
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med. , 05 July 2023
Sec. Cardio-Oncology
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1212983
This article is part of the Research Topic Far from Perfect: The long-lasting effects of cancer treatments View all 8 articles
 Paolo Morfino1*
Paolo Morfino1* Alberto Aimo1,2
Alberto Aimo1,2 Vincenzo Castiglione1,2
Vincenzo Castiglione1,2 Michela Chianca1
Michela Chianca1 Giuseppe Vergaro1,2
Giuseppe Vergaro1,2 Carlo Maria Cipolla3
Carlo Maria Cipolla3 Antonella Fedele3
Antonella Fedele3 Michele Emdin1,2
Michele Emdin1,2 Iacopo Fabiani1,2
Iacopo Fabiani1,2 Daniela Cardinale3
Daniela Cardinale3
Amyloid light-chain (AL) amyloidosis is a hematological disorder characterized by abnormal proliferation of a plasma cell clone producing monoclonal free light chains that misfold and aggregate into insoluble fibrils in various tissues. Cardiac involvement is a common feature leading to restrictive cardiomyopathy and poor prognosis. Current first-line treatments aim at achieving hematological response by targeting the plasma cell clones, and these have been adapted from multiple myeloma therapy. Patients with AL amyloidosis often exhibit multiorgan involvement, making them susceptible to cancer therapy-related cardiovascular toxicity. Managing AL amyloidosis is a complex issue that requires enhanced knowledge of the cardio-oncological implications of hematological treatments. Future research should focus on implementing and validating primary and secondary prevention strategies and understanding the biochemical basis of oncological therapy-related damage to mitigate cardiovascular toxicity.
Amyloid light-chain (AL) amyloidosis is a hematological disease caused by the proliferation of a plasma cell clone that overproduces immunoglobulin (Ig) λ (75%–80%) or κ free light chains (FLC) that are prone to misfolding and self-aggregation into amyloid fibrils (1). These fibrils accumulate in various tissues, leading to both direct tissue damage through their intrinsic proteotoxicity and architectural disruption (2).
The immunophenotype of plasma cells involved in AL amyloidosis is not much different from multiple myeloma (MM) and monoclonal gammopathy of uncertain significance (MGUS), even if the plasma cell burden, namely the bone marrow percentage of plasma cells, is generally lower than in MM (2, 3). The first-line treatment options for AL amyloidosis aim at obtaining a hematological response, defined as normalization of FLC ratio or reduction of differential FLC (difference between amyloidogenic and uninvolved circulating FLC), because the disruption of abnormal plasma cell clones results in slower disease progression and reduced amyloid deposition (4). On the other side, since the cardiac amyloid burden is the main determinant of prognosis, supportive care directed to cardiac dysfunction has a crucial role in patients with AL amyloidosis (2).
Treatment of AL amyloidosis has traditionally been readapted from that of MM. However, most MM cancer therapy regimens exhibit a potential cardiovascular (CV) toxicity leading to enhanced risk of heart failure (HF), arrhythmias and vascular disease (Figure 1) (5). Additionally, patients with AL amyloidosis often display a multiorgan involvement which makes them fragile and susceptible to cancer therapy-related CV toxicity (CTR-CT) (6).

Figure 1. Schematic of therapeutic targets and cardiovascular toxicity in light chain amyloidosis. The Figure summarizes the targets, mechanisms of action and cardiovascular toxicities of the most frequently used drugs in AL amyloidosis. PIs impair the function of the proteasome, inducing protein accumulation. IMiDs induce the proteasome-mediated degradation of IKZF transcription factors and exert both direct and indirect (through stimulation of immune response and anti-angiogenetic effect) cytotoxic effect. Daratumumab causes ADCC, CDC, and cellular apoptosis by targeting CD38 on plasma cells. Alkylating agents induce DNA damage, thus inhibiting cellular transcription and replication. ASCT consists of autologous bone marrow substitution, which represses the proliferation of abnormal plasma cell clones. ADCC, antibody-dependent cell cytotoxicity; AL, light chain amyloidosis; ASCT, autologous stem cell transplant; ATE, arterial thromboembolism; CDC, complement-dependent cytotoxicity; DM, diabetes mellitus; HF, heart failure; IMiD, immunomodulatory drug; MI, myocardial infarction; PD, pericardial disease; PH, pulmonary hypertension; PI, proteasome inhibitor; VTE, venous thromboembolism.
This review tries to elucidate the clinical manifestations and management of CV adverse effects (AEs) caused by cancer therapy in patients with AL amyloidosis.
AL amyloidosis is a rare condition, with 20-year incidence and prevalence rates of approximately 10 and 51 cases per million population, but it still represents the most common form of systemic amyloidosis (3, 7). Interestingly, although AL amyloidosis is considered 5–10-fold less common than MM, about 10%–15% of patients with a diagnosis of MM will develop AL amyloidosis, and a similar proportion of AL amyloidosis patients will manifest MM (7). Patients with a MGUS exhibit a 8.8-fold higher risk of developing AL amyloidosis compared to the general population (8). AL amyloidosis also represents one of the two most common causes of cardiac amyloidosis (CA), along with transthyretin amyloidosis, together accounting for 90%–95% of all cases of CA (9). Indeed, cardiac involvement can be detected in approximately 75% of AL patients (10). AL amyloidosis is usually diagnosed at a mean age of 63 years, and is slightly more common in males (55%) (7, 11).
The diagnosis of AL-CA requires the demonstration of light-chain amyloid within tissues. Suspecting CA is crucial to start the diagnostic workup for this condition. A non-invasive multiparametric echocardiography score has been proposed to detect cardiac involvement among patients with systemic AL (AL score) or in those with hypertrophic phenotype [increased wall thickness (IWT) score]. AL score included relative wall thickness, E/e′, longitudinal strain, and TAPSE, with an optimism-corrected area under the ROC curve (AUC) of 0.905; whereas the IWT score included relative wall thickness, E/e′, longitudinal strain, TAPSE and septal longitudinal systolic apex-to-base ratio, with an AUC of 0.864 (12).
Cardiac involvement is the main determinant of prognosis in AL patients, which exhibits a 3–6 month median survival in the absence of hematological treatments, while untreated patients without cardiac involvement have a median expected survival of 13 months (13–16). The mortality rate in AL amyloidosis has dramatically decreased at all stages of the disease because of therapeutic advancements. Indeed, the analysis of patient outcomes according to the date of diagnosis (1980–2020) revealed that the 6-month mortality rate reduced from 23% to 13% over the last 40 years (7, 17, 18). To date, patients treated for AL amyloidosis display a median survival rate ranging from 0.4 to 12 years (for Mayo stages I and IIIb, respectively), which strongly depends on the disease stage at diagnosis, thus opening new perspectives on the long-term AEs of hematological treatment (19, 20).
Treatment for AL amyloidosis relied on melphalan and steroids until the 1990s, when the combination of melphalan and autologous hematopoietic stem cell transplantation (ASCT) was introduced, emulating MM therapy with adaptations in terms of dose and schedule. Drug agents directed to specific molecular targets became available after 2,000, including immunomodulatory drugs (IMiDs) and proteasome inhibitors (PIs) (3). Daratumumab, a novel human IgG1κ monoclonal antibody (mAb) targeting CD38 on plasma cells, has been recently introduced, while antibodies directed against amyloid fibrils are under evaluation (21).
AL amyloidosis patients are primarily evaluated to assess if they meet eligibility criteria for ASCT (approximately 20%–25%) (22). High-dose melphalan (200 mg/m2) is recommended before ASCT, but patients with a plasma cell burden >10% may benefit from an induction therapy with chemotherapy regimens (23). Current guidelines do not recommend a maintenance therapy after transplantation, but high-dose melphalan may be considered as consolidation treatment in those not achieving complete hematological response (24).
Daratumumab-CyBorD (cyclophosphamide, bortezomib, dexamethasone) is the preferred regimen among AL amyloidosis patients ineligible for ASCT. When daratumumab is not available, a bortezomib-based regimen is recommended, such as CyBorD or BMDex (bortezomib-melphalan-dexamethasone). CyBorD may be considered as the first-line therapy, because its administration is more feasible and indicated even in patients with moderate or severe impairment of renal function (13). At least 2 cycles after the complete hematological response (normalization of FLC ratio) are recommended, while treatment can be prolonged to 6–8 cycles in patients with at least a very good partial response (differential FLC <40 mg/L) after 3 cycles (25). The drug regimen and intensity should be based on the disease and the overall status of patients. For example, patients with Mayo cardiac stage IIIb should receive dose-modified daratumumab-CyBorD or single-agent daratumumab or alternative dose-modified CyBorD or BMDex. In patients not achieving complete or very good partial response after 2–3 cycles, therapy should be reconsidered. Patients with relapsed or refractory AL amyloidosis may be treated with first- and second-generation PIs or IMiDs at lower doses than for MM, mAb, or bendamustine (mainly in IgM related AL amyloidosis with lymphoid component in the bone marrow) (25).
The definition of CV toxicity has changed over the years. The recent evolution of cardio-oncology has led to assessing CTR-CT with more rigid criteria, as reported by the European Society of Cardiology (ESC) guidelines (Table 1) (26). The risk of CTR-CT should be established before the administration of cancer therapy (6, 27, 28). Clinical response to cancer therapy is highly variable and the risk of developing CTR-CT during or after treatment depends on several factors: age, sex, genetics, history of CV disease or cancer or cardiotoxic therapies, anomalies of cardiac biomarkers or ECG or transthoracic echocardiogram, lifestyle risk factors and their treatment (29). Only a few risk scores have been validated and the gold standard is currently represented by the Heart Failure Association of the ESC in collaboration with the International Cardio-Oncology Society (HFA-ICOS) baseline risk stratification (6, 28, 30). Patients selected for cancer therapy can be stratified into low, moderate, high and very high risk. Interestingly, patients with a diagnosis of AL and cardiac infiltration are classified as very high risk patients independently from other conditions (6). Therefore, patients with AL-CA are recommended for cardiology referral (Class I, Level C) before the administration of cancer therapy. Specialists are invited to perform a multidisciplinary discussion about the risk-benefit ratio of cancer therapy (Class I, Level C) and to evaluate potential cardioprotective strategies (Class IIa) (Figure 2) (6).
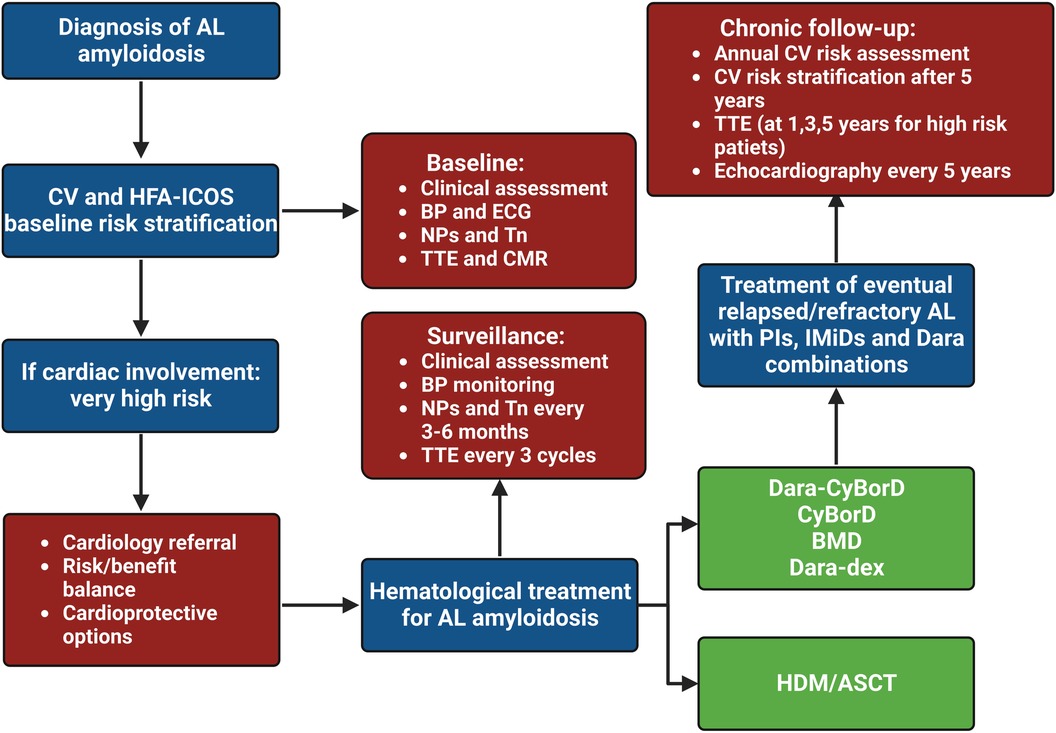
Figure 2. Clinical flow chart of cardio-oncological follow-up in patients with light chain amyloidosis. HDM/ASCT, high dose melphalan followed by autologous stem cell transplant; AL, light chain amyloidosis; BMD, bortezomib-melphalan-dexamethasone; BP, blood pressure; CyBorD, cyclophosphamide-bortezomib-dexamethasone; CV, cardiovascular, Dara, daratumumab; dex, dexamethasone; ECG, electrocardiogram; IMiD, immunomodulatory drug; NP, natriuretic peptide; PI, proteasome inhibitor; Tn, troponin; TTE, transthoracic echocardiogram.
Collecting baseline ECG, plasma cardiac biomarkers, imaging tests, medical history and clinical examination can furtherly help physicians to delineate the personalized CTR-CT risk profile of patients (Class I, Level C). Indeed, primary and secondary prevention (management of pre-existing CV disease) strategies should be pursued to reduce the risk of future CV disease (28, 31, 32).
Evidence from several studies suggests that a healthy lifestyle, such as not smoking, maintaining physical activity, a healthy diet (high intake of vegetables, fruits and whole grains), moderate consumption of alcohol, and modest sleep duration, results in a decreased risk of transition from cancer to subsequent CV disease (33). Moreover, adult patients at high and very high risk selected for cancer therapy, such as those with AL amyloidosis, may be treated with cardioprotective drugs including statins (Class IIa, Level B) and angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers plus beta-blockers (Class IIa, Level C) for primary CV prevention (6, 34). Finally, among patients with AL amyloidosis, an echocardiogram is recommended before starting cancer therapy (Class I, Level C) to assess left and right ventricular function, dilation, hypertrophy, wall motion abnormalities and other parameters which may influence the therapeutic strategy (6, 35).
CV monitoring for patients with AL-CA during hematological treatment consists of clinical assessment, with blood pressure monitoring, natriuretic peptides (NPs) and troponins (Tn) measurement every 3–6 months (Class I) (6). The routine measurement of cardiac biomarkers is crucial not only to detect CV toxicities but also to monitor the cardiac response (defined as reduction of N-terminal pro-B-type natriuretic peptide [NT-proBNP] values >30% and >300 ng/L if baseline levels >650 ng/L or New York Heart Association [NYHA] class response ≥2 if baseline class 3 or 4) to chemotherapy (36). Indeed, a BNP reduction to ≤200 ng/L during therapy is a significant predictor of improved survival in patients with AL-CA, while a >30% or >300 ng/L elevation in NT-proBNP or >33% increase in Tn reflect the progression of cardiac impairment (36, 37). Since cardiac involvement is the main predictor of mortality among AL patients, staging systems focus on parameters of heart damage. Cardiac biomarkers were also included in the most recent Mayo 2012 staging (I–IV) scoring system (TnT ≥0.025 ng/ml or high-sensitivity TnT ≥40 ng/L, NT-proBNP ≥1,800 pg/ml, and differential FLC ≥18 mg/dl) to characterize prognosis of patients with AL-CA and to properly administer risk-adapted therapies (38).
AL amyloidosis patients should also perform individual blood pressure home monitoring and an echocardiogram at the end of every 3 cycles (or every 3–6 months in patients treated with PIs) (Class IIa) (39). Finally, a specialist CV assessment is recommended for patients who exhibit new CV toxicity during and after cancer treatment (Class I, Level C) (6).
Physicians should periodically evaluate the CV toxicity risk profile of cancer survivors at the end of therapy (and 5 years after treatment in asymptomatic patients), according to recent ESC guidelines, to properly establish a follow-up plan (Class I, Level C). As reported in Table 2, stratification ranges from low to very high and divides early (<5 years) and late (>30 years) risks from completing cancer therapy (6). Asymptomatic patients should undergo an annual CV risk assessment, by collecting ECG, NPs and clinical history (Class I, Level B), while echocardiography should be performed every 5 years (or at 1, 3 and 5 years for patients at high or very high risk) (Class IIa/IIb, Level C) (6, 40). Finally, patients treated with chemo- or immunotherapy regimens may develop various CV diseases (HF, arrhythmias, pericardial disease, valvular heart disease, acute coronary syndrome, vascular disease and metabolic syndrome) which need to be treated according to relative guidelines (6).
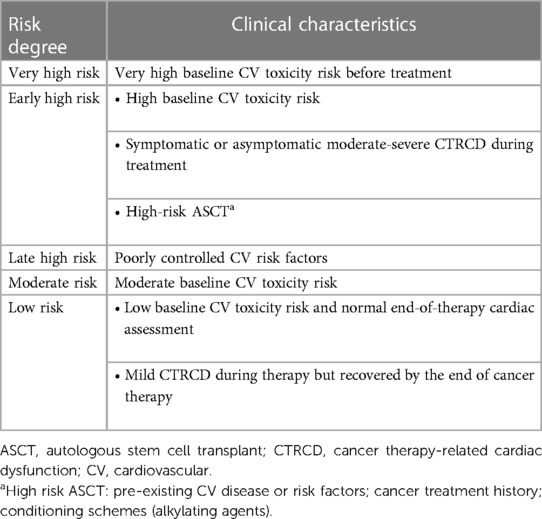
Table 2. Risk stratification for adults with light chain amyloidosis after cancer therapy (6).
Cardioprotective therapy consisting of an angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker and a beta-blocker, preferably carvedilol, is recommended (Class IIa) for secondary prevention of both symptomatic and asymptomatic cancer patients developing cardiac dysfunction [defined as a reduction of left ventricular ejection fraction (LVEF) ≥ 10%] according to guidelines for HF (6, 41, 42). Hypertension is another common complication of oncological treatment and, if poorly controlled despite the previously mentioned drugs, it can be treated with amlodipine or aldosterone inhibitors, avoiding negative inotropic agents (42).
In particular, patients with AL-CA amyloidosis require a supportive treatment to contrast the effects of amyloid infiltration in addition to the possible CTR-CT (4). For instance, the treatment of cardiac amyloidosis is mainly based on the management of symptoms related to HF, with loop diuretics (particularly torsemide and bumetanide), possibly coupled with mineralocorticoid receptor antagonists, being the cornerstone of therapy (41). Neuro-hormonal antagonists may be poorly tolerated in patients with AL-CA due to hypotension, conduction disorders or kidney disease (43). The management of arrhythmias and conduction disorders is complex as well. Patients with AL-CA frequently display atrial fibrillation, but catheter ablation is arduous due to the extensive atrial infiltration, with high rate of recurrence. Amiodarone and dofetilide are the preferred antiarrhythmic agents, because they do not exert negative inotropic effect (4). Moreover, anticoagulation must be prescribed to patients with atrial fibrillation regardless of the CHA2DS2-VASc score (44, 45). Although there is no evidence to support anticoagulation in patients with AL amyloidosis and sinus rhythm, patients with hematological malignancies show increased risk of thromboembolic events (45). For this reason, the use of aspirin or low molecular weight heparin is recommended in patients with MM receiving IMiDs or in those at high risk of thromboembolism (6, 46).
Only a limited number of studies have investigated the toxic effects of cancer therapy in AL amyloidosis. Therefore, most of the findings are based on studies conducted on patients with MM. Furthermore, the trials have used different definitions of CV toxicity, and patients with severe pre-existing comorbidities or CV disease were frequently excluded (47). In the case of AL amyloidosis, CTR-CT primarily includes cardiac dysfunction, arrhythmias, arterial vascular disease, venous thrombo-embolism and systemic hypertension, as summarized in Table 3 (48).
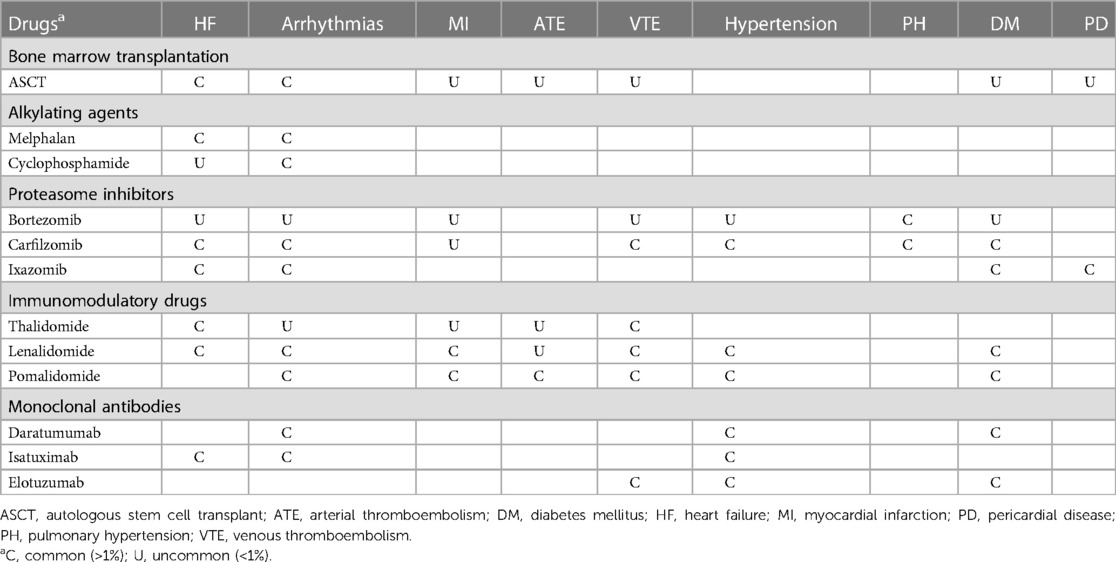
Table 3. Clinical manifestations of cancer therapy-related cardiovascular toxicity in light chain amyloidosis (49, 50).
The increased awareness of ASCT-related toxicity as well as the growing population of transplanted survivors are an important source of concern. It is estimated that approximately two-thirds of ASCT survivors develop at least one long-term toxic effect (51), and transplanted patients after 5-year survival have a 4–9-fold higher mortality rate than the general population for 30 years (52). CV adverse events following ASCT are not common (<10%), but significantly impact on survival and quality of life (53).
Short-term CV adverse events (i.e., within 100 days) are relatively rare (0.9%–8.9%) and mainly include arrhythmias (2%–10%), congestive HF (0.4%–2.2%), and rarely myocardial infarction and pericardial effusion (54–56). Long-term toxicities consist of development of ischemic heart disease, HF, diabetes mellitus, stroke, hypertension and vascular disease (57). An observational study which involved 1,244 patients undergoing autologous transplant for a hematologic malignancy reported that the cumulative incidence of congestive HF was 4.8% after 5 years and raised to 9.1% after 15 years from ASCT (58).
The only trial comparing ASCT with a chemotherapy regimen (melphalan-dexamethasone) did not show a significant prognostic benefit from ASCT. Indeed, this small phase III study (n = 100) reported an ASCT-related survival rate at 3 years of 58% compared to 80% in the group treated only with chemotherapy (p = 0.13) (59). In a retrospective analysis comparing AL amyloidosis patients (n = 241) treated with ASCT vs. Mayo stage-matched non-transplanted controls, 49% of transplanted patients showed any-grade cardiac arrhythmia (60). Moreover, a study demonstrated that AL patients (n = 101) undergoing peripheral blood hematopoietic stem cell mobilization before ASCT had a significant risk of developing at least one AE, such as cardiac (atrial or ventricular arrhythmias, ischemic heart disease, acute HF, and hypertensive emergencies: 7%) and thromboembolic events (deep venous thrombosis and pulmonary embolism: 5%), probably due to previous growth factors administration-related release of proinflammatory cytokines (61).
Alkylating agents inhibit the transcription and replication of DNA through alkylation, which impairs cell replication (3). Although alkylating agents are non-selective drugs associated with various AEs (mainly cytopenia and gastrointestinal toxicities), their administration combined with corticosteroids is a safe and tolerable option, but considered suboptimal (25).
Melphalan is not directly associated with CTR-CT in the context of AL amyloidosis, but treatment with ASCT and high-dose melphalan may cause atrial fibrillation and supraventricular arrhythmia, as suggested by clinical studies including patients with MM (62, 63). Among patients not eligible for transplantation, treatment with melphalan-dexamethasone (MelDex) results in significant hematological response but it is more effective when combined with bortezomib (BMDex) (64). An observational study comparing MelDex and BMDex in newly diagnosed AL patients (n = 109) demonstrated that treatment with melphalan is safe and well tolerated, but the addition of bortezomib was associated with a 2-fold increase in severe AEs (64).
Cyclophosphamide is a prodrug which is converted into active metabolites by cytochrome P450-2B6. Metabolites are likely to be involved in CTR-CT through various mechanisms, involving oxidative and nitrative stress, calcium imbalance, and formation of protein adducts leading to inflammation, apoptosis and dysregulation of signalling pathways (e.g., NFκB/p53/p38 MAPKs) (65). Cyclophosphamide is commonly used in MM ahead of bone marrow transplantation, but its significant CV toxicity is a contraindication for patients with AL amyloidosis (61). Indeed, patients undergoing cyclophosphamide show an increased risk of developing HF (7%–33% with a dose >150 mg/kg) (66, 67). Metabolites of cyclophosphamide may cause endothelial damage, edema, haemorrhage and thrombosis, thus resulting in myo(peri)carditis, pericardial effusion, pulmonary hypertension and acute coronary syndrome (6). In patients with advanced age and previous radiation or cardiotoxic treatment, high-dose cyclophosphamide correlates with an enhanced risk of CTR-CT (9). However, in the setting of AL amyloidosis, low-dose cyclophosphamide (500 mg per os) in CyBorD exhibits a good safety profile (68).
PIs tremendously improved the outcomes of patients with either newly diagnosed or relapsed/refractory AL amyloidosis (69). However, PIs administration is associated with an increased risk of systemic and pulmonary hypertension, HF, acute coronary syndrome, venous thromboembolism and cardiac arrhythmias (47, 70, 71). Interestingly, patients with MM receiving PIs plus IMiDs have an increased risk of thromboembolic events (both venous and arterial) compared with other therapies (hazard ratio [HR]: 1.31, confidence interval [CI]: 1.03–1.67) or PIs or IMiDs alone (HR: 1.37, 95% CI: 1.02–1.86) (72).
CTR-CT may be due to the induction of oxidative stress and endothelial dysfunction caused by the accumulation of proteinaceous species within CV tissues (73). Cardiomyocytes are susceptible to proteasome inhibition being non-proliferative cells. Therefore, proteolytic degradation represents a crucial strategy to reduce apoptosis associated with endoplasmic reticulum stress and to ensure long-term survival (74). Moreover, PIs may induce dysregulation of several intracellular pathways, leading to the activation of NFκB, impairment of endothelial nitric oxide synthase, mitochondrial dysfunction and fibrosis (47).
Bortezomib is a first-generation PI which reversibly binds to the β5 proteasome subunit. Bortezomib has revolutionized the treatment of AL amyloidosis, and it is currently included in the CyBorD regimen as first-line therapy for patients non-eligible for ASCT (3). Carriers of the t(11;14) mutation show a worse response to bortezomib-based schemes (75). Hypertension is a common (1%–10%) AE associated with bortezomib, followed by uncommon (0.1%–1%) toxicities, such as hyperglycemia or diabetes mellitus, HF, atrial fibrillation and venous thromboembolism; while myocardial infarction and pulmonary hypertension are even rarer (<0.1%) (6). Atrial arrhythmias may be more frequent following intravenous injection compared to subcutaneous bortezomib (76).
In a phase I/II trial on patients (n = 31) with relapsed AL amyloidosis, those treated with bortezomib twice-weekly had an increased risk of cardiac dysfunction (23%), defined as >10% reduction in LVEF, compared to patients treated weekly (9, 77). Bortezomib has been associated with HF, complete heart block and ischemic heart disease, but studies on larger cohorts failed to demonstrate these associations (78–84). In a metanalysis of 25 clinical trials on various malignancies (MM, lymphoma, non-small-cell lung cancer, Waldenström's macroglobulinemia and ovarian cancer), treatment with bortezomib was not associated with a significant risk of CV toxicity (HR: 1.14, 95% CI: 0.82–1.62) compared to control medications, with a mortality rate related to CTR-CT of 3%. However, patients with MM had a greater incidence of cardiotoxicity (decline in LVEF, HF, cardiac arrest and arrhythmias) compared to other subgroups (4.3% vs. 2.3%). Interestingly, bortezomib monotherapy resulted in a higher frequency of CTR-CT compared to bortezomib combinations (85). A retrospective analysis of phase II and III trials involving MM patients treated with bortezomib confirmed that bortezomib does not significantly increase the risk of cardiotoxicity (86). Peripheral neuropathy is a common reason for bortezomib discontinuation and patients with lung disease should be monitored because of a small risk of pulmonary toxicity (87).
Carfilzomib is a second-generation PI which irreversibly binds to the β5 subunit, resulting in a more sustained inhibition of proteasome than bortezomib. Carfilzomib shows an increased efficacy and toxicity compared to other PI (3). Hypertension is a very common (≥10% incidence) AE associated with carfilzomib, while hyperglycemia or diabetes mellitus, HF, atrial fibrillation, venous thromboembolism and pulmonary hypertension are classified as common toxicities; myocardial infarction is uncommon (6).
CV and renal toxicity often contraindicate carfilzomib in AL amyloidosis. In a phase I/II study conducted on relapsed or refractory AL patients (n = 28) with non-severe disease (MAYO stage ≤2) receiving 8 cycles of carfilzomib during 16 months, the regimen was effective and feasible but cardiac and renal toxicities were common, requiring cautious monitoring (88).
Carfilzomib may induce CTR-CT in a significant proportion of patients. Among 526 patients with relapsed or refractory MM, those treated with carfilzomib had a significant risk of developing cardiac AEs (22%), such as HF (7.2%), arrhythmias (13.3%), and ischemic heart disease (3%) (89). In a real-life study conducted on patients (n = 22) treated with previous lines of MM therapy, 23% showed symptoms of HF (90). Another real-world analysis of carfilzomib-related CV adverse events in patients with MM (n = 7,330 subjects with 815 on carfilzomib) reported an increased risk of HF, ischemic heart disease and hypertension (all p < 0.001) than non-users. In the same study, pulmonary hypertension was diagnosed in approximately 1% of patients administered with carfilzomib (91).
The phase III ASPIRE trial, which compared the effects of carfilzomib with lenalidomide and dexamethasone to lenalidomide and dexamethasone alone in patients (n = 792) with relapsed MM, found that the carfilzomib group had better progression-free survival but enhanced frequency of CTR-CT (92). A meta-analysis of 24 studies involving patients with MM confirmed that carfilzomib is associated with a high risk of CV adverse events (18%) in a dose-dependent manner. In particular, HF (4.1%) and hypertension (12.2%) were the most frequent AEs, compared to arrhythmias (2.4%) and ischemic heart disease (1.8%). Dyspnea (23.9%) and peripheral edema (24.7%) were frequently detected (93). A recent systematic review including 45 studies on patients with MM treated with carfilzomib-based regimens reported that the incidence of HF, peripheral edema, hypertension and ischemic heart disease was 5.1%, 20.7%, 13.2% and 4.6%, respectively. In addition, no differences in terms of toxicity were detected when carfilzomib was used as a single agent vs. combination therapy (94). Carfilzomib may also cause significant corrected QT interval prolongation compared with other PIs, thus increasing the risk of ventricular arrhythmias and sudden cardiac death (95).
In the observational PROTECT study, patients with relapsed MM treated with carfilzomib and bortezomib (n = 95) were monitored for CTR-CT through cardiac biomarkers and echocardiography for 18 months. The reported AEs were similar to other studies, but the majority were transient, and treatment discontinuation was often not required (71). Finally, another real-life study comparing carfilzomib-dexamethasone vs. carfilzomib-lenalidomide-dexamethasone in MM patients (n = 109) reported that the former regimen was associated with an increased risk of CV events. However, the association was not significant after adjusting for confounding factors, suggesting that the enhanced CV toxicity in patients under the carfilzomib-dexamethasone scheme was related to their greater frailty (96). Finally, some reports highlight the association between carfilzomib and pulmonary hypertension in MM, probably due to the less vasodilator effect mediated by nitric oxide (97).
Ixazomib is a second-generation PI with oral bioavailability and poor neurotoxic effects compared to bortezomib. Like bortezomib, ixazomib reversibly binds to the β5 proteasome subunit, with additional inhibition of β1 and β2 subunits at higher doses (3, 98). Ixazomib frequently results in peripheral edema (up to 18%) and hyperglycaemia when combined with lenalidomide or pomalidomide and dexamethasone (6).
In a phase I/II study including relapsed or refractory AL amyloidosis patients (n = 27), treatment with ixazomib was associated with a significant risk of NYHA class 3 dyspnea and fatigue (15%), but atrial fibrillation, congestive HF and pleural effusion were also common findings (7%), with 10% of patients manifesting NYHA class 3 HF (99). The phase III TOURMALINE-AL1 trial, comparing ixazomib plus dexamethasone to the treatment of choice (dexamethasone with/without melphalan, cyclophosphamide, lenalidomide or thalidomide) in patients with relapsed or refractory AL amyloidosis (n = 168), demonstrated that ixazomib-dexamethasone was well tolerated, with a similar safety profile between the two groups. Patients treated with ixazomib showed an increased risk of cardiac arrhythmias (26% vs. 15%), but the Authors assessed that the difference was mainly due to the greater frequency of non-drug-related atrial fibrillation (8% vs. 1%) (100). Although the study did not meet the primary endpoints of hematologic response, further analysis revealed that ixazomib administration is associated with similar or trended better quality of life and symptoms manifestation, compared with control regimens (101). In a phase I/II study, ixazomib-cyclophosphamide-dexamethasone was safe and well tolerated in patients with a new diagnosis of AL amyloidosis (102).
In the phase III TOURMALINE-MM1 trial, which evaluated ixazomib vs. placebo in combination with lenalidomide and dexamethasone in patients with relapsed or refractory MM (n = 722), CV adverse events (hypertension, HF, arrhythmias and ischemic heart disease) were not significantly different between the two arms. An important limitation of the study refers to the exclusion of patients with CV symptoms (hypertension, HF, unstable angina or ischemic heart disease) in the 6 months preceding the trial (103). A pharmacovigilance study comparing ixazomib with other approved drugs for MM reported an increased risk of atrial fibrillation (HR: 1.9, 95% CI: 1.5–2.3) among ixazomib users (104).
IMiDs refer to a group of compounds that includes thalidomide and its derivatives (lenalidomide and pomalidomide). The anti-cancer mechanisms of IMiDs rely on direct cytotoxicity and indirect modulation of the neoplasm microenvironment, with effective antiangiogenic and immunostimulatory features (3). IMiDs carry a black box warning for fetal malformations, risk in pregnancy, hemotoxicity, arterial and venous thromboembolic events (48). For this reason, patients are usually recommended to thromboprophylaxis with aspirin or anticoagulants (105).
Treatment with IMiDs usually exhibits poor response in patients with AL amyloidosis, but represents an important element of combination therapy (25). Thalidomide and pomalidomide are also associated with sinus bradycardia (106). Finally, a network metanalysis demonstrated that the incidence of AEs is greater among patients treated with combined IMiDs and PIs, compared to IMiDs alone, despite the difference was not significant (HR: 1.47; 95% CI: 1.19–1.82) (107).
Moreover, therapy with lenalidomide and pomalidomide may result in transient NPs elevation and worsening of renal function, thus requiring cautious monitoring (108, 109). Patients treated with IMiDs drugs often show a discrepancy between haematologic and/or cardiac response and adverse NPs elevation, which is probably due to a chemotherapy-related CV effect or fluid retention (110). In a study including AL patients (n = 68) treated with lenalidomide and dexamethasone, 86% of subjects experienced a >30% increase in BNP levels after therapy (111). Another study (n = 106) reported that treatment with lenalidomide or pomalidomide was associated with higher risk of NT-proBNP increase compared with MelDex regimen (58% vs. 29%), but there was no association between FLC and NT-proBNP response (112).
IMiDs bind to cereblon, which is the substrate receptor of a component of the E3 ubiquitin ligase, that triggers proteasome-mediated degradation of the IKZF1 and IKZF3 transcription factors, resulting in increased production of interleukin-2 and other cytokines known to modulate T cell activity (113, 114). It has to be fully understood if these biochemical pathways are linked with CV toxicity, but IMiDs are generally believed to cause damage to endothelial cells, enhanced platelet aggregation, and higher von Willebrand factor levels (115).
Thalidomide is a glutamic acid derivate largely used as an antiemetic for sickness during pregnancy until the 1960s, when its teratogenic potential was recognized, and the drug was withdrawn (3). The discovery of thalidomide's immunomodulatory and anti-angiogenetic effects led to its re-evaluation as an anti-cancer therapy (116). Thalidomide is associated with a high risk of neurological and gastrointestinal toxicity. Despite some promising results in terms of hematologic and organ response, the significant toxicities contraindicate its utilization in patients with AL amyloidosis (25, 117). Thalidomide therapy commonly results in HF and venous thromboembolism, while atrial fibrillation, myocardial infarction and arterial thromboembolism are uncommon findings (6).
Lenalidomide is generally effective, especially in combined therapies, but is poorly tolerated at the full 25 mg daily dose in patients with AL amyloidosis. Therefore, a reduced starting dose (5 mg) and closer monitoring are recommended (3, 6, 25). Notably, lenalidomide requires a dose adjustment based on renal status, since it is excreted through urine (3). Venous thromboembolism is a very common finding among patients treated with lenalidomide, as suggested by a trial evaluating lenalidomide plus high-dose dexamethasone vs. lenalidomide plus low dexamethasone in patients with a new diagnosis of MM (n = 445) (118). Hypertension, hyperglycemia, HF, atrial fibrillation and myocardial infarction are also common AEs (6). Arterial thromboembolism is uncommon. In a long-term follow-up of relapsed or refractory MM patients (n = 704) included in phase III trials, the risk of myocardial infarction and cerebrovascular events was higher in patients treated with lenalidomide-dexamethasone compared with placebo-dexamethasone (1.98% and 3.4% vs. 0.57% and 1.7%, respectively) (119).
The combination of PIs, lenalidomide, and steroids (lenalidomide-bortezomib-dexamethasone or ixazomib-lenalidomide-dexamethasone) often led to a hematological response, but the regimen was not well tolerated, leading to a high rate of therapy discontinuation despite low-dose lenalidomide (120, 121). Indeed, patients manifested a high risk of AEs, such as fluid overload (33.3%), arrhythmias (13.3%), and renal dysfunction (6.6%) (121). Lenalidomide is not significantly associated with peripheral neuropathy in AL amyloidosis patients, thus lenalidomide-based schemes may be considered for patients with neurologic involvement (25).
Pomalidomide is a third-generation IMiD which shows a safer renal profile and better tolerability compared to lenalidomide (3). Hypertension, atrial fibrillation, venous and arterial thromboembolism, and myocardial infarction are common findings (6). In a phase I/II trial including patients (n = 27) previously treated for AL amyloidosis, no thromboembolic events were registered, but kidney dysfunction and severe fatigue occurred in 26% and 18%, respectively (122). In a large retrospective series of relapsed or refractory AL amyloidosis patients (n = 153) treated with pomalidomide-dexamethasone, 33% of subjects showed severe AEs, including HF (7%), atrial fibrillation, suspected transient ischemic attack and atrial sinus block (with subsequent pacemaker implantation). Moreover, 19% of patients needed a dose reduction due to mild cytopenia (123).
Daratumumab causes cell death through several mechanisms, including complement-dependent cytotoxicity, antibody-dependent cellular phagocytosis, and antibody-dependent cell-mediated cytotoxicity, as well as direct cellular apoptosis (124). Hypertension and atrial fibrillation are common AEs (23).
The phase III ANDROMEDA trial included newly diagnosed patients with AL amyloidosis (n = 388) comparing traditional treatment with CyBorD vs. CyBorD plus subcutaneous daratumumab followed by daratumumab monotherapy for up to 24 cycles. The trial demonstrated the beneficial effects of daratumumab-CyBorD in terms of hematological response or survival free from major organ deterioration. The most common CV adverse events in the daratumumab group included cardiac failure (9.3% vs. 7.4%), syncope (5.2% vs. 6.4%), peripheral edema (3.1% vs. 5.9%), and hypokalemia (1.6% vs. 5.3%) (125). A recent metanalysis found that the most common severe AEs in AL amyloidosis are lymphocytopenia, HF (4%–13%), infection complications, pneumonia, fatigue (0%–9%), atrial fibrillation (0%–18%), neutropenia, and diarrhea (126).
Isatuximab is a chimeric IgG1κ mAb directed against CD38 targeting a different epitope compared to daratumumab, although both have proapoptotic effects with similar molecular mechanisms (21, 127). Isatuximab has a good hematological response rate and a good CV safety profile in relapsed disease (128). Isatuximab is being evaluated in a phase II trial (NCT03499808) in patients with relapsed or refractory AL and in a phase I trial (NCT04754945) in patients with high-risk AL amyloidosis. Hypertension is a very common AE in the context of MM therapy, whereas HF and atrial fibrillation are common findings (6). The main reasons for discontinuation were AEs in 26% of patients, including severe toxicities such as lymphopenia (9%), lung infection (6%), and an infusion-related reaction (3%) (129). Among patients with relapsed or refractory MM, the phase III IKEMA trial demonstrated that treatment with isatuximab plus carfilzomib-dexamethasone vs. carfilzomib-dexamethasone resulted in a similar rate of CV adverse events, including grade ≥3 hypertension (20%), HF (7%), and ischemic heart disease (5%) (130).
Elotuzumab is a humanized IgG1κ mAb targeting the signaling lymphocytic activation molecule family member F7 (SLAMF7) glycoprotein, normally expressed by plasma cells and amplified in patients with MM. Elotuzumab mainly acts through direct activation of NK cells and antibody-dependent cell-mediated cytotoxicity through the CD16 pathway (3). Hyperglicemia or diabetes are very common consequences of elotuzumab therapy; hypertension and venous thromboembolism are other common AEs (6).
A phase II trial (NCT03252600) is evaluating elotuzumab plus lenalidomide-dexamethasone with or without cyclophosphamide in relapsed AL amyloidosis. Small case series suggest that elotuzumab-lenalidomide-dexamethasone may prove safe and effective in previously treated AL patients (131). In the phase 3 ELOQUENT-2 trial, elotuzumab added to lenalidomide-dexamethasone in patients with relapsed or refractory MM was not associated with CV toxicities, and patients had a decreased risk of hypertension compared to those receiving lenalidomide-dexamethasone alone (1.3% vs. 2.2%) (132).
Cardio-oncology represents a novel multidisciplinary field, which is the result of both advancements in cancer therapy and improvement in the prognosis of cancer survivors. Patients with AL amyloidosis require hematological treatment to eradicate the abnormal plasma cell clones through chemo-immunotherapy regimens usually administered in MM therapy. Despite the beneficial effects in terms of prognosis and organ response, cancer therapy may lead to CV toxicities and severe AEs, such as HF, arrhythmias and vascular disease. Optimal personalized care involving close collaboration between both cardiology and oncology specialists should be performed to prevent and limit complications. After a diagnosis of AL amyloidosis, patients should undergo a rigorous evaluation to determine the feasibility and risk of ASCT, which unfortunately can be performed in a small minority of subjects. Patients not eligible for transplant are preferentially treated with daratumumab-CyBorD, which represents the most effective and safe strategy. Regimens including other PIs, such as carfilzomib, and IMiDs, such as lenalidomide, may prove useful in terms of organ response, especially among patients where first-line options are not feasible, but CV adverse events need to be cautiously monitored.
Considering that CV toxicity may compromise therapy due to interruption of hematological treatment, a baseline risk stratification assessment is crucial before starting with therapy. Patients with AL amyloidosis and cardiac involvement are considered at very high CV risk because of the cardiac amyloid infiltration, thus requiring close cardiologic monitoring (every 3–6 months). On the other side, a periodical CV risk assessment after therapy should be performed in order to both monitor AL disease progression and prevent the onset of long-term therapy-related toxicities.
Over the last decades, the prognosis of patients with AL amyloidosis has improved significantly, highlighting the importance of cardiac protection as well as the prevention of CTR-CT. Finally, the understanding of the biochemical basis of CV toxicity may lead to the implementation of selective agents which counteract the toxic effects of traditional regimens. Future research is warranted also to elaborate and validate novel prevention and surveillance options capable of attenuating therapy-related CV toxicity in the setting of AL amyloidosis.
PM, AA, and IF researched data for the article. PM, AA, VC, and IF contributed substantially to discussion of the content. PM, AA, VC, and IF wrote the article. AA, VC, MC, GV, CC, AF, ME, IF, and DC reviewed and/or edited the manuscript before submission. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Merlini G, Dispenzieri A, Sanchorawala V, Schönland SO, Palladini G, Hawkins PN, et al. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primer. (2018) 4(1):38. doi: 10.1038/s41572-018-0034-3
2. Wechalekar AD, Fontana M, Quarta CC, Liedtke M. AL amyloidosis for cardiologists. JACC CardioOncology. (2022) 4(4):427–41. doi: 10.1016/j.jaccao.2022.08.009
3. Bianchi G, Zhang Y, Comenzo RL. AL amyloidosis: current chemotherapy and immune therapy treatment strategies. JACC CardioOncology. (2021) 3(4):467–87. doi: 10.1016/j.jaccao.2021.09.003
4. Aimo A, Buda G, Fontana M, Barison A, Vergaro G, Emdin M, et al. Therapies for cardiac light chain amyloidosis: an update. Int J Cardiol. (2018) 271:152–60. doi: 10.1016/j.ijcard.2018.05.018
5. Bringhen S, Milan A, Ferri C, Wäsch R, Gay F, Larocca A, et al. Cardiovascular adverse events in modern myeloma therapy—incidence and risks. A review from the European myeloma network (EMN) and Italian society of arterial hypertension (SIIA). Haematologica. (2018) 103(9):1422–32. doi: 10.3324/haematol.2018.191288
6. Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European hematology association (EHA), the European society for therapeutic radiology and oncology (ESTRO) and the international cardio-oncology society (IC-OS). Eur Heart J. (2022) 43(41):4229–361. doi: 10.1093/eurheartj/ehac244
7. Kumar N, Zhang NJ, Cherepanov D, Romanus D, Hughes M, Faller DV. Global epidemiology of amyloid light-chain amyloidosis. Orphanet J Rare Dis. (2022) 17(1):278. doi: 10.1186/s13023-022-02414-6
8. Kyle RA, Larson DR, Therneau TM, Dispenzieri A, Kumar S, Cerhan JR, et al. Long-term follow-up of monoclonal gammopathy of undetermined significance. N Engl J Med. (2018) 378(3):241–9. doi: 10.1056/NEJMoa1709974
9. Kittleson MM, Ruberg FL, Ambardekar AV, Brannagan TH, Cheng RK, Clarke JO, et al. 2023 ACC expert consensus decision pathway on comprehensive multidisciplinary care for the patient with cardiac amyloidosis. J Am Coll Cardiol. (2023) 81(11):1076–126. doi: 10.1016/j.jacc.2022.11.022
10. Gertz MA, Dispenzieri A. Systemic amyloidosis recognition, prognosis, and therapy: a systematic review. JAMA. (2020) 324(1):79. doi: 10.1001/jama.2020.5493
11. Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Epidemiology of AL amyloidosis: a real-world study using US claims data. Blood Adv. (2018) 2(10):1046–53. doi: 10.1182/bloodadvances.2018016402
12. Boldrini M, Cappelli F, Chacko L, Restrepo-Cordoba MA, Lopez-Sainz A, Giannoni A, et al. Multiparametric echocardiography scores for the diagnosis of cardiac amyloidosis. JACC Cardiovasc Imaging. (2020) 13(4):909–20. doi: 10.1016/j.jcmg.2019.10.011
13. Wechalekar AD, Schonland SO, Kastritis E, Gillmore JD, Dimopoulos MA, Lane T, et al. A European collaborative study of treatment outcomes in 346 patients with cardiac stage III AL amyloidosis. Blood. (2013) 121(17):3420–7. doi: 10.1182/blood-2012-12-473066
14. Kyle RA, Greipp PR. Amyloidosis (AL). clinical and laboratory features in 229 cases. Mayo Clin Proc. (1983) 58(10):665–83.6353084
15. Skinner M, Anderson JJ, Simms R, Falk R, Wang M, Libbey CA, et al. Treatment of 100 patients with primary amyloidosis: a randomized trial of melphalan, prednisone, and colchicine versus colchicine only. Am J Med. (1996) 100(3):290–8. doi: 10.1016/S0002-9343(97)89487-9
16. Dubrey S. The clinical features of immunoglobulin light-chain (AL) amyloidosis with heart involvement. QJM. (1998) 91(2):141–57. doi: 10.1093/qjmed/91.2.141
17. Staron A, Zheng L, Doros G, Connors LH, Mendelson LM, Joshi T, et al. Marked progress in AL amyloidosis survival: a 40-year longitudinal natural history study. Blood Cancer J. (2021) 11(8):139. doi: 10.1038/s41408-021-00529-w
18. Muchtar E, Gertz MA, Kumar SK, Lacy MQ, Dingli D, Buadi FK, et al. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: cracking the glass ceiling of early death. Blood. (2017) 129(15):2111–9. doi: 10.1182/blood-2016-11-751628
19. Abdallah N, Dispenzieri A, Muchtar E, Buadi FK, Kapoor P, Lacy MQ, et al. Prognostic restaging after treatment initiation in patients with AL amyloidosis. Blood Adv. (2021) 5(4):1029–36. doi: 10.1182/bloodadvances.2020003782
20. Baker KR. Light chain amyloidosis: epidemiology, staging, and prognostication. Methodist DeBakey Cardiovasc J. (2022) 18(2):27–35. doi: 10.14797/mdcvj.1070
21. Popkova T, Hajek R, Jelinek T. Monoclonal antibodies in the treatment of AL amyloidosis: co-targetting the plasma cell clone and amyloid deposits. Br J Haematol. (2020) 189(2):228–38. doi: 10.1111/bjh.16436
22. Palladini G, Milani P, Merlini G. Management of AL amyloidosis in 2020. Blood. (2020) 136(23):2620–7. doi: 10.1182/blood.2020006913
23. Bomsztyk J, Khwaja J, Wechalekar AD. Recent guidelines for high-dose chemotherapy and autologous stem cell transplant for systemic AL amyloidosis: a practitioner’s perspective. Expert Rev Hematol. (2022) 15(9):781–8. doi: 10.1080/17474086.2022.2115353
24. Sanchorawala V, Boccadoro M, Gertz M, Hegenbart U, Kastritis E, Landau H, et al. Guidelines for high dose chemotherapy and stem cell transplantation for systemic AL amyloidosis: EHA-ISA working group guidelines. Amyloid. (2022) 29(1):1–7. doi: 10.1080/13506129.2021.2002841
25. Wechalekar AD, Cibeira MT, Gibbs SD, Jaccard A, Kumar S, Merlini G, et al. Guidelines for non-transplant chemotherapy for treatment of systemic AL amyloidosis: EHA-ISA working group. Amyloid. (2023) 30(1):3–17. doi: 10.1080/13506129.2022.2093635
26. Herrmann J, Lenihan D, Armenian S, Barac A, Blaes A, Cardinale D, et al. Defining cardiovascular toxicities of cancer therapies: an international cardio-oncology society (IC-OS) consensus statement. Eur Heart J. (2022) 43(4):280–99. doi: 10.1093/eurheartj/ehab674
27. Caro-Codón J, López-Fernández T, Álvarez-Ortega C, Zamora Auñón P, Rodríguez IR, Gómez Prieto P, et al. Cardiovascular risk factors during cancer treatment. Prevalence and prognostic relevance: insights from the CARDIOTOX registry. Eur J Prev Cardiol. (2022) 29(6):859–68. doi: 10.1093/eurjpc/zwaa034
28. Lyon AR, Dent S, Stanway S, Earl H, Brezden-Masley C, Cohen-Solal A, et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the cardio-oncology study group of the heart failure association of the European society of cardiology in collaboration with the international cardio-oncology society. Eur J Heart Fail. (2020) 22(11):1945–60. doi: 10.1002/ejhf.1920
29. Gilchrist SC, Barac A, Ades PA, Alfano CM, Franklin BA, Jones LW, et al. Cardio-oncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors: a scientific statement from the American heart association. Circulation. (2019) 139(21):e997–e1012. doi: 10.1161/CIR.0000000000000679
30. Battisti NML, Andres MS, Lee KA, Ramalingam S, Nash T, Mappouridou S, et al. Incidence of cardiotoxicity and validation of the heart failure association-international cardio-oncology society risk stratification tool in patients treated with trastuzumab for HER2-positive early breast cancer. Breast Cancer Res Treat. (2021) 188(1):149–63. doi: 10.1007/s10549-021-06192-w
31. Salem JE, Nguyen LS, Moslehi JJ, Ederhy S, Lebrun-Vignes B, Roden DM, et al. Anticancer drug-induced life-threatening ventricular arrhythmias: a world health organization pharmacovigilance study. Eur Heart J. (2021) 42(38):3915–28. doi: 10.1093/eurheartj/ehab362
32. Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. (2016) 133(11):1104–14. doi: 10.1161/CIRCULATIONAHA.115.020406
33. Michos ED, Marshall CH. Healthy lifestyle benefits both cancer and cardiovascular disease. JACC CardioOncology. (2021) 3(5):675–7. doi: 10.1016/j.jaccao.2021.11.002
34. Kim J, Nishimura Y, Kewcharoen J, Yess J. Statin use can attenuate the decline in left ventricular ejection fraction and the incidence of cardiomyopathy in cardiotoxic chemotherapy recipients: a systematic review and meta-analysis. J Clin Med. (2021) 10(16):3731. doi: 10.3390/jcm10163731
35. Čelutkienė J, Pudil R, López-Fernández T, Grapsa J, Nihoyannopoulos P, Bergler-Klein J,, et al. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: a position statement on behalf of the heart failure association (HFA), the European association of cardiovascular imaging (EACVI) and the cardio-oncology council of the European society of cardiology (ESC). Eur J Heart Fail. (2020) 22(9):1504–24. doi: 10.1002/ejhf.1957
36. Comenzo RL, Reece D, Palladini G, Seldin D, Sanchorawala V, Landau H, et al. Consensus guidelines for the conduct and reporting of clinical trials in systemic light-chain amyloidosis. Leukemia. (2012) 26(11):2317–25. doi: 10.1038/leu.2012.100
37. Ishiguro K, Hayashi T, Igarashi T, Maruyama Y, Ikeda H, Ishida T, et al. Decrease of B-type natriuretic peptide to less than 200 pg/mL predicts longer survival in cardiac immunoglobulin light chain amyloidosis. Int J Hematol. (2015) 102(2):200–4. doi: 10.1007/s12185-015-1814-0
38. Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Colby C, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and Serum free light chain measurements. J Clin Oncol. (2012) 30(9):989–95. doi: 10.1200/JCO.2011.38.5724
39. Garcia-Pavia P, Rapezzi C, Adler Y, Arad M, Basso C, Brucato A, et al. Diagnosis and treatment of cardiac amyloidosis: a position statement of the ESC working group on myocardial and pericardial diseases. Eur Heart J. (2021) 42(16):1554–68. doi: 10.1093/eurheartj/ehab072
40. Nolan MT, Marwick TH, Plana JC, Li Z, KirstenK N, Joshi VM, et al. Effect of traditional heart failure risk factors on myocardial dysfunction in adult survivors of childhood cancer. JACC Cardiovasc Imaging. (2018) 11(8):1202–3. doi: 10.1016/j.jcmg.2017.12.011
41. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42(36):3599–726. doi: 10.1093/eurheartj/ehab368
42. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC committee for practice guidelines: the task force for cancer treatments and cardiovascular toxicity of the European society of cardiology (ESC). Eur Heart J. (2016) 37(36):2768–801. doi: 10.1093/eurheartj/ehw211
43. Aimo A, Vergaro G, Castiglione V, Rapezzi C, Emdin M. Safety and tolerability of neurohormonal antagonism in cardiac amyloidosis. Eur J Intern Med. (2020) 80:66–72. doi: 10.1016/j.ejim.2020.05.015
44. Wechalekar AD, Gillmore JD, Bird J, Cavenagh J, Hawkins S, Kazmi M, et al. Guidelines on the management of AL amyloidosis. Br J Haematol. (2015) 168(2):186–206. doi: 10.1111/bjh.13155
45. Nicol M, Siguret V, Vergaro G, Aimo A, Emdin M, Dillinger JG, et al. Thromboembolism and bleeding in systemic amyloidosis: a review. ESC Heart Fail. (2022) 9(1):11–20. doi: 10.1002/ehf2.13701
46. Key NS, Khorana AA, Kuderer NM, Bohlke K, Lee AYY, Arcelus JI, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. (2020) 38(5):496–520. doi: 10.1200/JCO.19.01461
47. Wu P, Oren O, Gertz MA, Yang EH. Proteasome inhibitor-related cardiotoxicity: mechanisms. Diagnosis, and management. Curr Oncol Rep. (2020) 22(7):66. doi: 10.1007/s11912-020-00931-w
48. Herrmann J. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol. (2020) 17(8):474–502. doi: 10.1038/s41569-020-0348-1
51. Bhatia S. Long-term health impacts of hematopoietic stem cell transplantation inform recommendations for follow-up. Expert Rev Hematol. (2011) 4(4):437–54. doi: 10.1586/ehm.11.39
52. Martin PJ, Counts GW, Appelbaum FR, Lee SJ, Sanders JE, Deeg HJ, et al. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol Off J Am Soc Clin Oncol. (2010) 28(6):1011–6. doi: 10.1200/JCO.2009.25.6693
53. Ohmoto A, Fuji S. Cardiac complications associated with hematopoietic stem-cell transplantation. Bone Marrow Transplant. (2021) 56(11):2637–43. doi: 10.1038/s41409-021-01427-2
54. Tuzovic M, Mead M, Young PA, Schiller G, Yang EH. Cardiac complications in the adult bone marrow transplant patient. Curr Oncol Rep. (2019) 21(3):28. doi: 10.1007/s11912-019-0774-6
55. Zhao Y, He R, Oerther S, Zhou W, Vosough M, Hassan M. Cardiovascular complications in hematopoietic stem cell transplanted patients. J Pers Med. (2022) 12(11):1797. doi: 10.3390/jpm12111797
56. Gul Z, Bashir Q, Cremer M, Yusuf SW, Gunaydin H, Arora S, et al. Short-term cardiac toxicity of autologous hematopoietic stem cell transplant for multiple myeloma. Leuk Lymphoma. (2015) 56(2):533–5. doi: 10.3109/10428194.2014.926346
57. Leger KJ, Baker KS, Cushing-Haugen KL, Flowers MED, Leisenring WM, Martin PJ, et al. Lifestyle factors and subsequent ischemic heart disease risk after hematopoietic cell transplantation: lifestyle and heart disease after HCT. Cancer. (2018) 124(7):1507–15. doi: 10.1002/cncr.31227
58. Armenian SH, Sun CL, Shannon T, Mills G, Francisco L, Venkataraman K, et al. Incidence and predictors of congestive heart failure after autologous hematopoietic cell transplantation. Blood. (2011) 118(23):6023–9. doi: 10.1182/blood-2011-06-358226
59. Jaccard A, Moreau P, Leblond V, Leleu X, Benboubker L, Hermine O, et al. High-dose melphalan versus melphalan plus dexamethasone for AL amyloidosis. N Engl J Med. (2007) 357(11):1083–93. doi: 10.1056/NEJMoa070484
60. Girnius S, Seldin DC, Meier-Ewert HK, Sloan JM, Quillen K, Ruberg FL, et al. Safety and efficacy of high-dose melphalan and auto-SCT in patients with AL amyloidosis and cardiac involvement. Bone Marrow Transplant. (2014) 49(3):434–9. doi: 10.1038/bmt.2013.192
61. Yeh JC, Shank BR, Milton DR, Qazilbash MH. Adverse prognostic factors for morbidity and mortality during peripheral blood stem cell mobilization in patients with light chain amyloidosis. Biol Blood Marrow Transplant. (2018) 24(4):815–9. doi: 10.1016/j.bbmt.2017.11.040
62. Sureddi RK, Amani F, Hebbar P, Williams DK, Leonardi M, Paydak H, et al. Atrial fibrillation following autologous stem cell transplantation in patients with multiple myeloma: incidence and risk factors. Ther Adv Cardiovasc Dis. (2012) 6(6):229–36. doi: 10.1177/1753944712464102
63. Plummer C, Driessen C, Szabo Z, Mateos MV. Management of cardiovascular risk in patients with multiple myeloma. Blood Cancer J. (2019) 9(3):26. doi: 10.1038/s41408-019-0183-y
64. Kastritis E, Leleu X, Arnulf B, Zamagni E, Cibeira MT, Kwok F, et al. Bortezomib, melphalan, and dexamethasone for light-chain amyloidosis. J Clin Oncol. (2020) 38(28):3252–60. doi: 10.1200/JCO.20.01285
65. Iqubal A, Iqubal MK, Sharma S, MohdA A, Najmi AK, Ali SM, et al. Molecular mechanism involved in cyclophosphamide-induced cardiotoxicity: old drug with a new vision. Life Sci. (2019) 218:112–31. doi: 10.1016/j.lfs.2018.12.018
66. Goldberg M, Antin J, Guinan E, Rappeport J. Cyclophosphamide cardiotoxicity: an analysis of dosing as a risk factor. Blood. (1986) 68(5):1114–8. doi: 10.1182/blood.V68.5.1114.1114
67. Dhesi S, Chu MP, Blevins G, Paterson I, Larratt L, Oudit GY, et al. Cyclophosphamide-induced cardiomyopathy: a case report, review, and recommendations for management. J Investig Med High Impact Case Rep. (2013) 1(1):232470961348034. doi: 10.1177/2324709613480346
68. Palladini G, Sachchithanantham S, Milani P, Gillmore J, Foli A, Lachmann H, et al. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood. (2015) 126(5):612–5. doi: 10.1182/blood-2015-01-620302
69. Driscoll JJ, Girnius S. Proteasome inhibitors to treat AL amyloidosis. In: Fernandez-Escamilla AM, editors. Exploring new findings on amyloidosis. InTech (2016). Curatore. Disponibile su: http://www.intechopen.com/books/exploring-new-findings-on-amyloidosis/proteasome-inhibitors-to-treat-al-amyloidosis (Citato Aprile 7, 2023).
70. Gavazzoni M, Lombardi CM, Vizzardi E, Gorga E, Sciatti E, Rossi L, et al. Irreversible proteasome inhibition with carfilzomib as first line therapy in patients with newly diagnosed multiple myeloma: early in vivo cardiovascular effects. Eur J Pharmacol. (2018) 838:85–90. doi: 10.1016/j.ejphar.2018.09.014
71. Cornell RF, Ky B, Weiss BM, Dahm CN, Gupta DK, Du L, et al. Prospective study of cardiac events during proteasome inhibitor therapy for relapsed multiple myeloma. J Clin Oncol. (2019) 37(22):1946–55. doi: 10.1200/JCO.19.00231
72. Chen Y, Lairson DR, Chan W, Du XL. Risk of adverse events associated with front-line anti-myeloma treatment in medicare patients with multiple myeloma. Ann Hematol. (2018) 97(5):851–63. doi: 10.1007/s00277-018-3238-4
73. Zheng Y, Huang S, Xie B, Zhang N, Liu Z, Tse G, et al. Cardiovascular toxicity of proteasome inhibitors in multiple myeloma therapy. Curr Probl Cardiol. (2023) 48(3):101536. doi: 10.1016/j.cpcardiol.2022.101536
74. Patel MB, Majetschak M. Distribution and interrelationship of ubiquitin proteasome pathway component activities and ubiquitin pools in various porcine tissues. Physiol Res. (2007) 56:341–50. doi: 10.33549/physiolres.931005
75. Bochtler T, Hegenbart U, Kunz C, Granzow M, Benner A, Seckinger A, et al. Translocation t(11;14) is associated with adverse outcome in patients with newly diagnosed AL amyloidosis when treated with bortezomib-based regimens. J Clin Oncol. (2015) 33(12):1371–8. doi: 10.1200/JCO.2014.57.4947
76. Cole DC, Frishman WH. Cardiovascular complications of proteasome inhibitors used in multiple myeloma. Cardiol Rev. (2018) 26(3):122–9. doi: 10.1097/CRD.0000000000000183
77. Reece DE, Sanchorawala V, Hegenbart U, Merlini G, Palladini G, Fermand JP, et al. Weekly and twice-weekly bortezomib in patients with systemic AL amyloidosis: results of a phase 1 dose-escalation study. Blood. (2009) 114(8):1489–97. doi: 10.1182/blood-2009-02-203398
78. Reneau JC, Asante D, van Houten H, Sangaralingham LR, Buadi FK, Lerman A, et al. Cardiotoxicity risk with bortezomib versus lenalidomide for treatment of multiple myeloma: a propensity matched study of 1,790 patients: cardiotoxicity risk with bortezomib. Am J Hematol. (2017) 92(2):E15–7. doi: 10.1002/ajh.24599
79. Takamatsu H, Yamashita T, Kotani T, Sawazaki A, Okumura H, Nakao S. Ischemic heart disease associated with bortezomib treatment combined with dexamethasone in a patient with multiple myeloma. Int J Hematol. (2010) 91(5):903–6. doi: 10.1007/s12185-010-0586-9
80. Orciuolo E, Buda G, Cecconi N, Galimberti S, Versari D, Cervetti G, et al. Unexpected cardiotoxicity in haematological bortezomib treated patients. Br J Haematol. (2007) 138(3):396–7. doi: 10.1111/j.1365-2141.2007.06659.x
81. Bockorny M, Chakravarty S, Schulman P, Bockorny B, Bona R. Severe heart failure after bortezomib treatment in a patient with multiple myeloma: a case report and review of the literature. Acta Haematol. (2012) 128(4):244–7. doi: 10.1159/000340050
82. Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. (2005) 352(24):2487–98. doi: 10.1056/NEJMoa043445
83. Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. (2003) 348(26):2609–17. doi: 10.1056/NEJMoa030288
84. Jagannath S, Barlogie B, Berenson J, Siegel D, Irwin D, Richardson PG, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma: bortezomib for relapsed or refractory multiple myeloma. Br J Haematol. (2004) 127(2):165–72. doi: 10.1111/j.1365-2141.2004.05188.x
85. Xiao Y, Yin J, Wei J, Shang Z. Incidence and risk of cardiotoxicity associated with bortezomib in the treatment of cancer: a systematic review and meta-analysis. Moretti C, curatore. PLoS One. (2014) 9(1):e87671. doi: 10.1371/journal.pone.0087671
86. Laubach JP, San Miguel JF, Sonneveld P, Orlowski RZ, Moreau P, Rosiñol L, et al. Quantifying the risk of heart failure associated with proteasome inhibition: a retrospective analysis of heart failure reported in phase 2 and phase 3 studies of bortezomib (btz) in multiple myeloma (MM). Blood. (2013) 122(21):3187. doi: 10.1182/blood.V122.21.3187.3187
87. Bruce JT, Tran JM, Phillips G, Elder P, Mastronarde JG, Devine SM, et al. Chemotherapeutic agents increase the risk for pulmonary function test abnormalities in patients with multiple myeloma. Clin Lymphoma Myeloma Leuk. (2012) 12(5):325–9. doi: 10.1016/j.clml.2012.06.002
88. Cohen AD, Landau H, Scott EC, Liedtke M, Kaufman JL, Rosenzweig M, et al. Safety and efficacy of carfilzomib (CFZ) in previously-treated systemic light-chain (AL) amyloidosis. Blood. (2016) 128(22):645. doi: 10.1182/blood.V128.22.645.645
89. Siegel D, Martin T, Nooka A, Harvey RD, Vij R, Niesvizky R, et al. Integrated safety profile of single-agent carfilzomib: experience from 526 patients enrolled in 4 phase II clinical studies. Haematologica. (2013) 98(11):1753–61. doi: 10.3324/haematol.2013.089334
90. Danhof S, Schreder M, Rasche L, Strifler S, Einsele H, Knop S. ‘Real-life’ experience of preapproval carfilzomib-based therapy in myeloma—analysis of cardiac toxicity and predisposing factors. Eur J Haematol. (2016) 97(1):25–32. doi: 10.1111/ejh.12677
91. Bishnoi R, Xie Z, Shah C, Bian J, Murthy HS, Wingard JR, et al. Real-world experience of carfilzomib-associated cardiovascular adverse events: SEER-medicare data set analysis. Cancer Med. (2021) 10(1):70–8. doi: 10.1002/cam4.3568
92. Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Špička I, Oriol A, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. (2015) 372(2):142–52. doi: 10.1056/NEJMoa1411321
93. Waxman AJ, Clasen S, Hwang WT, Garfall A, Vogl DT, Carver J, et al. Carfilzomib-associated cardiovascular adverse events: a systematic review and meta-analysis. JAMA Oncol. (2018) 4(3):e174519. doi: 10.1001/jamaoncol.2017.4519
94. Latif A, Kapoor V, Lateef N, Ahsan MJ, Usman RM, Malik SU, et al. Incidence and management of carfilzomib-induced cardiovascular toxicity; a systematic review and meta-analysis. Cardiovasc Hematol Disord-Drug Targets. (2021) 21(1):30–45. doi: 10.2174/1871529X21666210412113017
95. Buck B, Kellett E, Addison D, Vallakati A. Carfilzomib-induced cardiotoxicity: an analysis of the FDA adverse event reporting system (FAERS). J Saudi Heart Assoc. (2022) 34(3):134–41. doi: 10.37616/2212-5043.1311
96. Astarita A, Mingrone G, Airale L, Cesareo M, Colomba A, Catarinella C, et al. Carfilzomib-based regimen and cardiotoxicity in multiple myeloma: incidence of cardiovascular events and organ damage in carfilzomib-dexamethasone versus carfilzomib-lenalidomide-dexamethasone. A real-life prospective study. Cancers (Basel). (2023) 15(3):955. doi: 10.3390/cancers15030955
97. Desmarais T, Yang J, Narezkina A, Fernandes T. Etiology of pulmonary hypertension in multiple myeloma: a case series and literature review. Respir Med. (2023) 206:107071. doi: 10.1016/j.rmed.2022.107071
98. Chauhan D, Tian Z, Zhou B, Kuhn D, Orlowski R, Raje N, et al. In vitro and in vivo selective antitumor activity of a novel orally bioavailable proteasome inhibitor MLN9708 against multiple myeloma cells. Clin Cancer Res. (2011) 17(16):5311–21. doi: 10.1158/1078-0432.CCR-11-0476
99. Sanchorawala V, Palladini G, Kukreti V, Zonder JA, Cohen AD, Seldin DC, et al. A phase 1/2 study of the oral proteasome inhibitor ixazomib in relapsed or refractory AL amyloidosis. Blood. (2017) 130(5):597–605. doi: 10.1182/blood-2017-03-771220
100. Dispenzieri A, Kastritis E, Wechalekar AD, Schönland SO, Kim K, Sanchorawala V, et al. A randomized phase 3 study of ixazomib–dexamethasone versus physician’s choice in relapsed or refractory AL amyloidosis. Leukemia. (2022) 36(1):225–35. doi: 10.1038/s41375-021-01317-y
101. Sanchorawala V, Wechalekar AD, Kim K, Schönland SO, Landau HJ, Kwok F, et al. Quality of life and symptoms among patients with relapsed/refractory AL amyloidosis treated with ixazomib-dexamethasone versus physician’s choice. Am J Hematol. (2023) 98(5):720–29. doi: 10.1002/ajh.26866
102. Sanchez L, Landau HJ, Rosenbaum CA, Abrahams A, Chin C, Liotta B, et al. A phase 1/2 study to assess safety and dose of ixazomib in combination with cyclophosphamide and dexamethasone in newly diagnosed patients with light chain (AL) amyloidosis. Blood. (2019) 134(Suppl 1):3128. doi: 10.1182/blood-2019-126023
103. Moreau P, Masszi T, Grzasko N, Bahlis NJ, Hansson M, Pour L, et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. (2016) 374(17):1621–34. doi: 10.1056/NEJMoa1516282
104. Al-Yafeai Z, Ghoweba M, Ananthaneni A, Abduljabar H, Aziz D. Cardiovascular complications of modern multiple myeloma therapy: a pharmacovigilance study. Br J Clin Pharmacol. (2023) 89(2):641–8. doi: 10.1111/bcp.15499
105. Fradley MG, Groarke JD, Laubach J, Alsina M, Lenihan DJ, Cornell RF, et al. Recurrent cardiotoxicity potentiated by the interaction of proteasome inhibitor and immunomodulatory therapy for the treatment of multiple myeloma. Br J Haematol. (2018) 180(2):271–5. doi: 10.1111/bjh.14970
106. Ridolfi RL, Bulkley BH, Hutchins GM. The conduction system in cardiac amyloidosis. Am J Med. (1977) 62(5):677–86. doi: 10.1016/0002-9343(77)90870-1
107. Das A, Dasgupta S, Gong Y, Shah UA, Fradley MG, Cheng RK, et al. Cardiotoxicity as an adverse effect of immunomodulatory drugs and proteasome inhibitors in multiple myeloma: a network meta-analysis of randomized clinical trials. Hematol Oncol. (2022) 40(2):233–42. doi: 10.1002/hon.2959
108. Basset M, Kimmich CR, Schreck N, Krzykalla J, Dittrich T, Veelken K, et al. Lenalidomide and dexamethasone in relapsed/refractory immunoglobulin light chain (AL) amyloidosis: results from a large cohort of patients with long follow-up. Br J Haematol. (2021) 195(2):230–43. doi: 10.1111/bjh.17685
109. Sanchorawala V, Wright DG, Rosenzweig M, Finn KT, Fennessey S, Zeldis JB, et al. Lenalidomide and dexamethasone in the treatment of AL amyloidosis: results of a phase 2 trial. Blood. (2007) 109(2):492–6. doi: 10.1182/blood-2006-07-030544
110. Castiglione V, Franzini M, Aimo A, Carecci A, Lombardi CM, Passino C, et al. Use of biomarkers to diagnose and manage cardiac amyloidosis. Eur J Heart Fail. (2021) 23(2):217–30. doi: 10.1002/ejhf.2113
111. Tapan U, Seldin DC, Finn KT, Fennessey S, Shelton A, Zeldis JB, et al. Increases in B-type natriuretic peptide (BNP) during treatment with lenalidomide in AL amyloidosis. Blood. (2010) 116(23):5071–2. doi: 10.1182/blood-2010-09-305136
112. Dispenzieri A, Dingli D, Kumar SK, Rajkumar SV, Lacy MQ, Hayman S, et al. Discordance between serum cardiac biomarker and immunoglobulin-free light-chain response in patients with immunoglobulin light-chain amyloidosis treated with immune modulatory drugs. Am J Hematol. (2010) 85(10):757–9. doi: 10.1002/ajh.21822
113. Lu G, Middleton RE, Sun H, Naniong M, Ott CJ, Mitsiades CS, et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of ikaros proteins. Science. (2014) 343(6168):305–9. doi: 10.1126/science.1244917
114. Li W, Garcia D, Cornell RF, Gailani D, Laubach J, Maglio ME, et al. Cardiovascular and thrombotic complications of novel multiple myeloma therapies: a review. JAMA Oncol. (2017) 3(7):980. doi: 10.1001/jamaoncol.2016.3350
115. Rosovsky R, Hong F, Tocco D, Connell B, Mitsiades C, Schlossman R, et al. Endothelial stress products and coagulation markers in patients with multiple myeloma treated with lenalidomide plus dexamethasone: an observational study. Br J Haematol. (2013) 160(3):351–8. doi: 10.1111/bjh.12152
116. Singhal S, Mehta J. Thalidomide in cancer: potential uses and limitations. BioDrugs. (2001) 15(3):163–72. doi: 10.2165/00063030-200115030-00003
117. Cohen AD, Zhou P, Chou J, Teruya-Feldstein J, Reich L, Hassoun H, et al. Risk-adapted autologous stem cell transplantation with adjuvant dexamethasone ± thalidomide for systemic light-chain amyloidosis: results of a phase II trial: risk-adapted ASCT + thal/dex for AL amyloidosis. Br J Haematol. (2007) 139(2):224–33. doi: 10.1111/j.1365-2141.2007.06783.x
118. Rajkumar SV, Jacobus S, Callander NS, Fonseca R, Vesole DH, Williams ME, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. Lancet Oncol. (2010) 11(1):29–37. doi: 10.1016/S1470-2045(09)70284-0
119. Lenalidomide: risk of thrombosis and thromboembolism (2016). Disponibile su: https://www.gov.uk/drug-safety-update/lenalidomide-risk-of-thrombosis-and-thromboembolism.
120. Kastritis E, Dialoupi I, Gavriatopoulou M, Roussou M, Kanellias N, Fotiou D, et al. Primary treatment of light-chain amyloidosis with bortezomib, lenalidomide, and dexamethasone. Blood Adv. (2019) 3(20):3002–9. doi: 10.1182/bloodadvances.2019000147
121. Cohen OC, Sharpley F, Gillmore JD, Lachmann HJ, Sachchithanantham S, Mahmood S, et al. Use of ixazomib, lenalidomide and dexamethasone in patients with relapsed amyloid light-chain amyloidosis. Br J Haematol. (2020) 189(4):643–9. doi: 10.1111/bjh.16401
122. Sanchorawala V, Shelton AC, Lo S, Varga C, Sloan JM, Seldin DC. Pomalidomide and dexamethasone in the treatment of AL amyloidosis: results of a phase 1 and 2 trial. Blood. (2016) 128(8):1059–62. doi: 10.1182/blood-2016-04-710822
123. Milani P, Sharpley F, Schönland SO, Basset M, Mahmood S, Nuvolone M, et al. Pomalidomide and dexamethasone grant rapid haematologic responses in patients with relapsed and refractory AL amyloidosis: a European retrospective series of 153 patients. Amyloid. (2020) 27(4):231–6. doi: 10.1080/13506129.2020.1767566
124. Donk NWCJ, Janmaat ML, Mutis T, van Bueren JJ L, Ahmadi T, Sasser AK, et al. Monoclonal antibodies targeting CD 38 in hematological malignancies and beyond. Immunol Rev. (2016) 270(1):95–112. doi: 10.1111/imr.12389
125. Kastritis E, Palladini G, Minnema MC, Wechalekar AD, Jaccard A, Lee HC, et al. Daratumumab-based treatment for immunoglobulin light-chain amyloidosis. N Engl J Med. (2021) 385(1):46–58. doi: 10.1056/NEJMoa2028631
126. Sun C, Wang X, Zhang R, Xu L, Wang B, Li J. Efficacy and safety of intravenous daratumumab-based treatments for AL amyloidosis: a systematic review and meta-analysis. Cancer Cell Int. (2022) 22(1):222. doi: 10.1186/s12935-022-02635-6
127. Zhu C, Song Z, Wang A, Srinivasan S, Yang G, Greco R, et al. Isatuximab acts through fc-dependent, independent, and direct pathways to kill multiple myeloma cells. Front Immunol. (2020) 11:1771. doi: 10.3389/fimmu.2020.01771
128. Deckert J, Wetzel MC, Bartle LM, Skaletskaya A, Goldmacher VS, Vallée F, et al. SAR650984, a novel humanized CD38-targeting antibody, demonstrates potent antitumor activity in models of multiple myeloma and other CD38+ hematologic malignancies. Clin Cancer Res. (2014) 20(17):4574–83. doi: 10.1158/1078-0432.CCR-14-0695
129. Parker TL, Rosenthal A, Sanchorawala V, Landau HJ, Campagnaro E, Kapoor P, et al. A phase II study of isatuximab (SAR650984) (NSC-795145) for patients with previously treated AL amyloidosis (SWOG S1702; NCT#03499808). Blood. (2020) 136(Suppl 1):20–1. doi: 10.1182/blood-2020-143180
130. Moreau P, Dimopoulos MA, Mikhael J, Yong K, Capra M, Facon T, et al. Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): a multicentre, open-label, randomised phase 3 trial. Lancet. (2021) 397(10292):2361–71. doi: 10.1016/S0140-6736(21)00592-4
131. Iqbal SM, Stecklein K, Sarow J, Krabak M, Hillengass J, McCarthy P. Elotuzumab in combination with lenalidomide and dexamethasone for treatment-resistant immunoglobulin light chain amyloidosis with multiple myeloma. Clin Lymphoma Myeloma Leuk. (2019) 19(1):e33–6. doi: 10.1016/j.clml.2018.08.021
132. Dimopoulos MA, Lonial S, White D, Moreau P, Palumbo A, San-Miguel J, et al. Elotuzumab plus lenalidomide/dexamethasone for relapsed or refractory multiple myeloma: ELOQUENT-2 follow-up and post-hoc analyses on progression-free survival and tumour growth. Br J Haematol. (2017) 178(6):896–905. doi: 10.1111/bjh.14787
Keywords: AL amyloidosis, cardiac amyloidosis, chemotherapy, cardiovascular toxicity, treatment
Citation: Morfino P, Aimo A, Castiglione V, Chianca M, Vergaro G, Cipolla CM, Fedele A, Emdin M, Fabiani I and Cardinale D (2023) Cardiovascular toxicity from therapies for light chain amyloidosis. Front. Cardiovasc. Med. 10:1212983. doi: 10.3389/fcvm.2023.1212983
Received: 3 May 2023; Accepted: 23 June 2023;
Published: 5 July 2023.
Edited by:
Jun-ichi Abe, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Anecita P. Fadol, University of Texas MD Anderson Cancer Center, United States© 2023 Morfino, Aimo, Castiglione, Chianca, Vergaro, Cipolla, Fedele, Emdin, Fabiani and Cardinale. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paolo Morfino cGFvbG8ubW9yZmlub0BzYW50YW5uYXBpc2EuaXQ=; cGFvbG9tb3JmaW5vMTJAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.