
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 26 June 2023
Sec. Cardiac Rhythmology
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1217745
 Roland R. Tilz1,2*†,‡
Roland R. Tilz1,2*†,‡ Christian H. Heeger1,2,†,‡
Christian H. Heeger1,2,†,‡ Julia Vogler1,‡
Julia Vogler1,‡ Charlotte Eitel1,‡
Charlotte Eitel1,‡ Marcel Feher1
Marcel Feher1 Huong-Lan Phan1,‡
Huong-Lan Phan1,‡ Ilias Mushfiq1
Ilias Mushfiq1 Sorin S. Popescu1,‡
Sorin S. Popescu1,‡ Leonie Zetzsch1
Leonie Zetzsch1 Anna Traub1
Anna Traub1 Sascha Hatahet1
Sascha Hatahet1 Kai Mortensen1
Kai Mortensen1 Karl-Heinz Kuck1
Karl-Heinz Kuck1 Bettina Kirstein1*‡
Bettina Kirstein1*‡
Background: Wide antral circumferential ablation (WACA) in comparison to ostial pulmonary vein (PV) isolation (PVI) has been attributed with improved rhythm outcome. We investigated the feasibility, lesion formation, and rhythm outcome of WACA-PVI in comparison to ostial-PVI using pulsed field ablation (PFA).
Methods: Symptomatic atrial fibrillation (AF) patients (69 years, 67% male; 67% paroxysmal AF) were prospectively enrolled into our single-center registry and underwent first-time ostial-PFA or WACA-PFA, N = 15 each. In all patients, eight pulse trains (2 kV/2.5 s, bipolar, biphasic, 4× basket/flower configuration each) were delivered to each PV. In WACA-PFA, two extra pulse trains in a flower configuration were added to the anterior and posterior antrum of the PVs. For comparison of PFA lesion size, pre- and post-ablation left atrial (LA) voltage maps were acquired using a multipolar spiral catheter together with a three-dimensional electroanatomic mapping system.
Results: WACA-PFA resulted in a significant larger lesion formation than ostial-PFA (45.5 vs. 35.1 cm2, p = 0.001) with bilateral overlapping butterfly shape-like lesions and concomitant posterior LA wall isolation in 73% of patients. This was not associated with increased procedure time, sedation dosage, or exposure to radiation. One-year freedom from AF recurrence was numerically higher after WACA-PFA than ostial-PFA (94% vs. 87%) but not statistically significant (p = 0.68). No organized atrial tachycardias (ATs) were observed. Ostial-PFA patients more often underwent re-ablation due to recurrent AF episodes.
Conclusion: WACA-PFA is feasible and resulted in significantly wider lesion sets than ostial-PFA. Concomitant posterior LA wall isolation occurred as an epiphenomenon in the majority of patients. The WACA approach was associated with neither increased procedure and fluoroscopy times nor statistically significant differences in 1-year rhythm outcome. ATs were absent.
Atrial fibrillation (AF) is the most common arrhythmia. Pulmonary vein isolation (PVI) is the gold standard for AF ablation procedures and an established, guideline-directed treatment option for symptomatic patients (1).
However, durable PVI by conventional ablation energy sources such as radiofrequency (RF), cryo, or laser energy is highly variable (2) accounting for pulmonary vein (PV) reconnection as the main cause of AF recurrence (3–5).
Wide antral circumferential ablation (WACA) has been investigated as an alternative ablation approach and attributed with an improved rhythm outcome (6). In comparison to ostial-PVI that addresses AF substrates arising from the muscular sleeves of the pulmonary veins (PVs), WACA-PVI also targets arrhythmogenic substrates at the junction between the left atrium (LA) and the posterior LA wall (4, 7–9). As compared to smaller isolation areas, larger isolation areas around the PVs result in a significantly higher PVI success rate (10).
Point-by-point RF energy ablation is the preferred ablation energy source for WACA-PVI. However, RF ablation struggles with potential gap creation, if wider areas and thicker myocardium are targeted. Single-shot devices such as the cryoballoon have been shown to be equally effective to RF energy for treatment of AF (11) but are technically and anatomically limited when WACA is desired.
With pulsed field ablation (PFA), different catheter shapes can be used to create space for more individual lesion set administration. We therefore investigated feasibility, lesion formation, and rhythm outcome during WACA-PVI in comparison to ostial-PVI using PFA.
Between August and December 2021, consecutive patients with symptomatic AF undergoing pulsed field ablation were prospectively enrolled in our observational, single-center registry. While the first 15 patients received ostial-PFA, all following patients were treated with WACA-PFA. For better comparison, 15 WACA-PFA patients were propensity score matched to the first 15 ostial-PFA patients with respect to age, gender, AF type, and CHA2DS2VASc score. The registry was approved by the local ethical review board (Lübeck ablation registry ethical review board number: WF-028/15), and all participants provided written informed consent. All investigations were performed in compliance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
All patients underwent pre-ablation investigation as per the center's standard of care. Briefly, intracardiac thrombi were ruled out using transesophageal echocardiography. In patients on vitamin K antagonists, the procedure was performed under therapeutic INR values of 2–3. In patients on direct oral anticoagulants (DOACs), the morning dose was omitted on the procedure day. All ablations were performed under analgosedation using propofol, midazolam, and fentanyl following the recommendations of a position paper by the German Cardiac Society (12). Two 8 Fr ultrasound-guided femoral vein punctures were performed. One diagnostic catheter was introduced and positioned deep inside the coronary sinus. A single transseptal puncture (TSP) was performed under fluoroscopic guidance using a modified Brockenbrough technique. After TSP, heparin boluses were administered targeting an activated clotting time of >300 s. An SL1 sheath was used for antegrade LA access. Prior to first pulse delivery, 1 mg atropine was given intravenously to avoid vagal reaction such as sinus arrest or intermittent atrioventricular block.
Detailed description of the optimized PFA procedure has been described previously (2). In brief, eight pulse trains (2 kV/2.5 s, bipolar, biphasic, 4× basket and 4× flower configuration each) were delivered to each PV starting on the left-sided veins using the 12-F over-the-wire pentasline PFA catheter (Farawave™; Farapulse-Boston Scientific Inc.). Two extra pulse trains in the flower configuration were added for WACA as per the center's specific protocol. Pulse trains in flower configuration were actively angulated covering a wider anterior and posterior aspect of each PV antrum. Visualization of the PFA catheter angulation for WACA is shown in Figure 1. For implementation, every third electrode on each PFA catheter spline was used for image integration into the 3D navigation system (CARTO 3 V7; Biosense Webster).
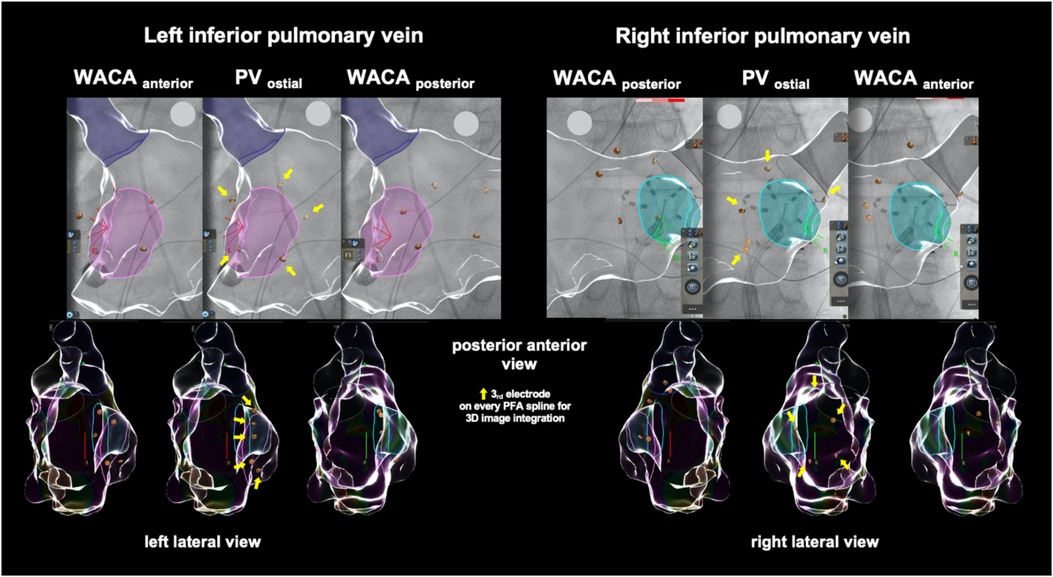
Figure 1. Example of the left atrial model reconstruction in one exemplary patient showing cardiac fluoroscopy and catheter visualization during wide antral circumferential pulsed field ablation (PFA, in flower configuration) of the left and right inferior pulmonary veins when integrated into a 3D navigation system. PFA, pulsed field ablation; PV, pulmonary vein; WACA, wide antral circumferential ablation.
Pre- and post-ablation, LA endocardial voltage maps using a three-dimensional electroanatomic reconstruction software (CARTO 3 V7; Biosense Webster or EnsiteX; Abbott) were performed via a multielectrode spiral mapping catheter (Lasso; Biosense Webster, I Advisor™ VL; Abbott Medical). Bipolar peak-to-peak electrogram amplitude <0.5 mV was defined as diseased low voltage signal or scar. No additional touch-up PFA pulses were performed after remapping. On post-ablation maps, the PVs were excluded following their anatomical margins. The ablated area was encircled and measured using vendor-specific software (Figure 2).
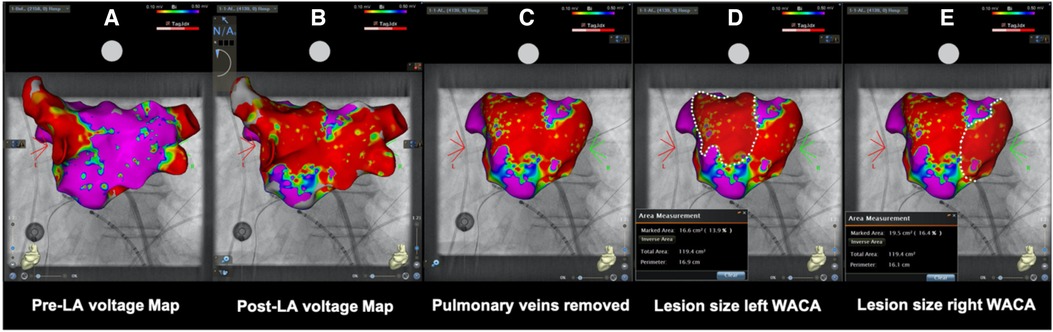
Figure 2. (A) Pre- and (B) post-ablation left atrial voltage maps in posterior–anterior view of the same patient showing the evaluation of ablation lesion size after wide antral circumferential pulsed field ablation with posterior wall isolation in a “butterfly-like” lesion shape. After anatomical (C) removal of the pulmonary veins, (D) left- and (E) right-sided WACA lesion size was encircled and calculated using dedicated inbuilt software. LA, left atrial/atria; WACA, wide antral circumferential ablation; Bi, bipolar; violet color, vital myocardium; red color, low voltage <0.10 mV indicating ablated myocardium/scar.
A vascular closure device or a figure-of-eight suture and a pressure bandage were used to prevent femoral bleeding. The pressure bandage was removed after 1–4 h depending on the closure technique used. The suture was removed the next day. Following ablation, all patients underwent transthoracic echocardiography immediately, after 2 h, and on the first day post ablation to rule out pericardial effusion. Oral anticoagulants were reinitiated 6 h post ablation and continued for at least 3 months and thereafter according to the CHA2DS2VASc score. Antiarrhythmic drugs were prescribed for 3 months post ablation, if there was no underlying contraindication, to prevent early AF recurrence during the blanking period. Rhythm follow-up (FU) was based on 24-h Holter ECG directly after the procedure as well as after 3, 6, and 12 months to report the 1-year rhythm outcome.
Continuous variables were tested for normal distribution using the Shapiro–Wilk test and summarized using descriptive statistics. In case of normal distribution, data were presented as mean ± standard deviation (SD), or as median and interquartile range (IQR) with first quartile (Q1), third quartile (Q3). Comparison of continuous data was performed using Student's t-test if normally distributed, or with the Wilcoxon signed-rank test.
Categorical data are reported as absolute (n) and relative frequencies (%) and were compared using the chi-square test or Fisher's exact test (small expected frequencies).
For group comparisons, normality of data was verified with the use of box plots and Kolmogorov–Smirnov normality test. For normally distributed data, comparisons were performed using Student's t-test for independent groups. In cases where data were not normally distributed, the nonparametric Wilcoxon rank sum test was used.
Results were expressed in terms of p-values. Exact two-sided statistical significance was considered for a p-value <0.01. All calculations were performed with the statistical analysis software DATAtab (DATAtab e.U., Graz, Austria).
Thirty patients with predominantly clinical symptomatic paroxysmal AF, a normal left ventricular ejection fraction, and a low burden of comorbidities (median CHA2DS2VASc score of 2) were prospectively enrolled into the registry. Prior pharmacological or electrical rhythm control using antiarrhythmic drugs or electrical cardioversion had been attempted in 43% and 37% of patients, respectively. Oral anticoagulation with a DOAC was established in the majority of patients. Baseline characteristics did not differ between both groups. Detailed patient baseline characteristics are shown in Table 1.
All PFA procedures were performed by two highly experienced EP operators. A median of 8 (IQR 8, 8) and 10 (IQR 9, 12) pulse trains per PV for ostial and WACA-PFA were applied. All PVs were isolated at the end of the procedure. The WACA-PFA approach was not associated with a significant increase in procedure time, sedation dosage, or exposure to radiation. Procedural data are depicted in Table 2.
Left atrial pre- and post-mapping point density did not differ between both groups. In the WACA-PFA group, a trend to usage of a smaller ablation catheter size was noted. WACA-PFA resulted in significantly larger lesion formation (45.5 ± 8.3 cm2) than ostial-PFA (35.1 ± 7.2 cm2), p = 0.001 as shown in Figure 3 with overlapping bilateral lesion sets and consecutive posterior LA wall isolation in 11/15 (73%) patients. In Figure 4, we provide examples highlighting the different lesion sets on pre- and post-ablation LA voltage maps for ostial-PFA, WACA-PFA, and WACA-PFA with an overlapping bilateral lesion set and electrical posterior LA wall isolation—the so-called “butterfly effect.”
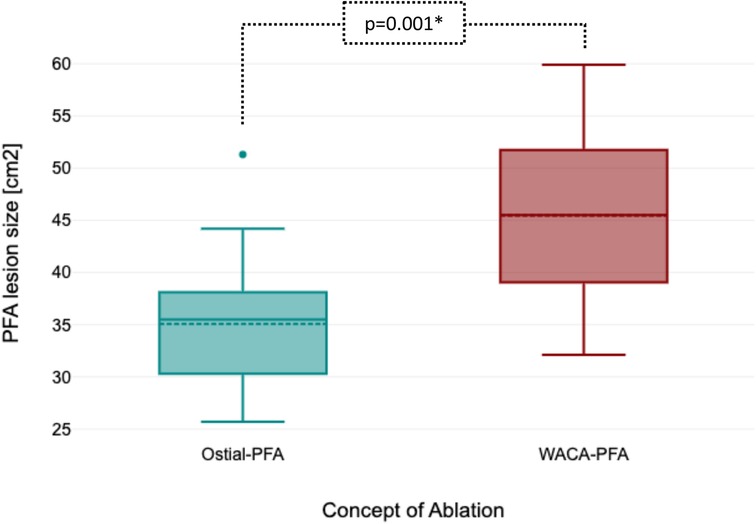
Figure 3. Boxplot showing significant larger ablation lesion size between ostial-PFA and WACA-PFA. PFA, pulsed field ablation; WACA, wide antral circumferential ablation.
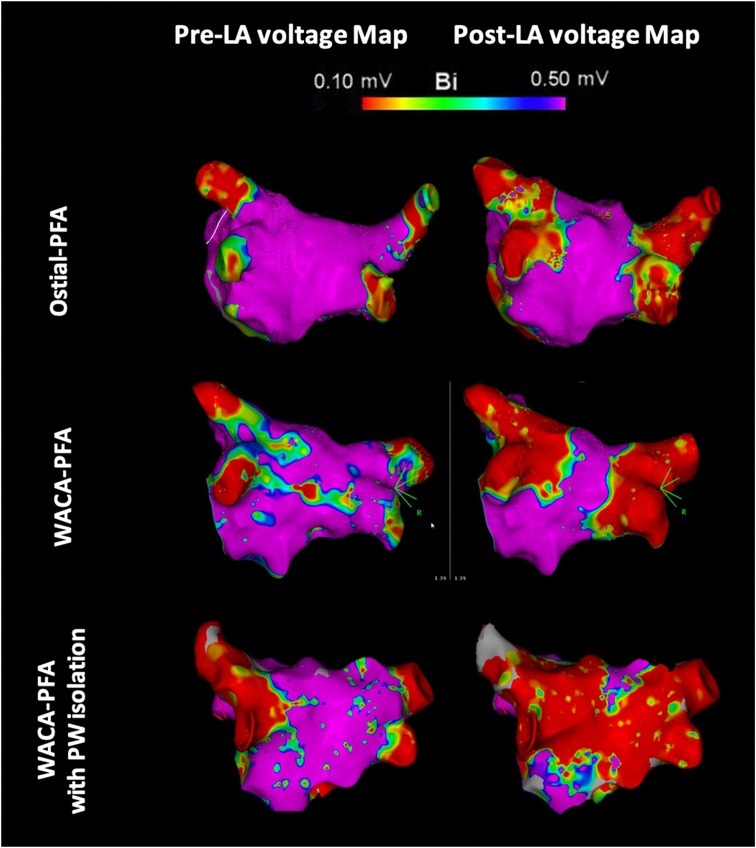
Figure 4. Pre- and post-ablation left atrial voltage maps in posterior–anterior view showing different lesion formation in ostial and wide antral circumferential pulsed field ablation with and without posterior wall isolation in a “butterfly-like” lesion shape. LA, left atrial/atria; PFA, pulsed field ablation; WACA, wide antral circumferential ablation; PW, posterior wall; Bi, bipolar; violet color, vital myocardium; red color, low voltage <0.10 mV indicating ablated myocardium/scar.
In the ostial-PFA group, four patients presented with transient phrenic nerve stunning—with full recovery the next day. One patient experienced acute postprocedural hemoptysis with no obvious bleeding source in the bronchoscopy, with spontaneous cessation without need for transfusion.
In the WACA-PFA group, one of each of the following events occurred either acute or during the further clinical follow-up: hemodynamically stable pericardial effusion recurrent under conservative therapy, groin hematoma and deep vein thrombosis at the venous access sight, decompensated heart failure with preserved ejection fraction despite sinus rhythm.
Completeness of Holter ECG recordings were 40% for all, 73% for at least three, 97% for at least two, and 100% for one Holter. Freedom from arrhythmia recurrence was 87% (N = 2/15) after ostial-PFA and 94% (N = 1/15) after WACA-PFA, which was not statistically different (p = 0.68). Both patients in the ostial-PFA group had recurrent symptomatic atrial arrhythmia episodes and underwent re-ablation. The patient in the WACA-PFA group experienced only one paroxysmal episode several days after the end of the blanking period and therefore was not scheduled for re-ablation so far. We did not observe organized atrial tachycardia (AT).
In our prospective registry, WACA-PFA in comparison to ostial-PFA resulted in significantly wider lesion sets. Using the currently available PFA catheter, we observed bilateral overlapping lesion sets with concomitant posterior LA wall isolation as an epiphenomenon in the majority of patients. This was not associated with prolonged procedure or fluoroscopy times. Rhythm outcome after 1 year was numerically better in the WACA-PFA group but was not statistically significant. However, only ostial-PFA patients underwent re-ablation due to recurrent episodes.
PFA is a novel ablation technology with promising results over existing ablation energies due to its unique myocardial tissue specificity (2, 13). Early preclinical and clinical data reported improved acute and chronic PVI rates up to 96% (14) without regression of the isolated area size between the acute and chronic phase (>75 days post-PFA) (15). On pathohistological assessment and gross anatomy models, PFA resulted in more homogeneous and transmural ablation lesion formation with less coagulation in comparison to RF energy, serving as a prerequisite for durable PVI (2, 16–18). Evaluation with high-density voltage mapping confirmed these findings. Sharp delineated lesion boundaries between scar and healthy tissue were described (19, 20). When lesion pattern between RF, cryo, and PFA energy on the posterior aspect of the PV antrum were compared against each other by electroanatomical mapping, PFA lesions resulted in an RF-like pattern, also encompassing the carina level of the ipsilateral PVs, which was left with notched-like areas of normal voltage when the cryoballon was used (21). In comparison to previously reported data on lesion size, ostial-PFA alone resulted in a larger ablated area than cryoballon ablation (35.1 vs. 28.9 cm2) (22).
Recently published data on high-density voltage mapping revealed the anterior and superior parts of both sided PVs as susceptible weak spots for gap formation after PFA (20). The authors attributed this to the over-the wire catheter design, which is prone to alignment to the posterior wall after transseptal puncture. Respecting this limitation, our modified WACA-PFA approach with active anterior and posterior catheter angulation resulted in larger lesion set formation. Subsequently, important arrhythmogenic foci on the anterior ridge, the carina level, and at the posterior aspects of the PV antrum were addressed. However, careful handling, tissue proximity, and experience in interventional left atrial procedures are needed to avoid collateral damage to the anterior and posterior LA wall, as seen in our population with the latter. This may lead to potentially proarrhythmogenic isthmus creation on the posterior wall and has been associated with the development of roof-dependent ATs. In our cohort, we did not observe organized ATs. In most of our WACA-PFA patients, bilateral overlapping areas of low voltage were assessed on acute electroanatomical remapping. This might have prevented the development of ATs as the type of arrhythmia recurrence. Overall, a more reliable catheter integration into available three-dimensional mapping systems would be desirable to better understand the site of energy delivery.
The variable opening degree and shapes of the current PFA catheter (from basket to olive to donut- and flower-like formation) enable individual lesion application, on the one hand, but can result in different pulsed field characteristics with divergent lesion characteristics, on the other hand. We therefore used the currently recommended and evaluated flower shape of the PFA catheter for WACA lesion application. Overall, this approach resulted in a high percentage of freedom from AF recurrence after 1 year with both approaches. This is in line with recently published 1-year outcome data reporting 78.5% freedom from atrial arrhythmia after PFA (23). However, patients with ostial-PFA had multiple AF recurrences and therefore underwent re-ablation. Further evaluation of the WACA-PFA approach in a larger number of patients is needed.
Our data present a single-center registry experience and encompass a small number of patients with early AF stages and low rate of comorbidities. No randomization or comparison to other ablation energy sources was performed. Data are only applicable to the vendor-specific PFA catheter design and technology. Potential bias might result from using variable PFA catheter sizes. No data on serum concentrations of highly sensitive troponin I as a surrogate for larger lesion extent were evaluated. Routine PFA catheter integration into the three-dimensional mapping system was not performed due to instable performance issues.
In our prospective registry, WACA-PFA in comparison to ostial-PFA was feasible. WACA-PVI resulted in significantly wider lesion sets and a bilaterally overlapping lesion formation on the posterior LA wall, the so-called “butterfly effect.” Concomitant posterior LA wall isolation occurred as an epiphenomenon in the majority of patients. This approach was not associated with a significant increase in the procedure time, sedation dosage, or exposure to radiation nor differences in rhythm outcome, especially ATs were absent. However, only ostial-PFA patients underwent re-ablation procedures due to recurrent symptomatic AF episodes. This needs to be evaluated further on longer follow-up and in a larger cohort of patients.
The datasets presented in this article are not readily available because non-digital data supporting this study are curated at the Study Center of the Department of Rhythmology, University Hospital Schleswig-Holstein, Germany. Requests to access the datasets should be directed to UKSH Campus Lübeck, Studienzentrale Rhythmologie, https://www.uksh.de/rhythmologie-luebeck/Forschung+_+Lehre/Studienzentrale.html.
The studies involving human participants were reviewed and approved by Lübeck ablation registry ethical review board number: WF-028/15. The patients/participants provided their written informed consent to participate in this study.
RRT and BK contributed to conception and design of the study. BK, CHH, JV, CE, MF, H-LP, IM, LZ, AT, SH and SSP collected the data and organized the database. BK performed the statistical analysis and wrote the first draft of the manuscript. BK, RRT, and CHH wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
BK reports educational grant support from Boston Scientific (Fellowship “Herzrhythmus”); travel and congress sponsoring from Biotronik, Abbott, Impulse Dynamics, and Pfizer; and speaker honoraria from Biotronik, Impulse Dynamics, CTI GmbH, and Doctrina Med outside the submitted work. CHH received travel grants and research grants from Boston Scientific, Liftech, Biosense Webster, and Cardiofocus and speaker honoraria from Boston Scientific, Biosense Webster, Cardiofocus, CTI GmbH, and Doctrina Med. H-LP received travel grants from Cardiofocus, Abbott, and CTI and an educational grant from Biosense Webster outside the submitted work. K-HK reports grants and personal fees from Abbott Vascular, Medtronic, and Biosense Webster outside the submitted work. RT is a consultant for Boston Scientific, Biotronik, and Biosense Webster and received speaker honoraria from Biosense Webster, Medtronic, Boston Scientific, and Abbot Medical. CE received travel grants from Biosense Webster, Medtronic, Biotronik, Abbott, and Daiichi Sankyo and speaker honoraria from Biosense Webster, Medtronic, Abbott, and Daiichi Sankyo.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1217745/full#supplementary-material
AF, atrial fibrillation; aHT, arterial hypertension; DOAC, direct oral anticoagulant; FU, follow-up; IQR, interquartile range; LA, left atrium/left atrial; PFA, pulsed field ablation; PV, pulmonary vein; PVI, pulmonary vein isolation; RF, radiofrequency; WACA, wide antral circumferential ablation.
1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. (2020) 42(5):373–498. doi: 10.1093/eurheartj/ehaa612
2. Reddy VY, Neuzil P, Koruth JS, Petru J, Funosako M, Cochet H, et al. Pulsed field ablation for pulmonary vein isolation in atrial fibrillation. J Am Coll Cardiol. (2019) 74:315–26. doi: 10.1016/j.jacc.2019.04.021
3. Ouyang F, Bänsch D, Ernst S, Schaumann A, Hachiya H, Chen M, et al. Complete isolation of left atrium surrounding the pulmonary veins: new insights from the double-lasso technique in paroxysmal atrial fibrillation. Circulation. (2004) 110(15):2090–6. doi: 10.1161/01.CIR.0000144459.37455.EE
4. Ouyang F, Antz M, Ernst S, Hachiya H, Mavrakis H, Deger FT, et al. Recovered pulmonary vein conduction as a dominant factor for recurrent atrial tachyarrhythmias after complete circular isolation of the pulmonary veins: lessons from double lasso technique. Circulation. (2005) 111:127–35. doi: 10.1161/01.CIR.0000151289.73085.36
5. Kowalski M, Grimes MM, Perez FJ, Kenigsberg DN, Koneru J, Kasirajan V, et al. Histopathologic characterization of chronic radiofrequency ablation lesions for pulmonary vein isolation. J Am Coll Cardiol. (2012) 59(10):930–8. doi: 10.1016/j.jacc.2011.09.076
6. Proietti R, Santangeli P, Di Biase L, Joza J, Bernier ML, Wang Y, et al. Comparative effectiveness of wide antral versus ostial pulmonary vein isolation: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol. (2014) 7(1):39–45. doi: 10.1161/CIRCEP.113.000922
7. Jaïs P, Haïssaguerre M, Shah DC, Chouairi S, Gencel L, Hocini M, et al. A focal source of atrial fibrillation treated by discrete radiofrequency ablation. Circulation. (1997) 95(3):572–6. doi: 10.1161/01.CIR.95.3.572
8. Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. (1998) 339:659–66. doi: 10.1056/NEJM199809033391003
9. Marrouche NF, Dresing T, Cole C, Bash D, Saad E, Balaban K, et al. Circular mapping and ablation of the pulmonary vein for treatment of atrial fibrillation: impact of different catheter technologies. J Am Coll Cardiol. (2002) 40:464–74. doi: 10.1016/S0735-1097(02)01972-1
10. Arentz T, Weber R, Bürkle G, Herrera C, Blum T, Stockinger J, et al. Small or large isolation areas around the pulmonary veins for the treatment of atrial fibrillation? Results from a prospective randomized study. Circulation. (2007) 115:3057–63. doi: 10.1161/CIRCULATIONAHA.107.690578
11. Kuck KH, Brugada J, Fürnkranz A, Metzner A, Ouyang F, Chun KJ, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. (2016) 374:2235–45. doi: 10.1056/NEJMoa1602014
12. Tilz RR, Chun KRJ, Deneke T, Kelm M, Piorkowski C, Sommer P, et al. Positionspapier der Deutschen Gesellschaft für Kardiologie zur Kardioanalgosedierung. Der Kardiologe. (2017) 11(5):369–82. doi: 10.1007/s12181-017-0179-4
13. Reddy VY, Koruth J, Jais P, Petru J, Timko F, Skalsky I, et al. Ablation of atrial fibrillation with pulsed electric fields: an ultra-rapid, tissue-selective modality for cardiac ablation. JACC Clin Electrophysiol. (2018) 4:987–95. doi: 10.1016/j.jacep.2018.04.005
14. Reddy VY, Dukkipati SR, Neuzil P, Anic A, Petru J, Funasako M, et al. Pulsed field ablation of paroxysmal atrial fibrillation. JACC Clin Electrophysiol. (2021) 7(5):614–27. doi: 10.1016/j.jacep.2021.02.014
15. Kawamura I, Neuzil P, Shivamurthy P, Petru J, Funasako M, Minami K, et al. Does pulsed field ablation regress over time? A quantitative temporal analysis of pulmonary vein isolation. Heart Rhythm. (2021) 18(6):878–84. doi: 10.1016/j.hrthm.2021.02.020
16. Koruth JS, Kuroki K, Iwasawa J, Enomoto Y, Viswanathan R, Brose R, et al. Preclinical evaluation of pulsed field ablation: electrophysiological and histological assessment of thoracic vein isolation. Circ Arrhythm Electrophysiol. (2019) 12:e007781. doi: 10.1161/CIRCEP.119.007781
17. Redd VY, Anic A, Koruth J, Petru J, Funasako M, Minami K, et al. Pulsed field ablation in patients with persistent atrial fibrillation. J Am Coll Cardiol. (2020) 76(9):1068–80. doi: 10.1016/j.jacc.2020.07.007
18. Reddy VY, Dukkipati SR, Neuzil P, Anic A, Petru J, Funasako M, et al. Pulsed field ablation of paroxysmal atrial fibrillation: 1-year outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin Electrophysiol. (2021) 7(5):614–27. doi: 10.1016/j.jacep.2021.02.014
19. Gunawardene MA, Schaeffer BN, Jularic M, Eickholt C, Maurer T, Akbulak RÖ, et al. Pulsed field ablation combined with ultra-high-density mapping in patients undergoing catheter ablation for atrial fibrillation: practical and electrophysiological considerations. J Cardiovasc Electrophysiol. (2022) 33:345–56. doi: 10.1111/jce.15349
20. Bohnen M, Weber R, Minners J, Jadidi A, Eichenlaub M, Neumann FJ, et al. Characterization of circumferential antral pulmonary vein isolation areas resulting from pulsed-field catheter ablation. Europace. (2022) 25(1):1–9. doi: 10.1093/europace/euac111
21. Kawamura I, Neuzil P, Shivamurthy P, Kuroki K, Lam J, Musikantow D, et al. How does the level of pulmonary venous isolation compare between pulsed field ablation and thermal energy ablation (radiofrequency, cryo, or laser)? Europace. (2021) 23(11):1–10. doi: 10.1093/europace/euab150
22. Rottner L, Heeger CH, Lemes C, Wohlmuth P, Maurer T, Reissmann B, et al. Quantification of left atrial fibrosis in patients after pulmonary vein isolation using the second generation cryoballoon. Int Heart J. (2021) 62:65–71. doi: 10.1536/ihj.20-301
Keywords: pulmonary vein ablation/isolation, WACA, pulsed field ablation, atrial fibrillation, catheter ablation
Citation: Tilz RR, Heeger CH, Vogler J, Eitel C, Feher M, Phan H-L, Mushfiq I, Popescu SS, Zetzsch L, Traub A, Hatahet S, Mortensen K, Kuck K-H and Kirstein B (2023) Wide antral circumferential vs. ostial pulmonary vein isolation using pulsed field ablation—the butterfly effect. Front. Cardiovasc. Med. 10:1217745. doi: 10.3389/fcvm.2023.1217745
Received: 5 May 2023; Accepted: 12 June 2023;
Published: 26 June 2023.
Edited by:
Michael Brunner, Artemed Kliniken Freiburg, GermanyReviewed by:
Felix Bourier, Centre Hospitalier Universitaire de Bordeaux, France© 2023 Tilz, Heeger, Vogler, Eitel, Feher, Phan, Mushfiq, Popescu, Zetzsch, Traub, Hatahet, Mortensen, Kuck and Kirstein. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bettina Kirstein aW5mby5iLmtpcnN0ZWluQGdtYWlsLmNvbQ== Roland R. Tilz dGlsejZAaG90bWFpbC5jb20=
†These authors share first authorship
‡ORCID Roland R. Tilz orcid.org/0000-0002-0122-7130 Christian H. Heeger orcid.org/0000-0002-9014-8097 Julia Vogler orcid.org/0000-0002-8386-9750 Charlotte Eitel orcid.org/0000-0002-8733-6791 Huong-Lan Phan orcid.org/0000-0002-6867-914X Sorin S. Popescu orcid.org/0000-0002-5469-8833 Bettina Kirstein orcid.org/0000-0002-6881-871X
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.