- 1College of Nursing and Public Health, Adelphi University, Garden City, NY, United States
- 2Biomedical Sciences, University of Padova, Padova, Italy
- 3Boston Scientific, Boston Scientific, Kerkrade, Netherlands
- 4Bioelectronic Medicine, Feinstein Institutes for Medical Research, Manhasset, NY, United States
Editorial on the Research Topic
Cardiovascular neuromodulation: mechanisms and therapies
High cardiovascular disease (CVDs) prevalence is projected to impact a large population across the world (1). Future therapeutic development efforts should take these estimates into account and provide new treatment modalities.
Modern neuromodulation therapies are an emerging non-pharmacological approach for the treatment of several disease conditions in basic research and clinical studies. The principial basis is to reduce or enhance, selectively, the altered neural activity that determined by the pathophysiological mechanisms, using neuro-medical devices (2). The main attractive subject is the autonomic nervous system (ANS) which maintains the body homeostasis, and the disruption of its integrity cornubites to the development and progression of many diseases including those affecting cardiovascular system and the immune components (3, 4).
The cardiac autonomic nervous system provides a closed-loop control of the heart and vascular system through a rich highly organized neural network composed of the brainstem, extracardiac sympathetic ganglia, vagus nerve and the intrinsic cardiac nerves system. Dysregulation at any level could lead ANS imbalance and, also, could trigger the chronic inflammatory process (5–7). Initially, dysregulation of the ANS may serve as a compensatory mechanism to maintain blood pressure and cardiac output in response to a cardiovascular insult, such as myocardial infarction and heart failure that associated with exaggerated sympathetic overflow and reduction of parasympathetic tone. However, if this dysregulation is not relieved by therapy, it becomes maladaptation and can lead to the development of a wide range of cardiovascular and non-cardiovascular conditions over time (8, 9). The beneficial effects of cardiac-brain-axis modulation were highlighted in multiple pre-clinical and clinical studies. For example, these studies included: renal denervation (RDN) to treat resistant hypertension, restore the baroreflex tone to treat orthostatic hypotension (OT), cardiac sympathectomy to suppress arrhythmias and vagus nerve stimulation (VNS) to reduce the progression of HF (Figure 1) (10–15).
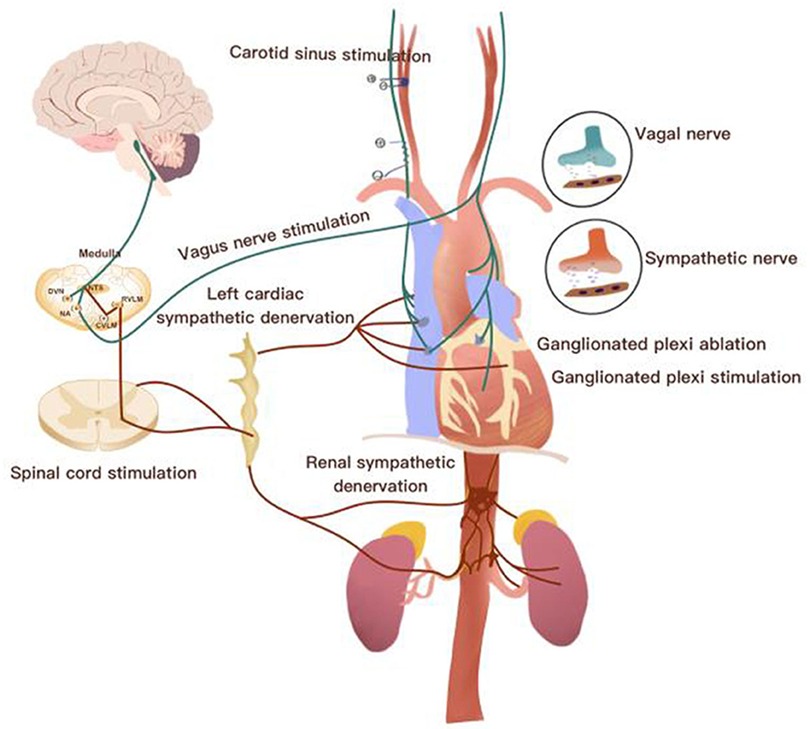
Figure 1. Overview of the neurocardiac axis and invasive neuromodulation approaches [Adapted from Chen et al., (10)].
In this issue the review by Ottaviani et al. has explored in detail the histological structure and the physiological role of vagus nerve, as the main tool to provide cardiovascular neuromodulation, and presented preclinical studies aimed at overcoming VNS limitations through optimization of anatomical targets, development of novel neural interface technologies, and design of efficient VNS closed-loop protocols. The interesting findings that were documented in the manuscript by Rodrigues et al. who measured blindly the effects of acute and short-term transcranial direct current stimulation (tDCS) sessions on blood pressure and autonomic modulation in RHT subjects and showed a reduction in the central blood pressure. The heart rate variability (HRV) as a measure of ANS balance was reduced in association with post MI arrhythmic events which increased the mortality in the study that was conducted by Pizzo et al. There was also a U-shaped association between HRVI and mortality in hemodialysis AF patients as found in the data from Braunisch et al. The sophisticated study by Neely et al. examined whether macrophages could drive the sympathetic phenotype in Spontaneously Hypertensive Rats (SHR), before animals develop high pressure; their findings showed that macrophages can be potent enhancers of sympathetic neuronal calcium responsiveness and plays a role in peripheral sympathetic hyperactivity observed in the initial stages of hypertension.
Cardiovascular neuromodulation is an emerging field with ongoing research and clinical trials to investigate its safety and efficacy in various cardiovascular conditions. It has the potential to offer new treatment options for patients with cardiovascular conditions that fail to respond to the traditional therapies. However, further research is needed to fully understand its mechanisms of action and long-term outcomes.
Author contributions
The first four authors are equally contributed. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Acknowledgments
We thank all authors for their contribution to our Research Topic.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mohebi R, et al. Cardiovascular disease projections in the United States based on the 2020 census estimates. J Am Coll Cardiol. (2022) 80(6):565–78. doi: 10.1016/j.jacc.2022.05.033
2. Pathak YJ, et al. Digital health integration with neuromodulation therapies: the future of patient-centric innovation in neuromodulation. Front Digit Health. (2021) 3:618959. doi: 10.3389/fdgth.2021.618959
3. McCorry LK. Physiology of the autonomic nervous system. Am J Pharm Educ. (2007) 71(4):78. doi: 10.5688/aj710478
4. Kenney MJ, Ganta CK. Autonomic nervous system and immune system interactions. Compr Physiol. (2014) 4(3):1177–200. doi: 10.1002/cphy.c130051
5. Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. (1999) 340(2):115–26. doi: 10.1056/NEJM199901143400207
6. Eskandari F, Webster JI, Sternberg EM. Neural immune pathways and their connection to inflammatory diseases. Arthritis Res Ther. (2003) 5(6):1–15. doi: 10.1186/ar1002
7. Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev. (2010) 90(2):513–57. doi: 10.1152/physrev.00007.2009
8. Zhang DY, Anderson AS. The sympathetic nervous system and heart failure. Cardiol Clin. (2014) 32(1):33–45. doi: 10.1016/j.ccl.2013.09.010
9. Iaccarino G, et al. Role of the sympathetic nervous system in cardiac remodeling in hypertension. Clin Exp Hypertens. (2001) 23(1–2):35–43. doi: 10.1081/CEH-100001195
10. Chen M, Wang S, Li X, Yu L, Yang H, Liu Q, et al. Non-invasive autonomic neuromodulation is opening new landscapes for cardiovascular diseases. Frontiers in physiology. (2020) 11:550578.33384606
11. Yoshida K, et al. Electrical vagal nerve stimulation ameliorates pulmonary vascular remodeling and improves survival in rats with severe pulmonary arterial hypertension. JACC Basic Transl Sci. (2018) 3(5):657–71. doi: 10.1016/j.jacbts.2018.07.007
12. Sabbah HN, et al. Vagus nerve stimulation in experimental heart failure. Heart Fail Rev. (2011) 16:171–8. doi: 10.1007/s10741-010-9209-z
13. Bertog SC, Sobotka PA, Sievert H. Renal denervation for hypertension. JACC Cardiovasc Interv. (2012) 5(3):249–58. doi: 10.1016/j.jcin.2011.12.011
14. O’Callaghan EL, et al. Deep brain stimulation for the treatment of resistant hypertension. Curr Hypertens Rep. (2014) 16:1–10. doi: 10.1007/s11906-014-0493-1
Keywords: heart, neuromodulation, autonomic nervous system, heart failiure, hypertension, vagus nerve stimulation
Citation: Hunt D, Mongillo M, Meo M, Zaglia T and Qanud K (2023) Editorial: Cardiovascular neuromodulation: mechanisms and therapies. Front. Cardiovasc. Med. 10:1214496. doi: 10.3389/fcvm.2023.1214496
Received: 29 April 2023; Accepted: 8 May 2023;
Published: 23 May 2023.
Edited and Reviewed by: Guido Iaccarino, University of Naples Federico II, Italy
© 2023 Hunt, Mongillo, Meo, Zaglia and Qanud. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Khaled Qanud a3FhbnVkQG5vcnRod2VsbC5lZHU=
†These authors have contributed equally to this work
 Deborah Hunt
Deborah Hunt Marco Mongillo
Marco Mongillo Marianna Meo
Marianna Meo Tania Zaglia
Tania Zaglia Khaled Qanud
Khaled Qanud