- 1Department of Vascular Surgery, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Outpatient, West China Hospital, Sichuan University, Chengdu, China
Objective: The aims of the present study were to explore the risk factors for type 2 endoleaks (T2ELs) after endovascular aneurysm repair (EVAR) and the association between T2ELs and the iliolumbar artery.
Materials and methods: A single-center, retrospective case–control study in West China Hospital was conducted among patients with infrarenal abdominal aortic aneurysm (AAA) who underwent EVAR between June 2010 and June 2019. The associations of patient characteristics, anatomical factors, internal iliac artery embolization, and ILA with the primary outcome were analyzed. The secondary objective was to analyze survival and reintervention between the T2EL group and the non-T2EL group. Kaplan–Meier survival, propensity matching analysis and multivariate logistic regression analysis were used.
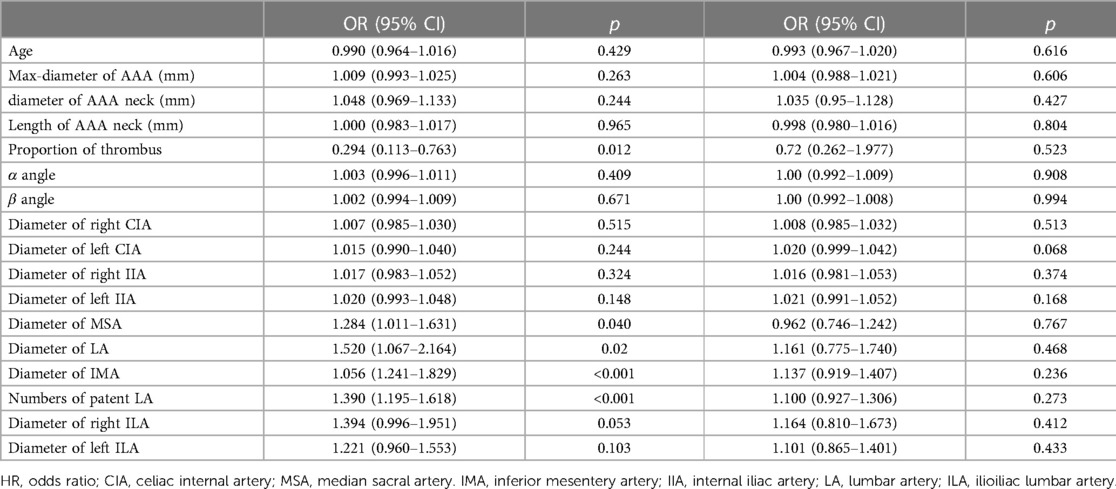
Results: A total of 603 patients were included. The median follow-up was 51 months (range 5.0–106.0). There was a significant difference in the diameter of the lumbar artery (LA), middle sacral artery (MSA) and inferior mesentery artery (IMA), proportion of thrombus and LA numbers. The univariate analysis showed that T2ELs were more likely to develop more thrombus in aneurysm cavity (OR = 0.294, p = 0.012), larger MSA (OR = 1.284, p = 0.04), LA (OR = 1.520, p = 0.015), IMA (OR = 1.056, p < 0.001) and more LAs (OR = 1.390, p = 0.019). The multivariate analysis showed that the number of LAs (HR: 1.349, 95% CI: 1.140–1.595, p < .001) and the diameter of the IMA (HR: 1.328, 95% CI: 1.078–1.636, p = 0.008) were significantly associated with T2ELs. There were no new findings from the propensity score matching. The reintervention-free survival rates were significantly different between the two groups (p = 0.048). Overall survival and AAA-related death rates were not different between the two group. This was consistent with the PSM analysis.
Conclusion: The iliolumbar artery and the different internal iliac artery interventions may not increase the incidence of T2ELs. But the numbers of LAs and IMA diameter were independent risk factors for T2Els. T2ELs was associated with the reintervention but did not affect long-term survival or increase aneurysm-related mortality after EVAR.
Introduction
Abdominal aortic aneurysm (AAA) is defined as a disease with an abdominal aortic diameter of more than 3 cm or 50% greater than the normal aortic diameter (1, 2). Although endovascular aortic aneurysm repair (EVAR) has become the first choice of treatment because of its advantages of less trauma, faster recovery and lower perioperative mortality, several studies have shown that the reintervention rate of EVAR is higher than that of OSR (3–5). Endoleaks, an important cause of reintervention, is a common and unique complication of EVAR and occurs in approximately 1/3 of postoperative patients (6). Type II endoleaks (T2ELs) are caused by retrograde blood flow from the side branches of the abdominal aorta entering the aneurysm sac after excluding the aneurysm, and they are the most common type of endoleaks, with an incidence rate between 8% and 44% (7–9).
The treatment methods for T2ELs include trans-lumbar direct embolization of the aneurysm sac, embolization of the aortic branches through the superior mesenteric artery or lumbar arteries, trans-cavity embolization, and open or laparoscopic clipping of side branches (10). Postoperative reintervention of the T2EL is challenging, while intervening in the anatomical risk factors seems to be more advantageous intraoperatively. Abdominal aortic collateral artery embolization can reduce the incidence of T2ELs and the reintervention rate (11–18) and promote the reduction of aneurysms after EVAR (12, 13, 16–18), with a lower incidence of complications (12). The high anatomical risk factors for T2ELs include patent IMA and LA (16, 19, 20). In addition, the incidence of T2ELs was also associated with the internal iliac artery ranging from 0 to 3.8% (21), and some investigators believe it was related to the iliolumbar artery (21, 22). The iliolumbar artery and lumbar artery are connected through collateral circulation and can communicate with the fourth lumbar artery (23–25). However, no studies have investigated the association between the iliolumbar artery and T2ELs after EVAR. The purpose of this study was to investigate the relationship between the iliolumbar artery and T2ELs after EVAR.
Method
Study design
This was a single-center, retrospective case–control study. The primary objective of this study was to investigate the relationship between the iliolumbar artery and T2ELs. The secondary objective was to investigate the effect of postoperative T2ELs on long-term mortality and reintervention rates. The patients with T2ELs were screened by a color Doppler ultrasound system and PACS system. The diameter of the ilio-lumbar artery was measured within approximately 1.5 cm of its origin, and the location of its origin was recorded.
Study population/participants
Patients with AAA who underwent EVAR in the Department of Vascular Surgery, West China Hospital, Sichuan University from June 2010 to June 2019 were enrolled. The exclusion criteria of this study were formulated as follows: (1) thoracoabdominal aortic aneurysm, para-renal abdominal aortic aneurysm, or suprarenal abdominal aortic aneurysm. (2) Patients undergoing hybrid abdominal aortic aneurysm surgery. (3) Abdominal aortic dissection aneurysm or pseudoaneurysm or perforating ulcer. (4) Ruptured abdominal aortic aneurysm or EVAR conversion to open surgery. (5) Patients who had no abdominal aortic CT before the operation and no follow-up records. (6) Patients with type I and III endoleaks.
Data collection
The standardized electronic data system of West China Hospital, HIS system of medical records and PACS system of imaging data were used to obtain the data of the research subjects. Data were collected from patient medical records and included the following baseline and anatomical variables and operation information: age, sex, preoperative AAA diameter, neck length, maximum iliac artery diameters, anatomical characteristics of the internal iliac artery and iliolumbar artery, anesthesia method, etc. To ensure the accuracy of the data, 20% of the data were randomly checked by senior physicians in vascular surgery. If the measurement deviation was more than 10%, the senior physicians remeasured and corrected the data. Patients were divided into two groups: with or without T2ELs (The case group was T2ELs and the control group was non-T2ELs).
Surveillance protocol
Ethics
This study was approved by the Ethics Committee of West China Hospital, Sichuan University. All the study participants provided written informed consent stating that the clinical data could be used in clinical research.
Analysis method
All statistical analyses were performed using SPSS version 25 (IBM Corporation, Armonk, NY). The data are presented as the mean ± standard deviation for continuous variables and as the frequency (percentage) for categorical variables, which were compared using the two-sample t-test, Fisher's exact test, and Pearson's χ2 test where appropriate. Overall survival and AAA-related mortality were generated using the Kaplan–Meier method, and the log-rank test was used to compare the differences. Differences with a p value <.05 were significant. The propensity matching score was used to calibrate the baseline.
Result
Baseline
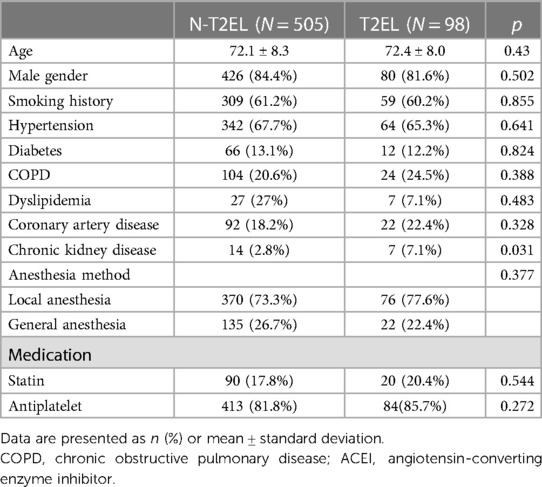
A total of 603 patients were included (T2EL, 505. N-T2EL, 98, Supplementary Figure S4). Baseline characteristics are depicted in Table 1. The mean patient age was 72.0 ± 8.3 years, and males comprised 83.9% of patients. No endoleaks were identified in 505 patients (83.7%), and T2ELs were found in 98 patients (16.3%). Except for CKD, there was no significant difference between the two groups in preoperative comorbidities. T2EL patients had a lower prevalence of CKD (2.8%) than N-T2EL patients (7.1%, p = 0.031). Oral beta-blockers were more common in the N-T2EL group than in the T2EL group (p = 0.01). The median follow-up duration was 49.0 months (IQR: 42; range 1.0–136.0) in the N-T2EL group and 54.2 months (IQR: 35.5; 1.0–138.0) in the T2EL group.
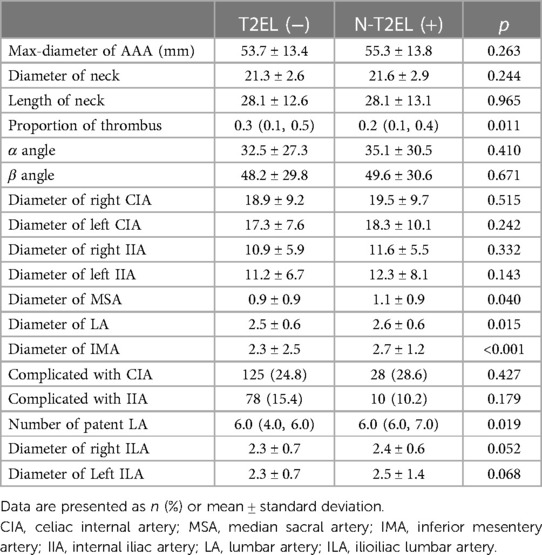
The vascular morphologic characteristics are shown in Table 2. Our results showed that the proportion of thrombus in the aneurysm cavity (p = 0.011), the diameter of the median sacral artery (p = 0.04), lumbar artery (p = 0.015), inferior mesenteric artery (p < 0.001) and the number of lumbar arteries (p = 0.019) were significantly different between the two groups. The diameter of the ILA in the N-T2EL group was 2.3 ± 0.7 mm, and in the T2EL group, the right ILA was 2.4 ± 0.6 mm, and the left ILA was 2.5 ± 1.4 mm (Table 3).
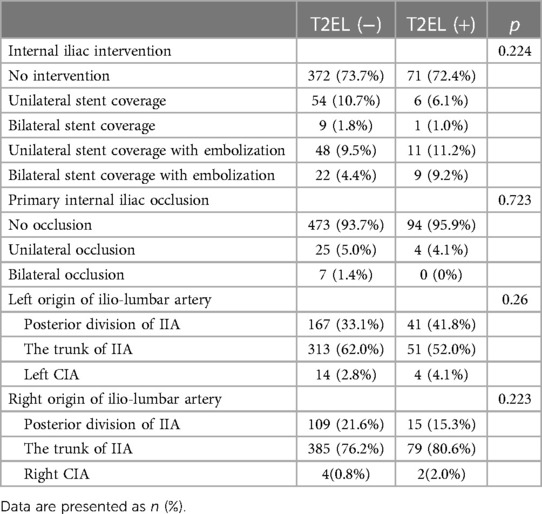
In Table 4, patient characteristics were compared based on the type of IIA embolization performed and primary IIA occlusion. A total of 443 individuals did not undergo any preoperative IIA intervention (T2EL, 72.4%. N-T2EL, 73.7%). In addition, 160 patients (26.5%) received the intervention. Sixty patients (T2EL, 10.7%. N-T2EL, 6.1%) accepted unilateral stent-covered IIA without embolization, and 10 patients (T2EL, 1.8%. N-T2EL, 1.0%) bilateral stent coverage without embolization. Unilateral stent coverage with embolization was performed in 59 patients (T2EL, 9.5%. N-T2EL, 11.2%), and bilateral stent coverage with embolization was completed in 31 patients (T2EL, 4.4%). N-T2EL, 9.2%). Our analysis showed no significant difference between the two groups in the intervention mode of IIA (p = 0.224). There was also no difference in primary iliac artery occlusion between the two groups (p = 0.723). No variable was significantly different in the anatomic origin of ILA between the two groups.
In the univariate analysis (Table 2), our results showed that T2ELs may have larger MSA (OR = 1.284, p = 0.04), LAs (OR = 1.520, p = 0.015), and IMA (OR = 1.056, p < 0.001) and more LAs (OR = 1.390, p = 0.019). And the patients with T2EL may have smaller proportion of thrombus in the aneurysm cavity (OR = 0.294, p = 0.012).
The factors with p < 0.1 in the univariate analysis in Tables 1, 2 were subjected to a subsequent multivariate analysis to evaluate the association with T2ELs. In Table 5, our multivariate analysis showed the number of LAs (before, OR: 1.351, 95% CI: 1.144–1.597, p < .001. After, OR: 1.349, 95% CI: 1.140–1.595, p < .001), and diameter of IMA (Before, OR: 1.330, 95% CI: 1.08–1.637, p = .007. After, OR: 1.328, 95% CI: 1.078–1.636, p = .008) were identified to be significantly associated with T2Els, which was consistent after adjusting for the IIA.

Table 5. Multivariate analysis before (M1) and after (M2) adjusting for primary hypogastric artery condition.
ROC analysis (Figure 1) showed that the cutoff value for the number of LAs was 6 (AUC = 0.640, 95% CI: 0.587–0.693, sensitivity = 0.714, specificity = 0.522) and for the diameter of the IMA was 2.5 mm (AUC = 0.642, 95% CI: 0.584–0.699, sensitivity = 0.786, specificity = 0.48).
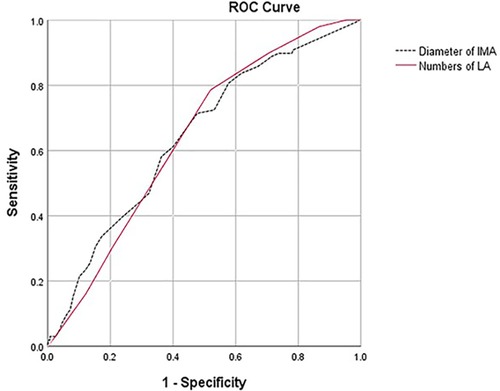
Figure 1. ROC analysis showed that there was no difference in predictive value between the number of LAs (AUC = 0.640, 95% CI: 0.587–0.693, sensitivity = 0.714, specificity = 0.522) and the diameter of the IMA (AUC = 0.642, 95% CI: 0.584–0.699, sensitivity = 0.786, specificity = 0.48, p = 0.972).
The Kaplan–Meier curves in Figures 2, 3 show that there were no differences between the T2EL group and N-T2EL group in overall survival (T2EL, 71.9%, N-T2EL, 59.9% at 8 years, p = 0.45) and freedom from AAA-related death (T2EL, 95%, N-T2EL, 91.3% at 8 years, p = 0.61). The reintervention-free survival rates at 8 years were 96.1% and 77.3% in patients with and without T2ELs, respectively, which were significantly different between the two groups (p = 0.049, Figure 4).
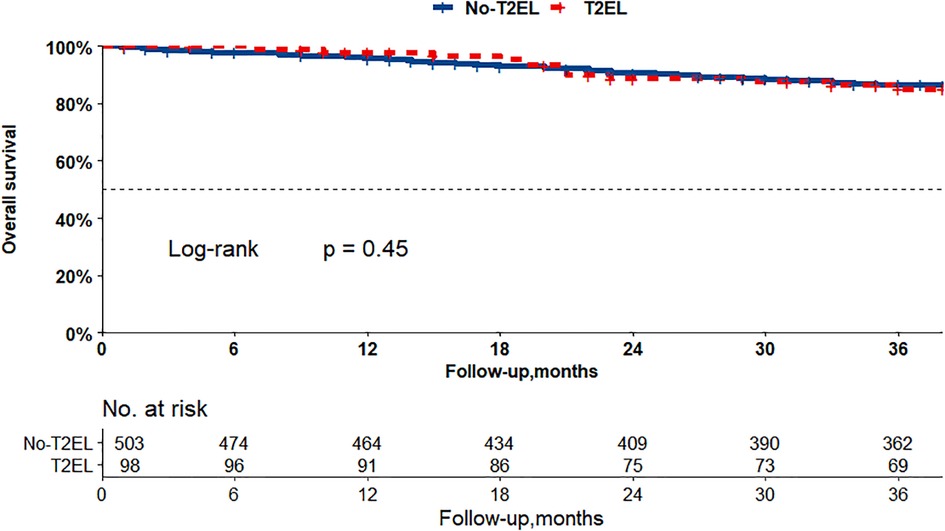
Figure 2. Overall survival did not differ between the patients with T2ELs and those without T2ELs. (p = 0.45, log rank test).
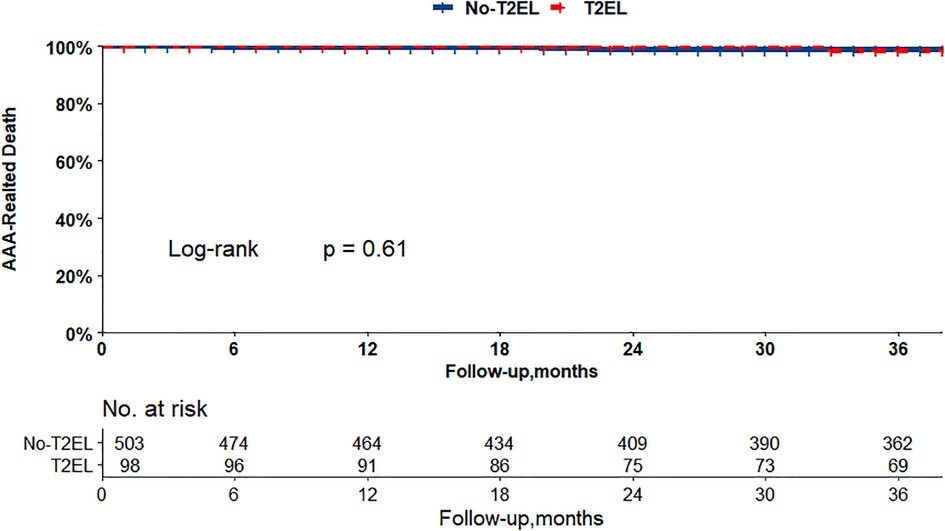
Figure 3. AAA-related survival did not differ between the patients with T2ELs and those without T2ELs. (p = 0.61, log rank test).
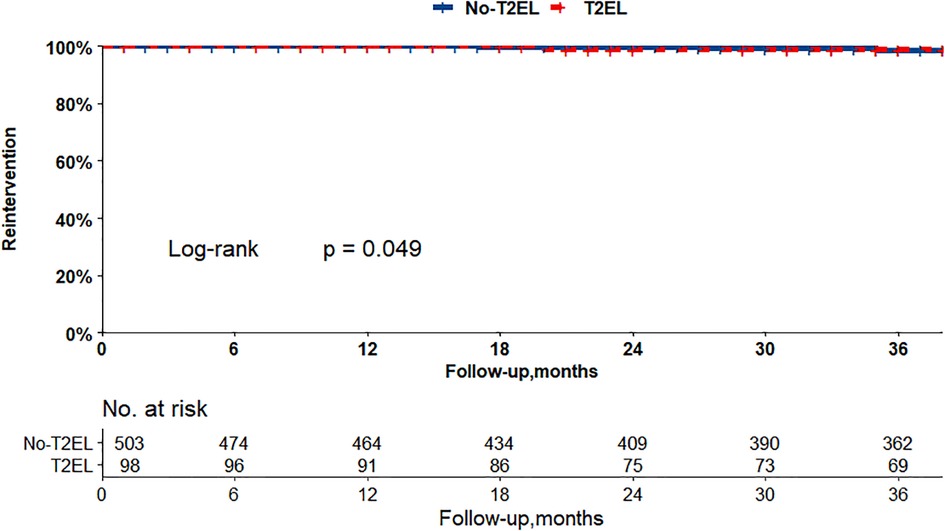
Figure 4. Patients with T2ELs had more reinterventions than those without T2ELs (p = 0.049, log rank test).
Variables of PSM included all anatomical data, age, sex, and preoperative comorbidities (Table 6, univariable analysis after PSM). The propensity score matching (PSM) included 391 patients (T2ELs vs. non-T2ELs: 91 vs. 297), and the multivariate analysis after PSM in Table 5 indicated that the independent risk factors for T2ELs were still the number of LAs (OR: 1.349, 95% CI: 1.140–1.595, p < 0.001) and the diameter of the IMA (OR: 1.328, 95% CI: 1.078–1.636, p = 0.008). There was no significant difference between the intervention modes of IIA and ILA in the two groups. Additionally, the Kaplan–Meier curves after PSM found that T2ELs group had a higher rate of reintervention (p = 0.034) in Supplementary Figure S3, but there was no difference in overall survival and AAA-related death between the two groups (Supplementary Figures S1 and S2).
Discussion
The management of T2EL remains controversial in the current literature, and it has several definitions, including “early”, “late”, “persistent” and “isolated” type II endoleaks. T2ELs were observed in 10.2% of patients after EVAR (9), and 30% to 50% of these resolved spontaneously (9, 26). In a Japanese nationwide analysis, persistent T2EL was defined as T2EL detected after EVAR on initial contrast-enhanced CT and during follow-up or new T2EL not documented at the end of EVAR but reported at any point during follow-up (27). A correlation between persistent T2ELs (p-T2ELs) and late adverse events, including aneurysm sac enlargement, reintervention, rupture, and abdominal aortic aneurysm-related mortality after endovascular aneurysm repair, was demonstrated. In addition to p-T2ELs, older age, female sex, chronic kidney disease, and dilated proximal neck were associated with sac enlargement.
Wang et al. (28) analyzed 10-year follow-up results and found that T2ELs were significantly associated with aneurysm sac growth, but no association with survival was observed. The low overall survival rate in our analysis may be related to COVID-19.
Current guidelines, such as the 2019 European Society for Vascular Surgery (ESVS) guideline and Society for Vascular Surgery implementation of clinical practice guidelines, have recommended conservative management, and intervention was indicated for significant sac expansion (≥10 mm or 5 mm) (1, 29). Although reintervention for T2ELs after EVAR could achieve some clinical effects (30–32), a study (33) found that embolization procedures were generally ineffective in preventing further expansion of abdominal aortic aneurysms in patients with T2ELs after EVAR. The risk of repeated intervention after reintervention for T2ELs exists, and the key to treating T2ELs has shifted from reintervention to prevention.
Therefore, identifying risk factors for T2ELs and early intervention in high-risk patients are key to treatment. Our study found that the IMA diameter and the number of LAs were independent risk factors, which was consistent with most studies (8, 13, 22, 34). Through ROC curve analysis, this study determined the number of lumbar arteries (≥6) and the cutoff of IMA diameter (≥2.5 mm). Some studies have identified IMA ≥3 mm as a risk factor for T2ELs (11, 12).
IIA embolization has also been suggested as a risk factor for T2ELs in some studies (35, 36). They thought IIA embolization was more likely to increase the redistribution of blood flow from the lumbar arteries and IMA branches than IIA stent coverage alone. The formation of collateral circulation may also be associated with ILA. Meishi et al. (22) thought that the iliolumbar artery arising from the IIA was a major source of T2ELs. In their study, no significant impact of IIA embolization on T2ELs was observed after analyzing 375 patients. Of all 603 patients in our article, 9.8% received unilateral and 5.1% bilateral IIA embolization. The multivariate analysis showed that the different interventions for IIA were not associated with T2ELs, regardless of the IIA status. Currently, iliac branch devices are used to preserve at least one IIA. From existing studies (37, 38) and the limited evidence presented in our article, preservation of the IIA does not appear to increase the incidence of T2ELs (22).
In addition, the relationship between ILA and T2ELs has not been compared, although it has sometimes been found to be the source of T2ELs during follow-up (Figure 5). In our study, the right ILA diameter measured based on abdominal CTA was 2.31 ± 0.65 mm, and the left ILA was 2.30 ± 0.66 mm, which was similar to previous results based on human anatomy, which reported that the diameter was 2.7 ± 0.6 mm (39). We found that 28.0% of ILAs originated from the posterior of the IIA, 69.9% from the main trunk of the IIA, and 0.2% from the CIA. Kiray et al. (40) reported an average ILA diameter of 3.7 ± 0.7 mm, and Teli et al. (25) reported an average ILA diameter of 3.5 ± 0.5 mm. Koc et al. (23) reported that the ILA diameter originating from the main trunk of the IIA was smaller than that originating from the posterior branch of the IIA. In addition, the iliac lumbar artery mainly originates from the main internal iliac artery and less from the posterior branch of the IIA and CIA (25, 39, 40). This finding was similar to our study.
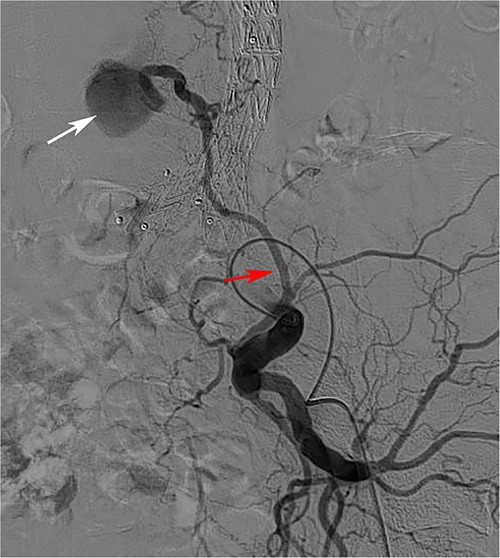
Figure 5. The iliolumbar artery was a source of type 2 endoleak. (Red arrow is the ILA, white arrow is the aneurysm cavity).
In the T2ELs caused by ILA, we found that ILA tended to be backward and upward, but only 2% of T2ELs were caused by ILA. There was no significant difference in the anatomical characteristics of the ILA in univariate analysis, and the right ILA diameter also showed no difference in multivariate analysis (p = 0.83). T2ELs from the IIA reported in the past are relatively rare, and the incidence of most previous report series is between 0% and 3.8% (21, 41), and no correlation was demonstrated in previous studies (22, 42). Other risk factors associated with T2ELs include chronic kidney disease, advanced age, aneurysm sac volume, and aneurysm sac thrombus volume (34, 43–46). Although our study did not find a statistical correlation between ILA and T2ELs, T2ELs caused by ILA still deserve attention.
Pre-embolization for the IMA or aneurysm sac in high-risk patients seems to be of greater benefit (14, 47, 48), and it could suppress aneurysm sac expansion and reduce the reintervention rate. A network meta-analysis (49) suggested that IMA embolization demonstrated benefits in achieving long-term aneurysm sac stability and lowering the risk of secondary surgery. Nonselective embolization of aneurysm sac side branches more effectively reduces the incidence of T2ELs, while IMA embolization alone or in combination with aneurysm sac coil embolization enhances the clinical benefits of EVAR. Sun et al. (49) analyzed whether nonselective preemptive aneurysm sac embolization could prevent T2ELs in the short and mid-term, and they interestingly found that the IMA diameter showed continuous regression in the embolization group.
However, Kontopodis et al. (50) in their meta-analysis that included four random studies, suggested that preemptive embolization confers no clinical benefits in EVAR, but their data were limited and had low certainty. Additionally, Väärämäki et al. (51) found that the strategy of routinely embolizing the IMA does not seem to yield any significant clinical benefit and should therefore be abandoned. Therefore, more standardized, and high-quality studies are needed to explore the therapeutic effects of preemptive embolization.
There are several other limitations that should be noted. The present study excluded patients with type I and III endoleaks at any time during follow-up. This may reduce the number of patients with T2ELs because some patients with T2ELs may also have type I or type III endoleaks. Another limitation is the definition of T2EL. Previous reports use various terms, such as “early”, “late”, and “persistent” T2ELs. However, we did not classify T2ELs in this study. On the one hand, there was recall bias during the telephone follow-up; on the other hand, some patients did not have regular follow-up after surgery, so the occurrence time of endoleaks was not clear.
Finally, the mode of IIA embolization was not a routine procedure and was only performed in a relatively specialized anatomical population. Aortoiliac aneurysms with insufficient distal anchor area of the common iliac artery may lead to type Ib endoleaks (52). In some aortoiliac abdominal aortic aneurysms or AAA with internal iliac aneurysms and with a short common iliac artery, stents need to extend to the external iliac artery or even embolize the ipsilateral internal iliac artery. Future studies may need to collect the anatomical characteristics of the patent lateral branches after EVAR to identify the risk factors further accurately for type II endoleaks through the changes before and after surgery.
Conclusion
The iliolumbar artery and the different internal iliac artery interventions may not increase the incidence of T2Els. But the number of patent lumbar arteries (≥6) and the diameter of the inferior mesenteric artery (≥2.5 mm) were independent risk factors for T2ELs. More rigorous studies are still needed to explore the risk factors for T2ELs.T2ELs was associated with the reintervention but did not affect long-term survival or increase aneurysm-related mortality after EVAR.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of West China Hospital, Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
GC and DL are joint first authors and wrote the manuscript. JZ, as the corresponding author, was responsible for the review and submission of the manuscript. CC and CW were responsible for the data analysis and data inspection and were in charge of statistical method correction. JW was responsible of the telephone follow-up. TW, DY and BH were responsible for anatomic data verification. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1210248/full#supplementary-material
SUPPLEMENTAL FIGURE 1
Overall survival did not differ between T2ELs group and non-T2Els group after PSM (p = 0.58, log rank test).
SUPPLEMENTAL FIGURE 2
AAA-related survival did not differ between T2ELs group and non-T2Els group after PSM (p = 0.6, log rank test).
SUPPLEMENTAL FIGURE 3
Patients with T2ELs had more reinterventions than those without T2ELs. (p = 0.034, log rank test).
SUPPLEMENTAL FIGURE 4.
Flow chart of cases inclusion and expulsion.
References
1. Wanhainen A, Verzini F, Van Herzeele I, Allaire E, Bown M, Cohnert T, et al. Editor’s choice - European society for vascular surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. (2019) 57(1):8–93. doi: 10.1016/j.ejvs.2018.09.020
2. US Preventive Services Task Force, Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, et al. Screening for abdominal aortic aneurysm: US preventive services task force recommendation statement. JAMA. (2019) 322(22):2211–8. doi: 10.1001/jama.2019.18928
3. Sweeting MJ, Patel R, Powell JT, Greenhalgh RM, Investigators ET. Endovascular repair of abdominal aortic aneurysm in patients physically ineligible for open repair: very long-term follow-up in the EVAR-2 randomized controlled trial. Ann Surg. (2017) 266(5):713–9. doi: 10.1097/SLA.0000000000002392
4. Antoniou GA, Antoniou SA, Torella F. Editor’s choice - endovascular vs. open repair for abdominal aortic aneurysm: systematic review and meta-analysis of updated peri-operative and long term data of randomised controlled trials. Eur J Vasc Endovasc Surg. (2020) 59(3):385–97. doi: 10.1016/j.ejvs.2019.11.030
5. Li B, Khan S, Salata K, Hussain MA, de Mestral C, Greco E, et al. A systematic review and meta-analysis of the long-term outcomes of endovascular versus open repair of abdominal aortic aneurysm. J Vasc Surg. (2019) 70(3):954–69 e30. doi: 10.1016/j.jvs.2019.01.076
6. Lal BK, Zhou W, Li Z, Kyriakides T, Matsumura J, Lederle FA, et al. Predictors and outcomes of endoleaks in the veterans affairs open versus endovascular repair (OVER) trial of abdominal aortic aneurysms. J Vasc Surg. (2015) 62(6):1394–404. doi: 10.1016/j.jvs.2015.02.003
7. Ultee KHJ, Buttner S, Huurman R, Bastos Goncalves F, Hoeks SE, Bramer WM, et al. Editor’s choice - systematic review and meta-analysis of the outcome of treatment for type II endoleak following endovascular aneurysm repair. Eur J Vasc Endovasc Surg. (2018) 56(6):794–807. doi: 10.1016/j.ejvs.2018.06.009
8. Guo Q, Du X, Zhao J, Ma Y, Huang B, Yuan D, et al. Prevalence and risk factors of type II endoleaks after endovascular aneurysm repair: a meta-analysis. PLoS One. (2017) 12(2):e0170600. doi: 10.1371/journal.pone.0170600
9. Sidloff DA, Stather PW, Choke E, Bown MJ, Sayers RD. Type II endoleak after endovascular aneurysm repair. Br J Surg. (2013) 100(10):1262–70. doi: 10.1002/bjs.9181
10. Bryce Y, Schiro B, Cooper K, Ganguli S, Khayat M, Lam CK, et al. Type II endoleaks: diagnosis and treatment algorithm. Cardiovasc Diagn Ther. (2018) 8(Suppl 1):S131–S7. doi: 10.21037/cdt.2017.08.06
11. Axelrod DJ, Lookstein RA, Guller J, Nowakowski FS, Ellozy S, Carroccio A, et al. Inferior mesenteric artery embolization before endovascular aneurysm repair: technique and initial results. J Vasc Interv Radiol. (2004) 15(11):1263–7. doi: 10.1097/01.RVI.0000141342.42484.90
12. Samura M, Morikage N, Otsuka R, Mizoguchi T, Takeuchi Y, Nagase T, et al. Endovascular aneurysm repair with Inferior mesenteric artery embolization for preventing type II endoleak: a prospective randomized controlled trial. Ann Surg. (2020) 271(2):238–44. doi: 10.1097/SLA.0000000000003299
13. Yu HYH, Lindstrom D, Wanhainen A, Tegler G, Hassan B, Mani K. Systematic review and meta-analysis of prophylactic aortic side branch embolization to prevent type II endoleaks. J Vasc Surg. (2020) 72(5):1783–92 e1. doi: 10.1016/j.jvs.2020.05.020
14. Li Q, Hou P. Sac embolization and Side branch embolization for preventing type II endoleaks after endovascular aneurysm repair: a meta-analysis. J Endovasc Ther. (2020) 27(1):109–16. doi: 10.1177/1526602819878411
15. Vaillant M, Barral PA, Mancini J, De Masi M, Bal L, Piquet P, et al. Preoperative inferior mesenteric artery embolization is a cost-effective technique that may reduce the rate of aneurysm sac diameter enlargement and reintervention after EVAR. Ann Vasc Surg. (2019) 60:85–94. doi: 10.1016/j.avsg.2019.03.012
16. Dosluoglu HH, Rivero M, Khan SZ, Cherr GS, Harris LM, Dryjski ML. Pre-emptive nonselective perigraft aortic sac embolization with coils to prevent type II endoleak after endovascular aneurysm repair. J Vasc Surg. (2019) 69(6):1736–46. doi: 10.1016/j.jvs.2018.10.054
17. Manunga JM, Cragg A, Garberich R, Urbach JA, Skeik N, Alexander J, et al. Preoperative inferior mesenteric artery embolization: a valid method to reduce the rate of type II endoleak after EVAR? Ann Vasc Surg. (2017) 39:40–7. doi: 10.1016/j.avsg.2016.05.106
18. Ward TJ, Cohen S, Fischman AM, Kim E, Nowakowski FS, Ellozy SH, et al. Preoperative inferior mesenteric artery embolization before endovascular aneurysm repair: decreased incidence of type II endoleak and aneurysm sac enlargement with 24-month follow-up. J Vasc Interv Radiol. (2013) 24(1):49–55. doi: 10.1016/j.jvir.2012.09.022
19. Otsu M, Ishizaka T, Watanabe M, Hori T, Kohno H, Ishida K, et al. Analysis of anatomical risk factors for persistent type II endoleaks following endovascular abdominal aortic aneurysm repair using CT angiography. Surg Today. (2016) 46(1):48–55. doi: 10.1007/s00595-015-1115-5
20. Piazza M, Squizzato F, Zavatta M, Menegolo M, Ricotta JJ 2nd, Lepidi S, et al. Outcomes of endovascular aneurysm repair with contemporary volume-dependent sac embolization in patients at risk for type II endoleak. J Vasc Surg. (2016) 63(1):32–8. doi: 10.1016/j.jvs.2015.08.049
21. Heye S. Preoperative internal iliac artery coil embolization for aneurysms involving the iliac bifurcation. Acta Chir Belg. (2006) 106(2):144–8. doi: 10.1080/00015458.2006.11679861
22. Meshii K, Sugimoto M, Niimi K, Kodama A, Banno H, Komori K. The association between perioperative embolization of hypogastric arteries and type II endoleaks after endovascular aortic aneurysm repair. J Vasc Surg. (2021) 73(1):99–107. doi: 10.1016/j.jvs.2020.04.505
23. Koc T, Gilan IY, Aktekin M, Kurtoglu Z, Dagtekin A, Aytac G, et al. Evaluation of the origin and branching patterns of the iliolumbar artery and its implications on pelvic and vertebral surgery. Saudi Med J. (2016) 37(4):457–60. doi: 10.15537/smj.2016.4.12665
24. Winters HA, van Harten SM, van Royen BJ. The iliolumbar artery as the nutrient pedicle for an iliac crest graft: a new technique in reconstruction of the lumbar spine. Plast Reconstr Surg. (2002) 109(1):249–52. doi: 10.1097/00006534-200201000-00039
25. Teli CG, Kate NN, Kothandaraman U. Morphometry of the iliolumbar artery and the iliolumbar veins and their correlations with the lumbosacral trunk and the obturator nerve. J Clin Diagn Res. (2013) 7(3):422–6. doi: 10.7860/jcdr/2013/4763.2789
26. Rokosh RS, Wu WW, Dalman RL, Chaikof EL. Society for vascular surgery implementation of clinical practice guidelines for patients with an abdominal aortic aneurysm: endoleak management. J Vasc Surg. (2021) 74(6):1792–4. doi: 10.1016/j.jvs.2021.04.042
27. Seike Y, Matsuda H, Shimizu H, Ishimaru S, Hoshina K, Michihata N, et al. Nationwide analysis of persistent type II endoleak and late outcomes of endovascular abdominal aortic aneurysm repair in Japan: a propensity-matched analysis. Circulation. (2022) 145(14):1056–66. doi: 10.1161/CIRCULATIONAHA.121.056581
28. Wang Y, Yuan F, Bai Y, Yao W, Zhou C, Liu J, et al. Natural history and influence on long-term outcomes of isolated type II endoleak after endovascular aneurysm repair: a 10-year experience at a single center. Rev Cardiovasc Med. (2022) 23(3):99. doi: 10.31083/j.rcm2303099
29. Chaikof EL, Dalman RL, Eskandari MK, Jackson BM, Lee WA, Mansour MA, et al. The society for vascular surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J Vasc Surg. (2018) 67(1):2–77. doi: 10.1016/j.jvs.2017.10.044
30. Akmal MM, Pabittei DR, Prapassaro T, Suhartono R, Moll FL, van Herwaarden JA. A systematic review of the current status of interventions for type II endoleak after EVAR for abdominal aortic aneurysms. Int J Surg. (2021) 95:106138. doi: 10.1016/j.ijsu.2021.106138
31. Guo Q, Zhao J, Ma Y, Huang B, Yuan D, Yang Y, et al. A meta-analysis of translumbar embolization versus transarterial embolization for type II endoleak after endovascular repair of abdominal aortic aneurysm. J Vasc Surg. (2020) 71(3):1029–34. doi: 10.1016/j.jvs.2019.05.074
32. Nana P, Spanos K, Heidemann F, Panuccio G, Kouvelos G, Rohlffs F, et al. Systematic review on transcaval embolization for type II endoleak after endovascular aortic aneurysm repair. J Vasc Surg. (2022) 76(1):282–91.e2. doi: 10.1016/j.jvs.2022.02.032
33. Iwakoshi S, Ogawa Y, Dake MD, Ono Y, Higashihara H, Ikoma A, et al. Outcomes of embolization procedures for type II endoleaks following endovascular abdominal aortic repair. J Vasc Surg. (2023) 77(1):114–21.e2. doi: 10.1016/j.jvs.2022.07.168
34. Ide T, Masada K, Kuratani T, Sakaniwa R, Shimamura K, Kin K, et al. Risk analysis of aneurysm sac enlargement caused by type II endoleak after endovascular aortic repair. Ann Vasc Surg. (2021) 77:208–16. doi: 10.1016/j.avsg.2021.06.013
35. Lo RC, Buck DB, Herrmann J, Hamdan AD, Wyers M, Patel VI, et al. Risk factors and consequences of persistent type II endoleaks. J Vasc Surg. (2016) 63(4):895–901. doi: 10.1016/j.jvs.2015.10.088
36. van Marrewijk CJ, Fransen G, Laheij RJ, Harris PL, Buth J. Is a type II endoleak after EVAR a harbinger of risk? Causes and outcome of open conversion and aneurysm rupture during follow-up. Eur J Vasc Endovasc Surg. (2004) 27(2):128–37. doi: 10.1016/j.ejvs.2003.10.016
37. Cao Z, Zhu R, Ghaffarian A, Wu W, Weng C, Chen X, et al. A systematic review and meta-analysis of the clinical effectiveness and safety of unilateral versus bilateral iliac branch devices for aortoiliac and iliac artery aneurysms. J Vasc Surg. (2022) 76(4):1089–98.e8. doi: 10.1016/j.jvs.2022.03.005
38. van der Veen D, Holewijn S, Bellosta R, van Sterkenburg SMM, Heyligers JMM, Ficarelli I, et al. One year outcomes of an international multicentre prospective cohort study on the gore excluder iliac branch endoprosthesis for aorto-iliac aneurysms. Eur J Vasc Endovasc Surg. (2021) 62(2):177–85. doi: 10.1016/j.ejvs.2021.04.006
39. Chen RS, Liu YX, Liu CB, Hu YS, Xu DC, Zhong SZ, et al. Anatomic basis of iliac crest flap pedicled on the iliolumbar artery. Surg Radiol Anat. (1999) 21(2):103–7. doi: 10.1007/s00276-999-0103-0
40. Kiray A, Akçalı O, Tayefi H, Koşay C, Ergür I. Anatomical variations of iliolumbar artery and its relation with surgical landmarks. Acta Orthop Traumatol Turc. (2010) 44(6):464–8. doi: 10.3944/AOTT.2010.2347
41. Cynamon J, Lerer D, Veith FJ, Taragin BH, Wahl SI, Lautin JL, et al. Hypogastric artery coil embolization prior to endoluminal repair of aneurysms and fistulas: buttock claudication, a recognized but possibly preventable complication. J Vasc Interv Radiol. (2000) 11(5):573–7. doi: 10.1016/S1051-0443(07)61608-X
42. Luo H, Huang B, Yuan D, Yang Y, Xiong F, Zeng G, et al. 8-Year long-term outcome comparison: two ways to exclude the internal iliac artery during endovascular aorta repair (EVAR) surgery. PLoS One. (2015) 10(7):e0130586. doi: 10.1371/journal.pone.0130586
43. Piazza M, Frigatti P, Scrivere P, Bonvini S, Noventa F, Ricotta JJ, et al. Role of aneurysm sac embolization during endovascular aneurysm repair in the prevention of type II endoleak-related complications. J Vasc Surg. (2013) 57(4):934–41. doi: 10.1016/j.jvs.2012.10.078
44. AbuRahma AF, Mousa AY, Campbell JE, Stone PA, Hass SM, Nanjundappa A, et al. The relationship of preoperative thrombus load and location to the development of type II endoleak and sac regression. J Vasc Surg. (2011) 53(6):1534–41. doi: 10.1016/j.jvs.2011.02.016
45. Fujii T, Banno H, Kodama A, Sugimoto M, Akita N, Tsuruoka T, et al. Aneurysm sac thrombus volume predicts aneurysm expansion with type II endoleak after endovascular aneurysm repair. Ann Vasc Surg. (2020) 66:85–94.e1. doi: 10.1016/j.avsg.2019.11.045
46. Müller-Wille R, Güntner O, Zeman F, Dollinger M, Hälg C, Beyer LP, et al. The influence of preoperative aneurysmal thrombus quantity and distribution on the development of type II endoleaks with aneurysm sac enlargement after EVAR of AAA. Cardiovasc Intervent Radiol. (2016) 39(8):1099–109. doi: 10.1007/s00270-016-1386-2
47. Aoki A, Maruta K, Omoto T, Masuda T. Midterm outcomes of endovascular abdominal aortic aneurysm repair with prevention of type 2 endoleak by intraoperative aortic Side branch coil embolization. Ann Vasc Surg. (2021) 78:180–9. doi: 10.1016/j.avsg.2021.06.037
48. Xiang Y, Huang B. Inferior mesenteric artery embolization reduced the incidence of persistent type 2 endoleak and related reintervention after EVAR without promoting aneurysm shrinkage. J Endovasc Ther. (2022) 30(4):641–2. doi: 10.1177/15266028221091887
49. Wu Y, Yin J, Hongpeng Z, Wei G. Systematic review and network meta-analysis of pre-emptive embolization of the aneurysm sac side branches and aneurysm sac coil embolization to improve the outcomes of endovascular aneurysm repair. Front Cardiovasc Med. (2022) 9:947809. doi: 10.3389/fcvm.2022.947809
50. Kontopodis N, Galanakis N, Kiparakis M, Ioannou CV, Kakisis I, Geroulakos G, et al. Pre-emptive embolization of the aneurysm sac or aortic Side branches in endovascular aneurysm repair: meta-analysis and trial sequential analysis of randomized controlled trials. Ann Vasc Surg. (2022) 9:90–107. doi: 10.1016/j.avsg.2022.10.027
51. Väärämäki S, Viitala H, Laukontaus S, Uurto I, Björkman P, Tulamo R, et al. Routine inferior mesenteric artery embolisation is unnecessary before endovascular aneurysm repair. Eur J Vasc Endovasc Surg. (2023) 65(2):264–70. doi: 10.1016/j.ejvs.2022.11.009
Keywords: abdominal aortic aneurysm, endovascular aortic repair, type 2 endoleaks, iliolumbar artery, risk factors
Citation: Chen GX, Liu D, Weng C, Chen C, Wan J, Zhao J, Yuan D, Huang B and Wang T (2023) Patent iliolumbar artery increase no risk of type II endoleaks after endovascular abdominal aortic aneurysm: a case-control study. Front. Cardiovasc. Med. 10:1210248. doi: 10.3389/fcvm.2023.1210248
Received: 22 April 2023; Accepted: 31 July 2023;
Published: 11 August 2023.
Edited by:
Wolf-Hans Eilenberg, Medical University of Vienna, AustriaReviewed by:
Konstantinos Spanos, University of Thessaly, GreeceDonglin Li, Zhejiang University, China
© 2023 Chen, Liu, Weng, Chen, Wan, Zhao, Yuan, Huang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jichun Zhao emhhb2ppY2h1bmRvY0AxNjMuY29t
†These authors share first authorship
 Guo Xin Chen
Guo Xin Chen Dan Liu2,†
Dan Liu2,† Chuwen Chen
Chuwen Chen Jichun Zhao
Jichun Zhao Ding Yuan
Ding Yuan Bin Huang
Bin Huang Tiehao Wang
Tiehao Wang