and then dCT was calculated.
The level of vascular superoxide production was evaluated by lucigenin-dependent chemiluminescence (LGCL) as previously described (26, 28). The vessels were cut to expose the endothelium and then aerated with 95% O2% and 5% CO2 in Krebs-HEPES buffer for 20 min. Measurements were made with 5 µM lucigenin (Millipore Sigma) in Krebs-HEPES buffer using a FB12 chemiluminometer (Berthold). ROS production was given in RLU per second per milligram of dry weight of the vessel (28).
Isometric tension studies were performed using tissue organ bath system 750TOBS (Danish Myo Technology) as previously described (4, 26). Segments of IMA rings were equilibrated in Krebs-Henseleit solution (in mM: 124 NaCl, 4.6 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 0.01 EDTA, 23 NaHCO3, and 11 glucose), with continuous gas (CO2 5%/O2 95%) and checked for viability with KCl (60 mN). Following pre-constriction with phenylephrine (Phe) (up to 70% of the maximum KCl contraction), the vessels were relaxed with acetylcholine (ACh) and sodium nitroprusside (SNP) to obtain endothelium-dependent (ACh) and endothelium-independent (SNP) vasodilatations.
Peripheral blood was collected in tubes containing ethylenediaminetetraacetic acid (EDTA) prior to the premedication for the surgery. Within 1 h after blood collection, samples were centrifuged at 400×g for 10 min. The platelet-rich plasma was then collected and centrifuged at 2,000×g for 15 min at 4°C. Plasma samples without pellets were then collected and stored at −80°C until analysis. The concentration of selected cytokines in plasma was measured using commercially available high-sensitivity (HS) ELISA kits for Human IL-1β/IL-1F2 (HSLB00D), IL-6 (HS600C) and TNF-α (HSTA00E). All kits were purchased from R&D Systems (Bio-Techne) and performed according to the manufacturer's instructions. The final absorbance readings were obtained using a Synergy H4 Hybrid Multi-Mode Microplate Reader (Thermo Fisher Scientific, Waltham, MA, USA). Plasma samples were also analysed for hs-CRP (sensitivity 0.024 µg/ml) with Luminex technology using a commercial assay (Human Luminex® Discovery Assay, LXSAHM, R&D) and were read on a Luminex 200 System (Merck, Millipore) in accordance with the manufacturer's instructions.
The deviation from normal distribution of variables was assessed using Shapiro-Wilk test. The continuous variables are presented as mean ± SD, whereas the categorical variables are presented as numbers and percentages. Continuous variables with non - normal distribution are presented as median (25th; 75th percentile). Continuous variables were compared using the Student's t-test. Pearson product-moment correlations were performed to analyse the correlation between IL-1β, IL-6 or TNF-α mRNA expression in the arteries and their corresponding plasma levels and hs-CRP. Sensitivity analyses were performed using non-parametric Spearman's rank order correlations and Mann–Whitney U-tests. This has been performed to assure results were not driven be skewed distribution of the variables. Spearman's rank order correlations were performed to analyse the correlation between vascular superoxide production, maximal relaxation to acetylcholine, and circulating or vascular proinflammatory cytokines. Multivariable regression analysis adjusting for age, BMI, sex and current smoking was used to determine the association between circulating inflammatory cytokines. These analyses used log10-transformed cytokine levels and normal distribution of regression-derived residuals was confirmed using Shapiro–Wilk test. A p-value below 0.05 was considered as statistically significant. All analyses were performed using IBM SPSS Statistics (New York, United States) package (version 28.0.1.0) and graphs were drawn using Graph-Pad Prism v9.5.1. A correlation matrix was performed using Statistical tools for high-throughput data analysis (STHDA, http://www.sthda.com/english/rsthda/correlation-matrix.php).
The clinical characteristics of the study participants in whom IMAs and human coronary arteries (LAD) were obtained are presented in Tables 1, 2, respectively. The mean age of patients undergoing CABG was 63.6 ± 8.8 years, and 68% of patients were males. Among patients undergoing cardiac transplantation, who were aged 50.2 ± 10.6 years, 94% were males. Ischemic heart disease (IHD) and dilatated cardiomyopathy (DCM) were diagnosed in 58.8% and 64.7% of patients with end-stage heart failure undergoing HTx. IL-1β plasma level was positively associated with hypertension, whereas the circulating level of IL-6 was positively correlated with the age in CABG cohort (Table 3 and Supplementary Table S1).
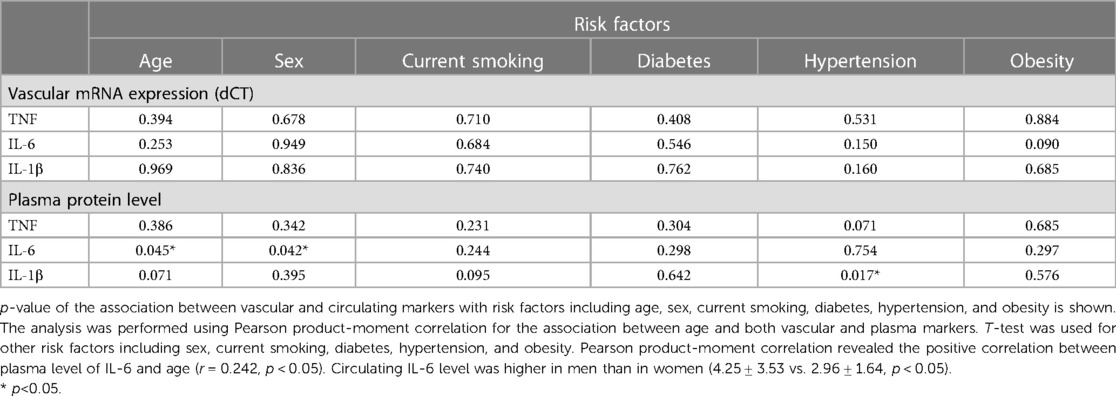
Table 3. Significance level of the differences in association between vascular and plasma markers with risk factors in CABG cohort.
The mRNA expression of TNF-α, IL-1β and IL-6 in the blood vessels was not correlated with plasma protein levels in both CABG and HTx groups (Figures 1A,B, Supplementary 1SA,1SB,2SA,2SB and Supplementary Table S2). The mRNA expression of all proinflammatory cytokines positively correlated with each other, both within IMA and LAD (Figures 1A,B and Supplementary Figure 2S). Additionally, we observed a positive correlation between the level of IL-6 and TNF-α (r = 0.45, p < 0.001 for Pearson product-moment correlation) in plasma collected from CABG patients (Figure 1A and Supplementary Figure 2SA), which remained significant in a multivariable linear regression analysis after adjustment for sex, age, BMI and current smoking status (B = 0.184 log10 (TNF-α)/llog10 (IL-6), SE = 0.051, p < 0.001). In the HTx patients we found a strong positive correlation between the plasma level of IL-6 and IL-1β (r = 0.82, p < 0.0001) (Figure 1B), which remained significant after adjustment for age and BMI in a multivariable analysis (B = 0.85 log10 (IL-1β)/llog10 (IL-6), SE = 0.29, p = 0.02). The positive corelations between hs-CRP and TNF-α and IL-6 have been observed in CABG cohort (Figure 1A and Supplementary Figure 2SA) and remained significant after adjustment for sex, age, BMI and current smoking status (respectively B = 1.60 log10 (hs-CRP)/llog10 (TNF-α), SE = 0.44, p < 0.001 and B = 1.08 log10 (hs-CRP)/llog10(IL-6), SE = 0.16, p < 0.001).There was a modest negative correlation between plasma hs-CRP and mRNA expression (dCT) of IL-6 in IMA (p < 0.05) (Figure 1A). However, this was not significant when Spearman's rank order correlation was used (Supplementary Figure 2S). In HTx group, hs-CRP positively correlated with TNF-α (Figure 1B and Supplementary Figure 2SB).
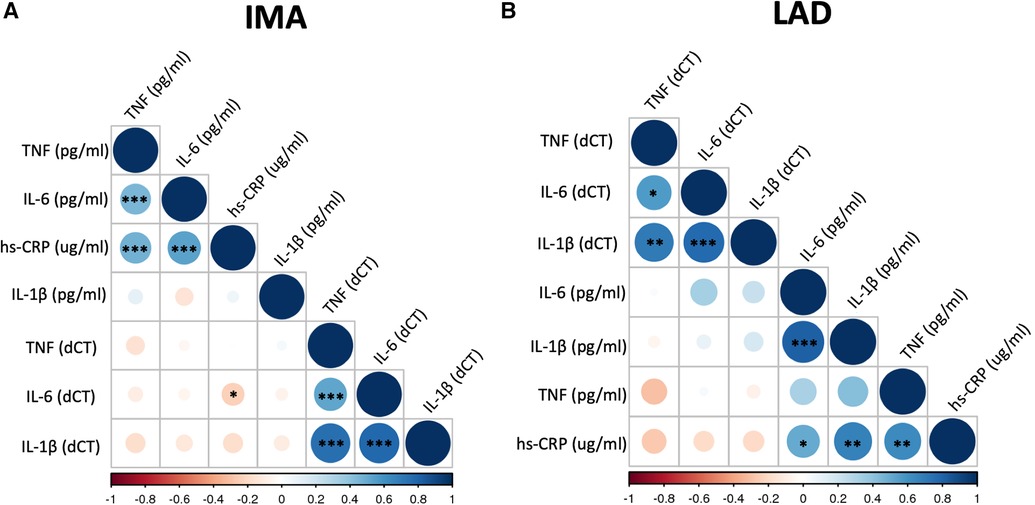
Figure 1. Correlation matrix of mRNA expression of selected biomarkers in blood vessels and their plasma levels. Correlation matrix shows the relationship between mRNA expression of TNF (TNF-α), IL-6, and IL-1β in the internal mammary arteries (IMA) (A) and left anterior descending coronary arteries (LAD) (B) and their plasma concentrations as well as hs-CRP level. Samples were obtained from patients undergoing CABG surgery (A) or heart transplantation (HTx) (B). The correlations were calculated using Pearson product-moment correlation. Colour and its intensity indicate r values i.e., direction and strength of correlation (blue positive correlation, red negative correlation), as per X axis. *p < 0.05, **p < 0.01, ***p < 0.001.
We did not observe any statistically significant correlations between superoxide production and vascular expression of TNF-α, IL-6 or IL-1β (Figure 2A). Circulating levels of these cytokines were not correlated to superoxide production in ex vivo vascular segments, measured by low concentration of lucigenin enhanced chemiluminescence (Figure 2B).
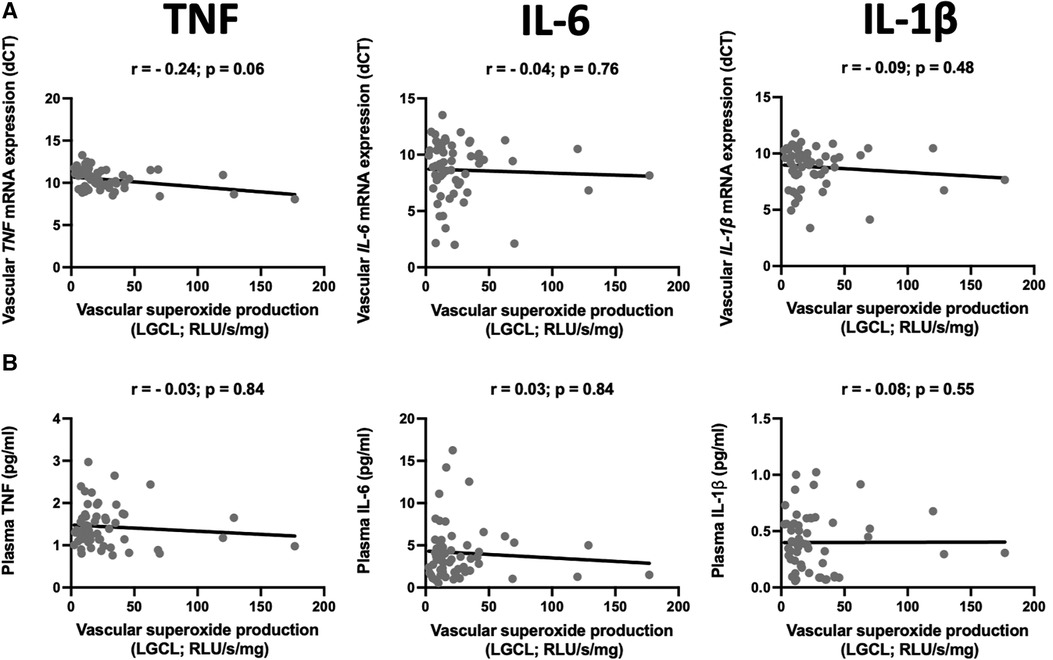
Figure 2. Relationship between mRNA expression and plasma levels of selected pro-inflammatory cytokines and superoxide production. The scatter plots show the correlations between mRNA expression of TNF (TNF-α), IL-6, and IL-1β in the internal mammary arteries (IMA) (A) and their plasma levels (B) and vascular superoxide production measured by the lucigenin-dependent chemiluminescence (LGCL). Samples were obtained from patients undergoing CABG surgery. The correlations were calculated using Spearman's rank order correlation. N = 59.
In the next step, we performed the additional analysis of the mRNA expression of TNF-α, IL-6 and IL-1β in the blood vessels and their plasma protein levels and both systolic or diastolic blood pressure (SBP, DBP, respectively) in CABG group. We observed that none of the studied circulating cytokines correlated with SBP or DBP (Table 4 and Supplementary Table S3). However, we observed the positive correlation between vascular expression of TNF-α (dCT) or IL-6 (dCT) and DBP (Table 4 and Supplementary Table S3). We did not find any significant correlation between vascular superoxide production and systolic or diastolic blood pressure (Table 4).
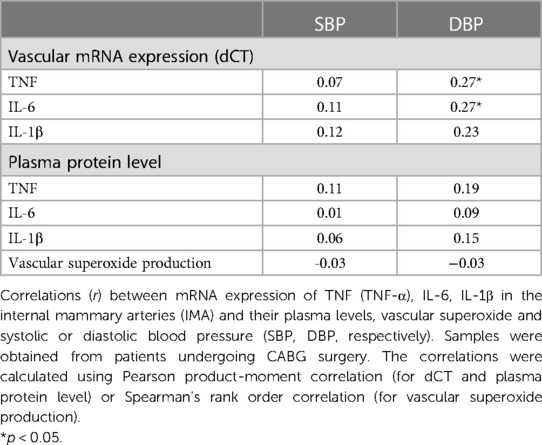
Table 4. Relationship between mRNA expression and plasma levels of selected pro-inflammatory cytokines, vascular superoxide, and blood pressure values.
Atherosclerosis has the heterogenous nature. Although the internal mammary arteries are resistant to the development of atherosclerosis, we observed that some of the vessels are characterized by endothelial dysfunction measured as a relaxation to acetylcholine. Interestingly, from all studied vascular and plasma cytokines, circulating IL-1β negatively correlated with the maximal relaxation (r = −0.37, p = 0.004, Figure 3).
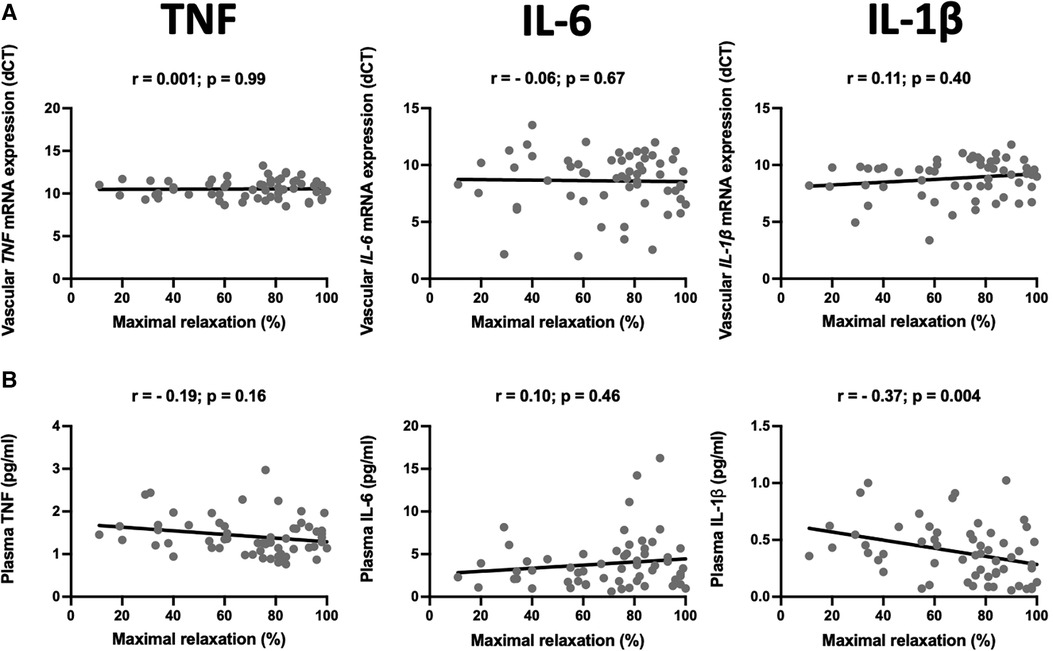
Figure 3. Relationship between mRNA expression and plasma levels of selected pro-inflammatory cytokines and endothelial function. The scatter plots show the correlations between mRNA expression of TNF (TNF-α), IL-6, and IL-1β in the internal mammary arteries (IMA) (A) and their plasma levels (B) and endothelial function measured as a maximal relaxation to acetylcholine. Samples were obtained from patients undergoing CABG surgery. The correlations were calculated using Spearman's rank order correlation. N = 59.
In this study we demonstrate that the levels of systemic TNF-α, IL-1β and IL-6 do not reflect their vascular mRNA expression in patients with advanced cardiovascular diseases, and that vascular expression of these cytokines is not associated with increased ROS generation in IMA in patients with coronary artery disease. Interestingly, we found that proinflammatory cytokine TNF-α positively correlated with high sensitivity C-reactive protein in patients with advanced heart failure undergoing heart transplantation. In CABG cohort the positive correlation of hs-CRP with TNF-α and IL-6 was observed. Serum CRP is considered as a marker of inflammation as well as a predictor of the development of cardiovascular events (29, 30). Our results are consistent with previous published data. It has been shown that human coronary artery smooth muscle cells are able to produce CRP in response to proinflammatory cytokines including TNF-α, IL-1β and IL-6 (29). Interestingly, locally produced CRP participated in the atherogenesis (29, 30). CRP was increased in patients with coronary artery lesions. Moreover, CRP positively correlated with IL-6 (31–33) and TNF-α (33–35).
In our study we observed no correlation between systolic blood pressure and systemic or vascular inflammatory cytokines. However, we found the correlation between diastolic blood pressure and vascular expression of TNF-α and IL-6. Previously, it has been shown that IL-6 and TNF-α, positively correlated with blood pressure in pregnancy induced hypertension (36, 37). Moreover, the positive correlation between IL-1β and mean blood pressure in patients with essential hypertension has been demonstrated (38). However, it should be noted that IL-1β inhibition by canakinumab reduces adverse cardiovascular events but this mechanism is not associated with changes in blood pressure (39). These discrepancies may be related to the fact that patients in our study had more advanced CVD, and comorbidities and medications may influence the relationship between blood pressure and inflammatory markers.
Previous studies have confirmed the role of TNF-α in vascular pathology (40, 41). TNF-α is synthesized initially by activated macrophages and T cells, it acts by binding to one of two types of receptors: TNFR1 or TNFR2 (tumor necrosis factor receptor) (42), and employs the stimulation of the release of IL-1β and IL-6, as well as upregulation of the expression of chemokines and endothelial adhesion molecules, and coordination of the migration of leukocytes to the inflamed targeted organs (40). In humans, evidence points towards TNF-α playing an important role in stimulating endothelial dysfunction. The positive correlation between increased plasma TNF-α levels and reduced reactivity to acetylcholine has been observed previously (41). In diabetic patients, TNF-α induces endothelial dysfunction, which is reversed by the PPAR-γ agonist (43). Moreover, the TNF-α inhibitors are particularly effective in blood pressure lowering in animal studies (44). ROS appears to be one of the key mediators involved in TNF-α induced endothelial activation. Mycophenolic acid, active metabolite of mycophenolate mofetil inhibits TNF-α-stimulated ROS generation in human aortic endothelial cells (45). TNFRs activate transcription factor NF-κB (nuclear factor-κB). ROS also function as second messengers in several signal transduction pathways including NF-κB signaling (46). ROS trigger activation of NF-κB but may also inhibit NF-κB activity. These opposite effects seem to be time and context dependent (47). In our study the vascular TNF expression (dCT) in IMA negatively correlated with vascular superoxide production, but this interaction did not reach the statistical significance. No correlation of TNF with maximal relaxation was found. Interestingly, we observed the strong negative correlation of plasma IL-1β with the maximal relaxation. This observation is consistent with previous study showing the link between IL-1β and coronary endothelial dysfunction (48).
Discordance between levels of systemic cytokines and their vascular expression may be partially explained by the complexity of the inflammatory process. Cytokines are local mediators, and their production can be compartmentalized (49). Patterns of cytokine production differ under various clinical conditions, particularly in low-grade chronic inflammation, and cellular mRNA may not correspond to circulation proteins. Although elevations in inflammatory markers commonly occur together, they may not be synchronized. Additionally, concentrations of some inflammatory markers change relatively slowly, while other can change rapidly. Accurate detection of cytokines is challenging because of their dynamic secretion processes and short half-lives. The majority of cytokines have a short half-life in vivo and may be subjected to immediate degradation during sample collection and preparation (50).
Interestingly, multiple distinct endothelial cells subtypes have been revealed in aorta by single-cell sequencing (51). At the same time, vascular dysfunction can correlate between different arteries, as well as between venous and arterial endothelium, suggesting that endothelial dysfunction may be systemic (52). Furthermore, genetic and epigenetic modifications may influence endothelial function both in healthy individuals and in patients with cardiovascular diseases (53). Immune response can also vary within the individual, between local and systemic scales and across the tissues.
The analysis of biomarkers in patients with atrial fibrillation (AF) has revealed compelling associations between various mechanisms and cardiovascular death. These mechanisms include oxidative stress, cardiorenal dysfunction, inflammation, vascular and renal dysfunction, calcium balance, apoptosis, and fibrinolysis (54). Additionally, the associations between biological processes related to IFN-γ production, T-cell co-stimulation, and all-cause mortality have been demonstrated. In light of these findings, the potential therapeutic targets may involve enhancing IFN-γ production and targeting CD28, CD70, TNF superfamily member-14 (TNFSF14), and inducible costimulator ligand (ICOSLG) (55). Furthermore, it is worth noting that circulating cardiovascular disease biomarkers may also be associated with an increased risk of cancer and overall mortality (56).
Our study does have certain limitations. Firstly, we have evaluated the association of plasma biomarker concentrations and their vascular mRNA expression but not vascular protein level. It should be noted that during the surgery there were collected very small fragments of the vessel, and we were unable to perform the additional analyses, including the measurements of cytokine vascular protein levels. This may be the limitation of our study. On the other hand, cytokines are small, secreted proteins, which are produced by immune cells as well as a number of vascular cells (57). For this reason, the vascular cytokine mRNA expression may be more representative than its vascular protein level. Moreover, examining mRNA levels could offer valuable insights into the interplay between inflammation and oxidation, as ROS may regulate cytokine transcription in vascular cells. In addition, no histological data were available on the human vessels.
Secondly, it is important to note that the patients included in our study had advanced CVD and multimorbidity, which indicates the presence of several potential confounding factors that could influence the systemic levels of peripheral inflammatory markers more than local vascular expression. These confounders may include factors such as adipose tissue in obesity, medication usage, and other comorbidities. We do not have data on the imaging of other arteries which could show the extent of atherosclerosis. Although, it may be speculated that presence of clinical atherosclerosis in one vascular territory largely indicates an increased likelihood of its presence in other vessels.
Another limitation is a relatively small number of HTx patients included for this study. We have collected 17 samples of the left anterior descending artery from HTx cohort. However, it should be noted that, other studies on the human cardiac tissue and coronary arteries were performed on a smaller number of samples, collected from 10 to 14 patients (27, 58, 59).
Furthermore, our study involved a single systemic cytokine measurement. This may partially explain the lack of relationship observed with local production, as systemic levels can vary depending on environmental and other factors. Therefore, our analysis may not fully capture the potential variability over time. However, it is worth noting that in practical terms, our aim was to identify biomarkers that could be implemented clinically without the need for multiple consecutive measurements. Requiring frequent measurements could hinder their feasibility and practical use in a clinical setting.
In conclusion, our findings suggest that while individual cytokines show correlation within their respective compartments, indicating consistency in local processes, we observed a lack of correlation between cytokine levels across compartments. This lack of correlation between local cytokine expression and their circulating levels highlights the complex nature of inflammation, which may involve intricate aspects that cannot be fully captured by measuring circulating inflammatory cytokines.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Local Bioethics Committee of the Jagiellonian University (KBET124/B/2012 and Approval No. 122.6120.46.2017 and 1072.6120.162.2019). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
JS-G: have conceived and designed the study and contributed to data interpretation. JS-G, TG, and TM: have drafted the manuscript. ŁN and BG: have crucially participated in data collection and enrolment of CAD patients. PS, EJ, KU, MS, and TM: have analyzed all the biological parameters. GF, BK, and KW: have contributed with patients’ enrolment and samples collection. MK: has revised critically the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
This work was supported by the Polish National Centre for Research and Development (ERA-CVD/NEMO/7/2019 - TPM; Gut-brain-immune; ERA-CVD/JTC2020/25/ImmuneHyperCog/2022; NCBiR, Poland).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1230051/full#supplementary-material
1. Gil-Pulido J, Amézaga N, Jorgacevic I, Manthey HD, Rösch M, Brand T, et al. Interleukin-23 receptor expressing γδ T cells locally promote early atherosclerotic lesion formation and plaque necrosis in mice. Cardiovasc Res. (2022) 118(14):2932–45. doi: 10.1093/cvr/cvab359
2. Alexander Y, Osto E, Schmidt-Trucksäss A, Shechter M, Trifunovic D, Duncker DJ, et al. Endothelial function in cardiovascular medicine: a consensus paper of the European society of cardiology working groups on atherosclerosis and vascular biology, aorta and peripheral vascular diseases, coronary pathophysiology and microcirculation, and thrombosis. Cardiovasc Res. (2021) 117(1):29–42. doi: 10.1093/cvr/cvaa085
3. Libby P, Nahrendorf M, Swirski FK. Leukocytes link local and systemic inflammation in ischemic cardiovascular disease: an expanded “cardiovascular Continuum”. J Am Coll Cardiol. (2016) 67(9):1091–103. doi: 10.1016/j.jacc.2015.12.048
4. Mikolajczyk TP, Nosalski R, Szczepaniak P, Budzyn K, Osmenda G, Skiba D, et al. Role of chemokine RANTES in the regulation of perivascular inflammation, T-cell accumulation, and vascular dysfunction in hypertension. Faseb J. (2016) 30(5):1987–99. doi: 10.1096/fj.201500088R
5. Pan XX, Wu F, Chen XH, Chen DR, Chen HJ, Kong LR, et al. T-cell senescence accelerates angiotensin II-induced target organ damage. Cardiovasc Res. (2021) 117(1):271–83. doi: 10.1093/cvr/cvaa032
6. Guzik TJ, West NE, Black E, McDonald D, Ratnatunga C, Pillai R, et al. Vascular superoxide production by NAD(P)H oxidase: association with endothelial dysfunction and clinical risk factors. Circ Res. (2000) 86(9):E85–90.10807876
7. Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, et al. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. (2002) 105(14):1656–62. doi: 10.1161/01.CIR.0000012748.58444.08
8. Gimbrone MA Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. (2016) 118(4):620–36. doi: 10.1161/CIRCRESAHA.115.306301
9. González-Amor M, García-Redondo AB, Jorge I, Zalba G, Becares M, Ruiz-Rodríguez MJ, et al. Interferon-stimulated gene 15 pathway is a novel mediator of endothelial dysfunction and aneurysms development in angiotensin II infused mice through increased oxidative stress. Cardiovasc Res. (2022) 118(16):3250–68. doi: 10.1093/cvr/cvab321
10. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. (2017) 377(12):1119–31. doi: 10.1056/NEJMoa1707914
11. Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. (2019) 381(26):2497–505. doi: 10.1056/NEJMoa1912388
12. Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, et al. Colchicine in patients with chronic coronary disease. N Engl J Med. (2020) 383(19):1838–47. doi: 10.1056/NEJMoa2021372
13. Hettwer J, Hinterdobler J, Miritsch B, Deutsch MA, Li X, Mauersberger C, et al. Interleukin-1β suppression dampens inflammatory leucocyte production and uptake in atherosclerosis. Cardiovasc Res. (2022) 118(13):2778–91. doi: 10.1093/cvr/cvab337
14. Tang X, Zhao S, Liu J, Liu X, Sha X, Huang C, et al. Mitochondrial GSNOR alleviates cardiac dysfunction via ANT1 denitrosylation. Circ Res. (2023).
15. Higashikuni Y, Liu W, Numata G, Tanaka K, Fukuda D, Tanaka Y, et al. NLRP3 Inflammasome activation through heart-brain interaction initiates cardiac inflammation and hypertrophy during pressure overload. Circulation. (2023) 147(4):338–55. doi: 10.1161/CIRCULATIONAHA.122.060860
16. Michaëlsson E, Lund LH, Hage C, Shah SJ, Voors AA, Saraste A, et al. Myeloperoxidase inhibition reverses biomarker profiles associated with clinical outcomes in HFpEF. JACC Heart Fail. (2023).
17. Yang CM, Yang CC, Hsu WH, Hsiao LD, Tseng HC, Shih YF. Tumor necrosis factor-α-induced C-C motif chemokine ligand 20 expression through TNF receptor 1-dependent activation of EGFR/p38 MAPK and JNK1/2/FoxO1 or the NF-κB pathway in human cardiac fibroblasts. Int J Mol Sci. (2022) 23(16).
18. Li C, Qin D, Hu J, Yang Y, Hu D, Yu B. Inflamed adipose tissue: a culprit underlying obesity and heart failure with preserved ejection fraction. Front Immunol. (2022) 13:947147. doi: 10.3389/fimmu.2022.947147
19. Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, et al. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation. (2019) 139(10):1289–99. doi: 10.1161/CIRCULATIONAHA.118.038010
20. Józefczuk E, Guzik TJ, Siedlinski M. Novel biomarkers and emerging tools to identify causal molecular pathways in hypertension and associated cardiovascular diseases. Kardiol Pol. (2023) 81(3):221–31. doi: 10.33963/KP.a2023.0037
21. Sulicka J, Surdacki A, Strach M, Kwater A, Gryglewska B, Ćwiklińska M, et al. Elevated asymmetric dimethylarginine in young adult survivors of childhood acute lymphoblastic leukemia: a preliminary report. Dis Markers. (2012) 33(2):69–76. doi: 10.1155/2012/250286
22. Surdacki A, Sulicka J, Korkosz M, Mikolajczyk T, Telesinska-Jasiówka D, Klimek E, et al. Blood monocyte heterogeneity and markers of endothelial activation in ankylosing spondylitis. J Rheumatol. (2014) 41(3):481–9. doi: 10.3899/jrheum.130803
23. Sarwar N, Butterworth AS, Freitag DF, Gregson J, Willeit P, Gorman DN, et al. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. (2012) 379(9822):1205–13. doi: 10.1016/S0140-6736(11)61931-4
24. Yang X, Zhang D, Zhao Y, Liu D, Li Q, Guo C, et al. Association between serum level of C-reactive protein and risk of cardiovascular events based on cohort studies. J Hum Hypertens. (2021) 35(12):1149–58. doi: 10.1038/s41371-021-00546-z
25. Guzik TJ, Sadowski J, Guzik B, Jopek A, Kapelak B, Przybylowski P, et al. Coronary artery superoxide production and nox isoform expression in human coronary artery disease. Arterioscler Thromb Vasc Biol. (2006) 26(2):333–9. doi: 10.1161/01.ATV.0000196651.64776.51
26. Urbanski K, Ludew D, Filip G, Filip M, Sagan A, Szczepaniak P, et al. CD14(+)CD16(++) “nonclassical” monocytes are associated with endothelial dysfunction in patients with coronary artery disease. Thromb Haemost. (2017) 117(5):971–80. doi: 10.1160/TH16-08-0614
27. Canonico F, Pedicino D, Severino A, Vinci R, Flego D, Pisano E, et al. GLUT-1/PKM2 loop dysregulation in patients with non-ST-segment elevation myocardial infarction promotes metainflammation. Cardiovasc Res. (2022).
28. Szczepaniak P, Siedlinski M, Hodorowicz-Zaniewska D, Nosalski R, Mikolajczyk TP, Dobosz AM, et al. Breast cancer chemotherapy induces vascular dysfunction and hypertension through a NOX4-dependent mechanism. J Clin Invest. (2022) 132(13). doi: 10.1172/JCI149117
29. Calabró P, Willerson JT, Yeh ET. Inflammatory cytokines stimulated C-reactive protein production by human coronary artery smooth muscle cells. Circulation. (2003) 108(16):1930–2. doi: 10.1161/01.CIR.0000096055.62724.C5
31. Mitani Y, Sawada H, Hayakawa H, Aoki K, Ohashi H, Matsumura M, et al. Elevated levels of high-sensitivity C-reactive protein and serum amyloid-A late after kawasaki disease: association between inflammation and late coronary sequelae in kawasaki disease. Circulation. (2005) 111(1):38–43. doi: 10.1161/01.CIR.0000151311.38708.29
32. McArdle PA, McMillan DC, Sattar N, Wallace AM, Underwood MA. The relationship between interleukin-6 and C-reactive protein in patients with benign and malignant prostate disease. Br J Cancer. (2004) 91(10):1755–7. doi: 10.1038/sj.bjc.6602211
33. Shrivastava AK, Singh HV, Raizada A, Singh SK, Pandey A, Singh N, et al. Inflammatory markers in patients with rheumatoid arthritis. Allergol Immunopathol. (2015) 43(1):81–7. doi: 10.1016/j.aller.2013.11.003
34. Brennan-Bourdon LM, De la Cruz-Mosso U, Reyes-Castillo Z, Martínez-Bonilla GE, Ramírez-Dueñas MG, Islas-Carbajal MC, et al. MIF And TNFα serum levels in rheumatoid arthritis patients treated with disease-modifying antirheumatic drugs: a cross-sectional study. Immunopharm Immunot. (2015) 37(2):207–13. doi: 10.3109/08923973.2015.1017645
35. Li G, Ma H, Yin Y, Wang J. CRP, IL-2 and TNF-α level in patients with uremia receiving hemodialysis. Mol Med Rep. (2018) 17(2):3350–5.29257244
36. Jia XQ, Xu S, Tian MR, Ma YY. The relationship between inflammatory factor expression and blood pressure and urinary protein in the placenta of gestational hypertension rats. Exp Ther Med. (2018) 16(5):3793–8.30344654
37. Kong D, Wang H, Liu Y, Li H, Wang H, Zhu P. Correlation between the expression of inflammatory cytokines IL-6, TNF-α and hs-CRP and unfavorable fetal outcomes in patients with pregnancy-induced hypertension. Exp Ther Med. (2018) 16(3):1982–6.30186428
38. Dalekos GN, Elisaf M, Bairaktari E, Tsolas O, Siamopoulos KC. Increased serum levels of interleukin-1beta in the systemic circulation of patients with essential hypertension: additional risk factor for atherogenesis in hypertensive patients? J Lab Clin Med. (1997) 129(3):300–8. doi: 10.1016/S0022-2143(97)90178-5
39. Rothman AM, MacFadyen J, Thuren T, Webb A, Harrison DG, Guzik TJ, et al. Effects of interleukin-1β inhibition on blood pressure, incident hypertension, and residual inflammatory risk: a secondary analysis of CANTOS. Hypertension. (2020) 75(2):477–82. doi: 10.1161/HYPERTENSIONAHA.119.13642
40. Roach DR, Bean AG, Demangel C, France MP, Briscoe H, Britton WJ. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol. (2002) 168(9):4620–7. doi: 10.4049/jimmunol.168.9.4620
41. Holm T, Aukrust P, Andreassen AK, Ueland T, Brosstad F, Frøland SS, et al. Peripheral endothelial dysfunction in heart transplant recipients: possible role of proinflammatory cytokines. Clin Transplant. (2000) 14(3):218–25. doi: 10.1034/j.1399-0012.2000.140307.x
42. Zhang G. Tumor necrosis factor family ligand-receptor binding. Curr Opin Struct Biol. (2004) 14(2):154–60. doi: 10.1016/j.sbi.2004.03.003
43. Martens FM, Rabelink TJ, op ‘t Roodt J, de Koning EJ, Visseren FL. TNF-alpha induces endothelial dysfunction in diabetic adults, an effect reversible by the PPAR-gamma agonist pioglitazone. Eur Heart J. (2006) 27(13):1605–9. doi: 10.1093/eurheartj/ehl079
44. Murray EC, Nosalski R, MacRitchie N, Tomaszewski M, Maffia P, Harrison DG, et al. Therapeutic targeting of inflammation in hypertension: from novel mechanisms to translational perspective. Cardiovasc Res. (2021) 117(13):2589–609. doi: 10.1093/cvr/cvab330
45. Olejarz W, Bryk D, Zapolska-Downar D, Małecki M, Stachurska A, Sitkiewicz D. Mycophenolic acid attenuates the tumour necrosis factor-α-mediated proinflammatory response in endothelial cells by blocking the MAPK/NF-κB and ROS pathways. Eur J Clin Invest. (2014) 44(1):54–64. doi: 10.1111/eci.12191
46. Nakajima S, Kitamura M. Bidirectional regulation of NF-κB by reactive oxygen species: a role of unfolded protein response. Free Radic Biol Med. (2013) 65:162–74. doi: 10.1016/j.freeradbiomed.2013.06.020
47. Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. (2011) 21(1):103–15. doi: 10.1038/cr.2010.178
48. Chibana H, Kajimoto H, Ueno T, Yokoyama S, Sasaki KI, Ohtsuka M, et al. Interleukin-1β is associated with coronary endothelial dysfunction in patients with mTOR-inhibitor-eluting stent implantation. Heart Vessels. (2017) 32(7):823–32. doi: 10.1007/s00380-017-0947-x
49. Walsh CP, Lindsay EK, Grosse P, Natale BN, Fairlie S, Bwint A, et al. A systematic review and meta-analysis of the stability of peripheral immune markers in healthy adults. Brain Behav Immun. (2023) 107:32–46. doi: 10.1016/j.bbi.2022.09.011
50. Liu C, Chu D, Kalantar-Zadeh K, George J, Young HA, Liu G. Cytokines: from clinical significance to quantification. Adv Sci. (2021) 8(15):e2004433. doi: 10.1002/advs.202004433
51. Li Z, Solomonidis EG, Meloni M, Taylor RS, Duffin R, Dobie R, et al. Single-cell transcriptome analyses reveal novel targets modulating cardiac neovascularization by resident endothelial cells following myocardial infarction. Eur Heart J. (2019) 40(30):2507–20. doi: 10.1093/eurheartj/ehz305
52. Guzik TJ, Sadowski J, Kapelak B, Jopek A, Rudzinski P, Pillai R, et al. Systemic regulation of vascular NAD(P)H oxidase activity and nox isoform expression in human arteries and veins. Arterioscler Thromb Vasc Biol. (2004) 24(9):1614–20. doi: 10.1161/01.ATV.0000139011.94634.9d
53. Ordovás JM, Smith CE. Epigenetics and cardiovascular disease. Nat Rev Cardiol. (2010) 7(9):510–9. doi: 10.1038/nrcardio.2010.104
54. Pol T, Hijazi Z, Lindbäck J, Oldgren J, Alexander JH, Connolly SJ, et al. Using multimarker screening to identify biomarkers associated with cardiovascular death in patients with atrial fibrillation. Cardiovasc Res. (2022) 118(9):2112–23. doi: 10.1093/cvr/cvab262
55. Markousis-Mavrogenis G, Tromp J, Ouwerkerk W, Ferreira JP, Anker SD, Cleland JG, et al. Multimarker profiling identifies protective and harmful immune processes in heart failure: findings from BIOSTAT-CHF. Cardiovasc Res. (2022) 118(8):1964–77. doi: 10.1093/cvr/cvab235
56. Jovani M, Liu EE, Paniagua SM, Lau ES, Li SX, Takvorian KS, et al. Cardiovascular disease related circulating biomarkers and cancer incidence and mortality: is there an association? Cardiovasc Res. (2022) 118(10):2317–28. doi: 10.1093/cvr/cvab282
57. Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. (2009) 78(6):539–52. doi: 10.1016/j.bcp.2009.04.029
58. Litviňuková M, Talavera-López C, Maatz H, Reichart D, Worth CL, Lindberg EL, et al. Cells of the adult human heart. Nature. (2020) 588(7838):466–72. doi: 10.1038/s41586-020-2797-4
Keywords: cardiovascular disease, cytokine, inflammation, oxidative stress, superoxide, hs-CRP
Citation: Sulicka-Grodzicka J, Szczepaniak P, Jozefczuk E, Urbanski K, Siedlinski M, Niewiara Ł, Guzik B, Filip G, Kapelak B, Wierzbicki K, Korkosz M, Guzik TJ and Mikolajczyk TP (2023) Systemic and local vascular inflammation and arterial reactive oxygen species generation in patients with advanced cardiovascular diseases. Front. Cardiovasc. Med. 10:1230051. doi: 10.3389/fcvm.2023.1230051
Received: 27 May 2023; Accepted: 28 August 2023;
Published: 7 September 2023.
Edited by:
Yasutomi Higashikuni, The University of Tokyo, JapanReviewed by:
Hiroyuki Kiriyama, The University of Tokyo Hospital, Japan© 2023 Sulicka-Grodzicka, Szczepaniak, Jozefczuk, Urbanski, Siedlinski, Niewiara, Guzik, Filip, Kapelak, Wierzbicki, Korkosz, Guzik and Mikolajczyk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tomasz P. Mikolajczyk dG9tYXN6cC5taWtvbGFqY3p5a0B1ai5lZHUucGw=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.