
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 01 August 2023
Sec. Cardiovascular Biologics and Regenerative Medicine
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1208227
This article is part of the Research Topic Application of Stem Cells in the Treatment of Myocardial Infarction View all 6 articles
 Ping Lai1,2,†
Ping Lai1,2,† Ming Sheng3,†
Ming Sheng3,† Jin-hua Ye4,†
Jin-hua Ye4,† Zhi-xian Tang5
Zhi-xian Tang5 Shuo Hu6
Shuo Hu6 Bei Wang1
Bei Wang1 Jing-lin Yuan1
Jing-lin Yuan1 Yi-hong Yang1
Yi-hong Yang1 Yi-ming Zhong1,2*
Yi-ming Zhong1,2* Yong-ling Liao1,2*
Yong-ling Liao1,2*
Background: Cardiovascular tissue engineering (CTE) is a promising technique to treat incurable cardiovascular diseases, such as myocardial infarction and ischemic cardiomyopathy. Plenty of studies related to CTE have been published in the last 30 years. However, an analysis of the research status, trends, and potential directions in this field is still lacking. The present study applies a bibliometric analysis to reveal CTE research trends and potential directions.
Methods: On 5 August 2022, research articles and review papers on CTE were searched from the Web of Science Core Collection with inclusion and exclusion criteria. Publication trends, research directions, and visual maps in this field were obtained using Excel (Microsoft 2009), VOSviewer, and Citespace software.
Results: A total of 2,273 documents from 1992 to 2022 were included in the final analysis. Publications on CTE showed an upward trend from 1992 [number of publications (Np):1] to 2021 (Np:165). The United States (Np: 916, number of citations: 152,377, H-index: 124) contributed the most publications and citations in this field. Research on CTE has a wide distribution of disciplines, led by engineering (Np: 788, number of citations: 40,563, H-index: 105). “Functional maturation” [red cluster, average published year (APY): 2018.63, 30 times], “cell-derived cardiomyocytes” (red cluster, APY: 2018.43, 46 times), “composite scaffolds” (green cluster, APY: 2018.54, 41 times), and “maturation” (red cluster, APY: 2018.17, 84 times) are the main emerging keywords in this area.
Conclusion: Research on CTE is a hot research topic. The United States is a dominant player in CTE research. Interdisciplinary collaboration has played a critical role in the progress of CTE. Studies on functional maturation and the development of novel biologically relevant materials and related applications will be the potential research directions in this field.
Cardiovascular diseases, including myocardial infarction, ischemic cardiomyopathy, and heart failure, are caused by malfunctioning valves, blockage of blood vessels, or damaged heart muscle, and are the leading cause of death globally (1). Recent decades have seen significant advances in the treatment of these diseases (2, 3). However, novel therapies are required for regenerating the cardiomyocytes or tissues in the diseased heart tissues of patients with myocardial infarction or heart valve diseases (4).
Stem cells, such as induced pluripotent stem cells (iPSCs) (5) and embryonic stem cells (ESCs) (6), are characterized by their ability to self-renew and differentiate into various cell types in the human body (7), including cardiomyocytes (8). Currently, stem cells are increasingly being used in the field of regenerative medicine (9), especially in cardiovascular diseases (10). Several methods have been developed for delivering stem cells to the injury site to replace the lost cardiomyocytes in patients with cardiovascular diseases (11). However, the direct delivery of stem cells has not been successful because the percentage of the delivered stem cells differentiating into functional cardiomyocytes was significantly low and several cases showed the formation of teratomas (12, 13). Therefore, to overcome these issues, stem cells were first in vitro differentiated into cardiomyocytes and then transferred to the impaired site (14). However, the results were unsatisfactory because of a high rate of cell death and a limited number of viable cardiomyocytes after in-vitro differentiation (15).
The emergence of tissue engineering has further highlighted the clinical application of stem cells. For example, the survival of cardiomyocytes was significantly increased by attachment to a suitable supporting surface (16). The goal of tissue engineering is to assemble functional scaffolds with biologically acceptable materials, which provide a viable cellular environment for restoring, maintaining, or improving damaged tissues or whole organs (17). Tissue engineering takes into account the 3-dimensional (3D) structure of the tissue and combines the scaffolding biomaterials with the stem cells to gradually develop functional tissues, either completely or partly (18). Cardiovascular tissue engineering (CTE) involves the generation of specific high-differentiating cardiac tissues including heart muscles, valves, or vessels for the study and treatment of cardiovascular disease (19, 20). In the last three decades, the development of CTE has been rapid, including studies on novel biomaterials (21), mechanisms of cardiovascular disease (22), and the recovery of heart function (23).
Bibliometrics is the statistical analysis of publications in the area of interest and includes quantitative analysis of the literature regarding the characteristics of the publications, author output and impact, keywords, and references (24). Bibliometrics is widely used in many fields of medicine, including surgery (25), internal medicine (26), signaling pathways (27), and stem cell research (28).
In this study, we performed bibliometric analysis to determine the current status and challenges, evolutionary path, research hotspots, and future research direction in the area of CTE.
The Web of Science Core Collection (WoSCC) was used as the data source for our research. WoSCC is the world's leading citation database with the most comprehensive collection of articles from high-impact journals, open-source journals, conference meetings, and books. Therefore, we performed the following search in the WoSCC database on 5 August 2022: TI = “engineered tissue or tissue engineering” AND TS = “cardiovascular or heart or cardiac”; dates, “1992-01-01 to 2022-07-31”. Only articles and reviews that were published in English were extracted. The search records, including names of all the authors, titles of the articles or reviews, time of publication, journal title, affiliations, keywords, citations, and references were exported as plain text and Excel files. The flow chart of the bibliometric analysis is shown in Figure 1.
Microsoft Excel 2009 was used to analyze the citations over time (each year) and the distribution of countries or regions, top authors, research institutions, research areas, and journals. The number of publications (Np) was calculated for all authors, countries, journals, and other specified parameters. The number of citations without self-citations (Nc) and the average citation number (ACN; total citations/total publications) were used for estimating the degree of influence (high or low) of the author or the journal. The h-index from WoSCC was used to assess the scholarly contributions of the researchers and forecast the future scientific achievements of the investigators, countries, or institutions. The h-index was also used to determine the influence of the journal's publications. The main bibliometric indicators included the total number of publications, main authors (including institutions and countries), and citations. VOSviewer (v.1.6.18, CWTS, Leiden University) was used to visualize the network of co-citations, authors, countries, and keywords (without the search items). JAVA-based VOSviewer and CiteSpace (version 6.1.R3) tools were used to analyze the clusters of keywords from publications with high citation bursts. CiteSpace parameters were as follows: period (1992–2022); years per slice (one year); term source (title, abstract, author keyword, and keyword plus); node types (keyword); links (strength: cosine, scope: within slices); selection criteria (g-index: k = 25); and pruning (minimum spanning tree, pruning sliced networks, and pruning the merged network).
The final bibliometric analysis included 2,273 publications, of which 1,670 were articles and 603 were reviews. The total number of publications on CTE per year showed an upward trend from 1992 (Np:1) to 2021 (Np:165). Since 2009, more than 100 publications were published on CTE every year except in 2010 (Np:83). The highest number of publications on CTE was published in 2019 (Np:173). The citations of all the publications on CTE matched the trend of the publications on CTE. The total number of citations on CTE increased significantly after 2015 and peaked in 2021 with 14,189 citations (Figures 2A,B).
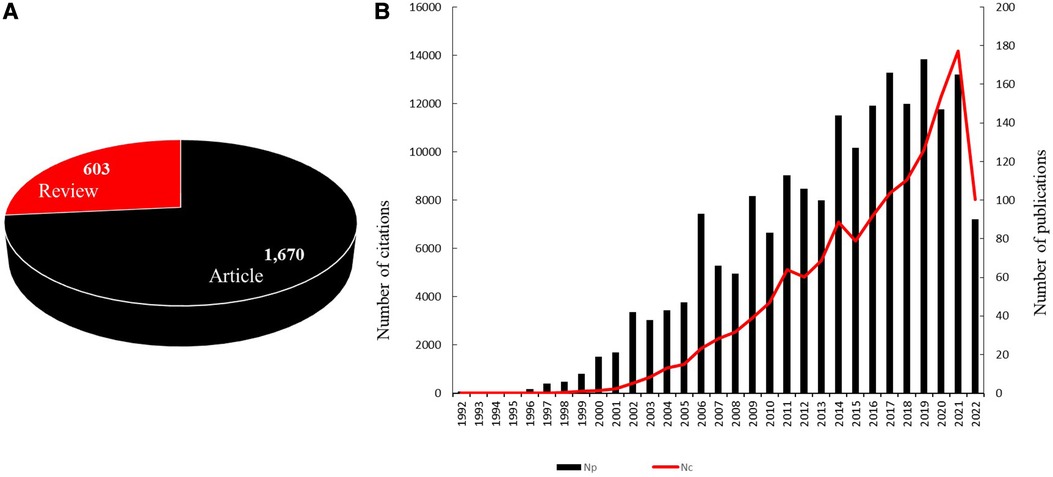
Figure 2. (A) Type of publications included in the current study. (B) The trend of publications and citations globally in the CTE research area between 1992 and 2022.
The United States (Np:916), Germany (Np:315), and China (Np:223) were the top three countries with the highest number of citations in the field of CTE. The top three countries accounted for 1,454 (63.97%) publications out of the 2,273 publications analyzed. The top three countries for the ACN index were the USA, China, and Germany (Table 1). This indicated robust research regarding CTE in these three countries.
Figure 3 shows the map of countries/regions with more than 20 publications over time. The highest number of publications were from research labs in the USA. Furthermore, in the early period, the USA and Germany led the research regarding CTE, whereas China and Iran are emerging countries in this area. Furthermore, researchers from the USA collaborated significantly with researchers from South Korea, Japan, Canada, and Australia; researchers from Germany collaborated closely with researchers from England, the Netherlands, France, Switzerland, and Spain; researchers from China collaborated with researchers from Singapore, Malaysia, Poland, and Spain (Figure 3).
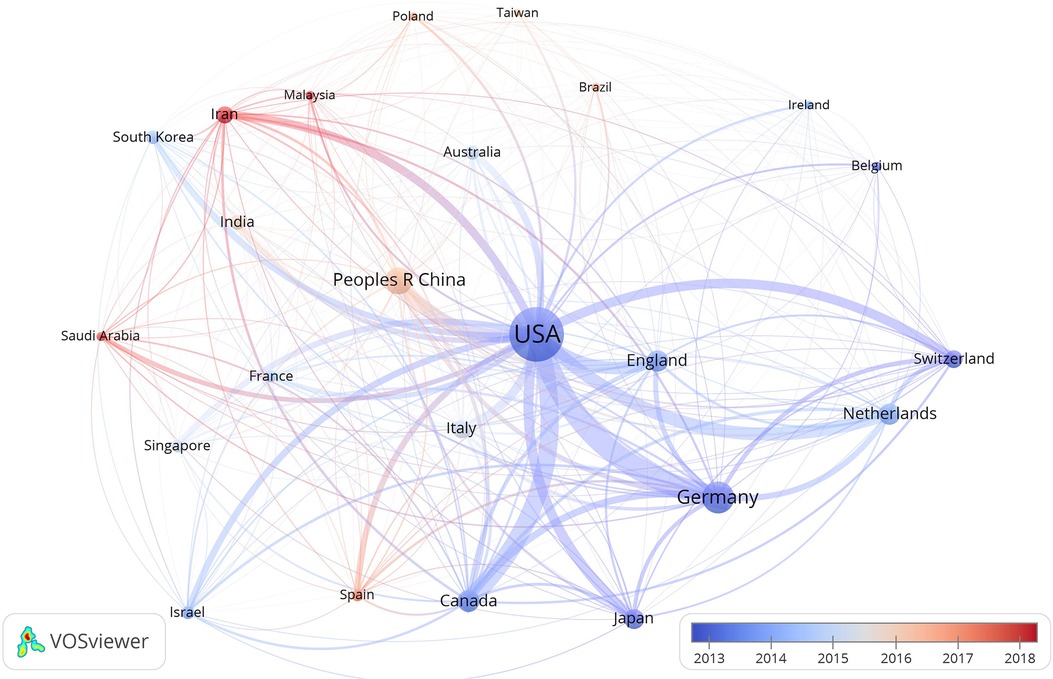
Figure 3. Map of countries/regions with more than 20 publications on the topic of CTE. The node size indicates the number of papers; the color of the node indicates the year in which the country/region started research in CTE, with blue denoting early, and red denoting late; cooperation between the countries/regions is represented by a line.
Among the top ten productive authors, Hoerstrup SP (Np:75) from Switzerland ranked first, followed by Eschenhagen T (Np:65) from Germany and Radisic M (Np:58) from Canada. Furthermore, four of the top ten productive authors were from the USA, and two each were from Germany and the Netherlands (Table 2). Eschenhagen T worked closely with Eder A, and Hoerstrup SP collaborated with Vacanti JP and Turina M (Figures 4A,B).
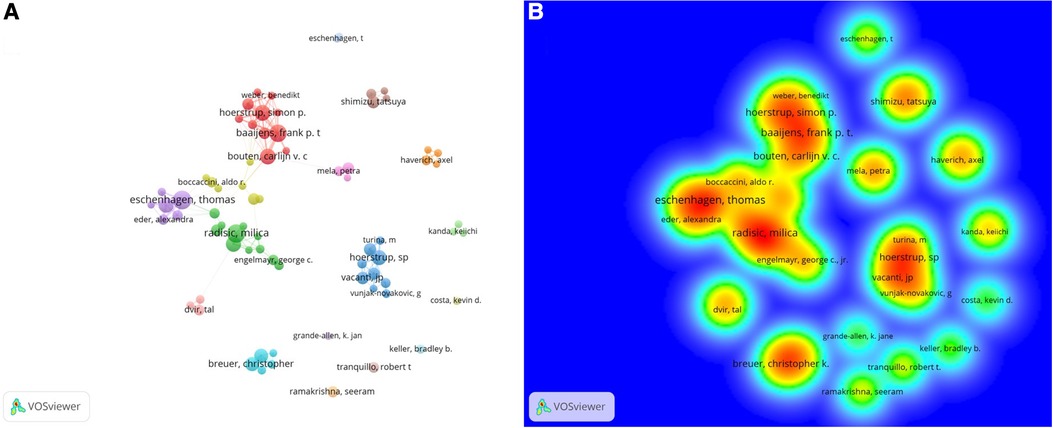
Figure 4. (A) Visual network of co-authors in CTE research. Each node represents an author; authors with close relationships are allocated to one cluster with a specific color. (B) The density visualization map shows the co-citation relationship between authors and co-authors.
Harvard University was the top research institution with 154 publications, followed by the Eindhoven University of Technology with 93 publications and the University of Toronto with 79 publications. The Massachusetts Institute of Technology (MIT) from the USA showed the highest ACN (125.17), thereby suggesting a significant influence of the USA in CTE research (Table 2).
The top three research disciplines with the highest number of publications were engineering (Np: 788, Nc: 40,563, H-index: 105), materials science (Np: 752, Nc:41,762, H-index: 105), and cell biology (Np: 539, Nc: 23,259, H-index: 79) (Table 3).
Among the top ten journals with the greatest number of publications, Tissue Engineering Part A (Np: 84, Nc: 3,049, ACN: 36.30) ranked first and was followed by Biomaterials (Np: 82, Nc:14,138, ACN: 172.41) and Acta Bio-material (Np: 64, Nc:4,156, ACN: 64.94). Furthermore, three of the top ten journals, namely, Biomaterials, Acta Biomaterial, and Circulation, were high-impact factor journals with an impact factor ≥10.0 (Table 4). Biomaterials showed the highest ACN value of 172.41 (Table 4).
Among the top ten papers with the most co-citations, nine were articles and one was a review. Seven of these were published after the year 2000. The earliest article was published in 1993 and the most recent article among the top ten was published in 2008. Zimmermann WH was the only author with two publications among the top ten most co-cited papers. Circulation, Science, and Nature Medicine had two articles each featuring among the top ten most co-cited papers (Table 5). Furthermore, 151 papers had more than 50 co-citations (Figure 5).
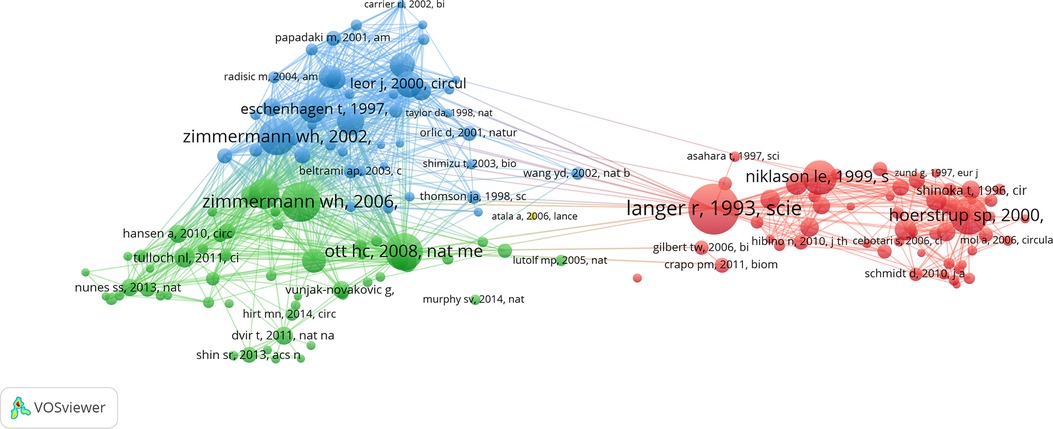
Figure 5. Map of the most co-cited publications (more than 50 times). The node size denotes the number of citations, with a larger node representing a higher number of citations. The clusters are represented by different colors. The lines connecting different nodes show the relationship between different publications.
The first important research paper in this area was a review in Science by Langer et al. titled “Tissue Engineering”, which was published in 1993. This review laid the foundations in tissue engineering and addressed potential challenges and applications in tissue regeneration and repair. Another highly cited paper was published in Nature Medicine by Zimmermann et al., in 2006, and was titled “Engineered Heart Tissue Grafts Improve Systolic and Diastolic Function in Infarcted Rat Hearts”. This study demonstrated the construction of large contractile cardiac tissue grafts in vitro and confirmed their viability after implantation and their ability to enhance the contractile function of infarcted hearts. In 2008, Ott et al. published a groundbreaking paper in Nature Medicine titled “Perfusion-Decellularized Matrix: Using Nature's Platform to Engineer a Bioartificial Heart”. This was the third-highest cited paper in this field. This study introduced the concept of perfusion decellularization for the production of a complex and biocompatible cardiac extracellular matrix scaffold, which was characterized by a perfusable vascular architecture, competent acellular valves, and an intact four-chamber geometry that provided a biomimetic template for tissue engineering (Table 5).
We analyzed 6,970 keywords extracted from 2,273 papers using the VOSviewer but excluded search terms, synonyms, and duplicates (e.g., tissue engineering, heart, cardiac tissue engineering, cardiac tissue, heart tissue, and engineered heart tissue). The top five keywords were in-vitro (474 times), scaffolds (295 times), extracellular-matrix (277 times), mesenchymal stem cells (266 times), and cardiomyocytes (223 times) (Table 6).
The most frequent keywords (≥30 times) were categorized into four clusters that represented four different directions in the CTE research area (Figure 6A). The most frequent keywords in the red cluster included cardiomyocytes (223 times), differentiation (216 times), transplantation (177 times), model (151 times), myocardial infarction (139 times), progenitor cells (118 times), and regeneration (116 times).
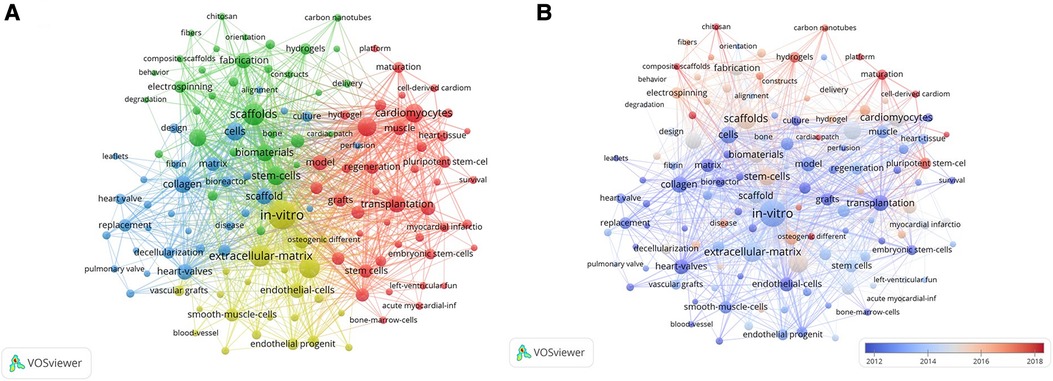
Figure 6. (A) Network map of high-frequency keywords that occur more than 30 times. A larger node size denotes a higher frequency of keyword occurrence. Different colors represent different clusters, and the lines between different nodes stand for the relationship between different keywords; (B) A visual map of high-frequency keywords that occurred more than 30 times over time. The node size denotes the frequency of the keyword with a larger node size representing a higher frequency. The average time of occurrence of the keywords is indicated by different colors with the blue color denoting older keywords, and red denoting the newer keywords. The relationship between different keywords is denoted by lines between different nodes.
The most frequent keywords in the green cluster included scaffolds (295 times), stem cells (214 times), biomaterials (185 times), mechanical properties (184 times), and fabrication (147 times).
The main research topics in the blue cluster were cells (200 times), collagen (186 times), scaffold (168 times), heart valves (160 times), matrix (133 times), replacement (105 times), and extracellular matrix (101 times).
The main keywords in the yellow cluster were in-vitro (474 times), extracellular matrix (277 times), mesenchymal stem cells (266 times), endothelial cells (143 times), regenerative medicine (135 times), and smooth muscle cells (114 times). Furthermore, significant overlap was observed between the four clusters, thereby indicating high complementation between various aspects of research studies related to CTE.
The average published year (APY) indicated the novelty of the keywords. The keywords with a high APY index included functional maturation (red cluster, APY: 2018.63, 30 times), cell-derived cardiomyocytes (red cluster, APY: 2018.43, 46 times), maturation (red cluster, APY: 2018.17, 84 times), composite scaffolds (green cluster, APY: 2018.54, 41 times), patch (blue cluster, APY: 2017.34, 32 times), and osteogenic differentiation (yellow cluster, APY: 2018.32, 50 times). These keywords are considered potential hotspots in this research area.
Early keywords (2012 to 2014) in this research area included in-vitro, collagen, and transplantation; the emerging keywords were maturation, hydrogels, platform, multipotent stem cells, and cell-derived cardiomyocytes, which showed strong association with the other keywords and potentially represent current study frontiers in CTE (Figure 6B).
The top 35 keywords with high burst intensities and the burst year were generated by CiteSpace. The main emerging keywords with a high citation strength in the field of CTE were pluripotent stem cells (citation strength: 14.14, 2017–2023), maturation (citation strength: 13,84, 2018–2023), composite scaffolds (citation strength: 11.16, 2018–2023), hydrogels (citation strength: 9.94, 2016–2023), and nanofibrous scaffolds (citation strength: 9.45, 2019–2023) (Figure 7).
To the best of our knowledge, this study represents the first bibliometric analysis conducted on CTE. This analysis resulted in some key findings. First, CTE studies have increased over time. Second, CTE studies involve collaborative research between diverse scientific disciplines. Third, studies originating from the United States and Europe have significantly influenced the research findings on CTE so far. Moreover, China has emerged as a prominent contributor in recent years with a significantly higher number of publications among emerging countries. Lastly, functional maturation of stem cells and the application of biomaterials research are potential research hotspots in this area.
CTE offers a promising therapeutic strategy for patients with myocardial infarction and ischemic cardiomyopathy, especially in cases showing limited differentiation of the injected stem cells into cardiomyocytes even after reaching the injury site (29). There has been a significant upward trend in CTE-related research over the last three decades as indicated by 165 publications in 2021 compared to a single publication in 1992. There have been several seminal research papers in this area. In 1993, a review by Langer et al. in Science summarized the foundations and challenges of this interdisciplinary field and addressed the quest for solutions in the area of tissue regeneration and repair (30). Subsequent studies have contributed significantly to progress in CTE through novel findings and innovations. In 1997, Eschenhagen T et al. reported the generation of a novel heart muscle model system based on the three-dimensional reconstitution of embryonic cardiomyocytes (31). Additionally, Niklason L et al. showed in vitro growth of functional arteries using tissue engineering approaches (32). These pioneering studies laid the groundwork for further advancements in CTE. The use of grafts (33) and bioengineered scaffold (34) has further propelled rapid progress in the field.
Similar to other research areas (35, 36), the United States emerged as a dominant leader in CTE research, with 916 publications. This can be attributed to the following three factors: (1) Early research in CTE was initiated in the United States and a solid foundation was established for subsequent progress; (2) There has been significant emphasis on advancing CTE research in the United States as is evident from the notable contributions of authors such as Vunjak-Novakovic G (37), Shinoka T (38), Breuer CK (39), and Mayer JE (40); and (3) the United States has invested significantly in engineered heart tissue (EHT) research as evidenced by ∼700 publications in this field being supported by US-based funding sources. This financial support has fueled the progress and innovation in CTE research within the United States.
China is an emerging country in the field of CTE research, as evidenced by 223 publications between 1992 and 2021. The National Natural Science Foundation of China ranked fourth among the top ten funding organizations. This highlighted China's commitment to CTE research. Chinese researchers have primarily focused on several hot topics within the field, including the application of scaffolds (41), hydrogel (42), nanomaterial (43), and the development of injectable and conductive cardiac patches (44). These trends suggest that China will contribute significantly to the field of CTE in the future and the number of citations for publications from China is expected to increase over time.
The United States is the most influential country in the field of CTE because research papers from the USA have the highest number of citations (52,377 times). The average citation rate of papers from the United States is 57.18 times/paper. This influence is attributed to a number of high-quality studies conducted by American researchers. Moreover, several highly cited papers in the field of CTE, including “Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart” (45), “Functional living trileaflet heart valves grown in vitro” (46), “Functional arteries grown in vitro” (32), and “Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds” (34), were authored by researchers from the United States. Furthermore, four of the top ten influential institutions in the research area of CTE are from the United States. This further reiterates the high impact of the United States in this field. Researchers from Harvard University focused primarily on the three-dimensional bioprinting of engineered tissue (47) and engineered three-layer scaffolds (48). Researchers from the Massachusetts Institute of Technology (MIT) focused on the application of hydrogels (49) and nano-biomaterials (50) in EHT. Researchers from the Pennsylvania Commonwealth System of Higher Education specialized in the area of musculoskeletal tissue repair (51). Researchers from the University of California focused on the application of injectable microporous gel scaffolds in tissue engineering (52) and drugs related to the biological function of the engineered tissue (53). Our data indicated that researchers from the United States established extensive collaborations with researchers from Canada, France, and England. Cooperation between different countries and institutions was instrumental in the advances made in this field.
Multidisciplinary investigations are common in studies related to CTE and have played a pivotal role in advancing the field. CTE research encompasses a wide range of disciplines, including biochemistry, molecular biology, materials science, and cell biology. Research scholars have actively investigated various areas within these disciplines for the application of siliceous sponges (54)and virus-based scaffolds (55) in engineering cardiac tissues and gaining a deeper understanding of the processes involved in tissue transplantation (56). The development of these related disciplines has significantly advanced CTE research, fostered collaboration, and driven the progress of this field.
Keywords are words or phrases that represent the main content and ideas represented in the paper and indicate the research focus and direction. The high-frequency keywords were “in vitro” (474 times), “scaffolds” (295 times), and “extracellular-matrix” (277 times), which highlighted the main research areas in CTE. The occurrence of keywords changes over time and the emerging keywords in top journals signify the potential research directions in the field. For example, emerging keywords such as “functional maturation” (57, 58), “platform” (59), “scaffold” (60), “hydrogel” (61), and “cell-derived cardiomyocytes” (62) were not listed in the top 20 most frequently mentioned keywords but demonstrate potential research directions. This aligns with those keywords with higher average published year and strength, which further indicates those are the potential future research directions in CTE.
In summary, the CTE technique shows significant promise in treatment for patients with cardiovascular diseases, including myocardial infarction and ischemic cardiomyopathy. Since 2011, publication rates in the field of CTE have increased significantly, thereby suggesting its status as a hot research topic. The United States is a dominant player in CTE research with a significantly high number of publications and citations compared to other countries. China is emerging as a significant contributor in the field. Interdisciplinary collaboration, especially between engineering and material sciences, has played a critical role in the progress of CTE. The future directions of research in CTE include the functional maturation of stem cells and the development of novel biologically relevant materials and related applications.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
PL, YL, and YZ designed the study. PL, MS, and JY collected and chose the data. PL, MS, ZT, JY, SH, BW, JY, and YY analyzed the data and drew the figures; PL, MS, and JY drafted the manuscript. PL, YL, and YZ revised the final version of the manuscript. All authors contributed to the article and approved the submitted version.
A grant from The National Public Fund for Study Abroad and CSC Scholarship (Grant No. 202008360179), the Open Project of Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases, Ministry of Education (No. XN201804), Science and Technology Research Project of Education Department of Jiangxi Province (No.170865), and Gannan Medical University Project (No. YB201803), and the National Natural Science Foundation of China (81860337, 81960326, and 82060384) supported this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. (2020) 76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010
2. Collet JP, Thiele H, Barbato E, Barthelemy O, Bauersachs J, Bhatt DL, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. (2020) 42(14):1289–367. doi: 10.1093/eurheartj/ehaa575
3. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42(36):3599–726. doi: 10.1093/eurheartj/ehab368
4. Cho HM, Cho JY. cardiomyocyte death and genome-edited stem cell therapy for ischemic heart disease. Stem Cell Rev Rep. (2021) 17(4):1264–79. doi: 10.1007/s12015-020-10096-5
5. Campostrini G, Windt LM, van Meer BJ, Bellin M, Mummery CL. Cardiac tissues from stem cells: new routes to maturation and cardiac regeneration. Circ Res. (2021) 128(6):775–801. doi: 10.1161/CIRCRESAHA.121.318183
6. Menasche P, Vanneaux V, Hagege A, Bel A, Cholley B, Parouchev A, et al. Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J Am Coll Cardiol. (2018) 71(4):429–38. doi: 10.1016/j.jacc.2017.11.047
7. Chagastelles PC, Nardi NB. Biology of stem cells: an overview. Kidney Int Suppl. (2011) 1(3):63–7. doi: 10.1038/kisup.2011.15
8. Zakrzewski W, Dobrzynski M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. (2019) 10(1):68. doi: 10.1186/s13287-019-1165-5
9. Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. (2013) 45:e54. doi: 10.1038/emm.2013.94
10. Roshanbinfar K, Esser TU, Engel FB. Stem cells and their cardiac derivatives for cardiac tissue engineering and regenerative medicine. Antioxid Redox Signal. (2021) 35(3):143–62. doi: 10.1089/ars.2020.8193
11. Guo R, Morimatsu M, Feng T, Lan F, Chang D, Wan F, et al. Stem cell-derived cell sheet transplantation for heart tissue repair in myocardial infarction. Stem Cell Res Ther. (2020) 11(1):19. doi: 10.1186/s13287-019-1536-y
12. Bhattacharyya S, Kumar A, Lal Khanduja K. The voyage of stem cell toward terminal differentiation: a brief overview. Acta Biochim Biophys Sin. (2012) 44(6):463–75. doi: 10.1093/abbs/gms027
13. Nussbaum J, Minami E, Laflamme MA, Virag JA, Ware CB, Masino A, et al. Transplantation of undifferentiated murine embryonic stem cells in the heart: teratoma formation and immune response. FASEB J. (2007) 21(7):1345–57. doi: 10.1096/fj.06-6769com
14. Hwang NS, Varghese S, Elisseeff J. Controlled differentiation of stem cells. Adv Drug Deliv Rev. (2008) 60(2):199–214. doi: 10.1016/j.addr.2007.08.036
15. Abdelwahid E, Kalvelyte A, Stulpinas A, de Carvalho KA, Guarita-Souza LC, Foldes G. Stem cell death and survival in heart regeneration and repair. Apoptosis. (2016) 21(3):252–68. doi: 10.1007/s10495-015-1203-4
16. Curtis MW, Russell B. Cardiac tissue engineering. J Cardiovasc Nurs. (2009) 24(2):87–92. doi: 10.1097/01.JCN.0000343562.06614.49
17. Ikada Y. Challenges in tissue engineering. J R Soc Interface. (2006) 3(10):589–601. doi: 10.1098/rsif.2006.0124
18. Kim J, Koo BK, Knoblich JA. Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol. (2020) 21(10):571–84. doi: 10.1038/s41580-020-0259-3
19. Jackman CP, Shadrin IY, Carlson AL, Bursac N. Human cardiac tissue engineering: from pluripotent stem cells to heart repair. Curr Opin Chem Eng. (2015) 7:57–64. doi: 10.1016/j.coche.2014.11.004
20. Maher B. Tissue engineering: how to build a heart. Nature. (2013) 499(7456):20–2. doi: 10.1038/499020a
21. Hirt MN, Hansen A, Eschenhagen T. Cardiac tissue engineering: state of the art. Circ Res. (2014) 114(2):354–67. doi: 10.1161/CIRCRESAHA.114.300522
22. Rogers AJ, Miller JM, Kannappan R, Sethu P. Cardiac tissue chips (CTCs) for modeling cardiovascular disease. IEEE Trans Biomed Eng. (2019) 66(12):3436–43. doi: 10.1109/TBME.2019.2905763
23. Giraud MN, Guex AG, Tevaearai HT. Cell therapies for heart function recovery: focus on myocardial tissue engineering and nanotechnologies. Cardiol Res Pract. (2012) 2012:971614. doi: 10.1155/2012/971614
24. Rousseau R. Library science: forgotten founder of bibliometrics. Nature. (2014) 510(7504):218. doi: 10.1038/510218e
25. Musbahi A, Rao CB, Immanuel A. A bibliometric analysis of robotic surgery from 2001 to 2021. World J Surg. (2022) 46(6):1314–24. doi: 10.1007/s00268-022-06492-2
26. Lai P, Xue JH, Xie MJ, Ye JH, Tian KJ, Ling JY, et al. Emerging trends in sacubitril/valsartan research: a bibliometric analysis of the years 1995-2021. Medicine (Baltimore). (2022) 101(31):e29398. doi: 10.1097/MD.0000000000029398
27. Xu JQ, Tang N, Zhang LF, Tan C, Su Y, George DM, et al. A bibliometric analysis of Wnt signaling pathway: from the top-100 cited articles to emerging trends. Ann Transl Med. (2021) 9(13):1065. doi: 10.21037/atm-21-174
28. Chen C, Lou Y, Li XY, Lv ZT, Zhang LQ, Mao W. Mapping current research and identifying hotspots on mesenchymal stem cells in cardiovascular disease. Stem Cell Res Ther. (2020) 11(1):498. doi: 10.1186/s13287-020-02009-7
29. Mingliang R, Bo Z, Zhengguo W. Stem cells for cardiac repair: status, mechanisms, and new strategies. Stem Cells Int. (2011) 2011:310928. doi: 10.4061/2011/310928
30. Langer R, Vacanti JP. Tissue engineering. Science. (1993) 260(5110):920–6. doi: 10.1126/science.8493529
31. Eschenhagen T, Fink C, Remmers U, Scholz H, Wattchow J, Weil J, et al. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. FASEB J. (1997) 11(8):683–94. doi: 10.1096/fasebj.11.8.9240969
32. Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, et al. Functional arteries grown in vitro. Science. (1999) 284(5413):489–93. doi: 10.1126/science.284.5413.489
33. Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. (2006) 12(4):452–8. doi: 10.1038/nm1394
34. Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, et al. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci U S A. (2004) 101(52):18129–34. doi: 10.1073/pnas.0407817101
35. Lai P, Liu YH, Xue JH, He PC, Qiu YQ. The 100 most-cited articles on aortic dissection. BMC Cardiovasc Disord. (2017) 17(1):30. doi: 10.1186/s12872-016-0426-9
36. Xue JH, Hu ZP, Lai P, Cai DQ, Wen ES. The 100 most-cited articles in Parkinson’s disease. Neurol Sci. (2018) 39(9):1537–45. doi: 10.1007/s10072-018-3450-y
37. Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, et al. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. (2018) 556(7700):239–43. doi: 10.1038/s41586-018-0016-3
38. Shinoka T, Breuer C. Tissue-engineered blood vessels in pediatric cardiac surgery. Yale J Biol Med. (2008) 81(4):161–6.19099046
39. Zbinden JC, Blum KM, Berman AG, Ramachandra AB, Szafron JM, Kerr KE, et al. Effects of braiding parameters on tissue engineered vascular graft development. Adv Healthc Mater. (2020) 9(24):e2001093. doi: 10.1002/adhm.202001093
40. Sacks MS, Schoen FJ, Mayer JE. Bioengineering challenges for heart valve tissue engineering. Annu Rev Biomed Eng. (2009) 11:289–313. doi: 10.1146/annurev-bioeng-061008-124903
41. Yang H, Wei L, Liu C, Zhong W, Li B, Chen Y, et al. Engineering human ventricular heart tissue based on macroporous iron oxide scaffolds. Acta Biomater. (2019) 88:540–53. doi: 10.1016/j.actbio.2019.02.024
42. Li Q, Bai Y, Jin T, Wang S, Cui W, Stanciulescu I, et al. Bioinspired engineering of poly(ethylene glycol) hydrogels and natural protein fibers for layered heart valve constructs. ACS Appl Mater Interfaces. (2017) 9(19):16524–35. doi: 10.1021/acsami.7b03281
43. Ma N, Cheung DY, Butcher JT. Incorporating nanocrystalline cellulose into a multifunctional hydrogel for heart valve tissue engineering applications. J Biomed Mater Res A. (2022) 110(1):76–91. doi: 10.1002/jbm.a.37267
44. Wang L, Liu Y, Ye G, He Y, Li B, Guan Y, et al. Injectable and conductive cardiac patches repair infarcted myocardium in rats and minipigs. Nat Biomed Eng. (2021) 5(10):1157–73. doi: 10.1038/s41551-021-00796-9
45. Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. (2008) 14(2):213–21. doi: 10.1038/nm1684
46. Hoerstrup SP, Sodian R, Daebritz S, Wang J, Bacha EA, Martin DP, et al. Functional living trileaflet heart valves grown in vitro. Circulation. (2000) 102(19 Suppl 3):III44–49. doi: 10.1161/01.cir.102.suppl_3.iii-44
47. Kolesky DB, Homan KA, Skylar-Scott MA, Lewis JA. Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci U S A. (2016) 113(12):3179–84. doi: 10.1073/pnas.1521342113
48. Masoumi N, Annabi N, Assmann A, Larson BL, Hjortnaes J, Alemdar N, et al. Tri-layered elastomeric scaffolds for engineering heart valve leaflets. Biomaterials. (2014) 35(27):7774–85. doi: 10.1016/j.biomaterials.2014.04.039
49. Choi SW, Guan W, Chung K. Basic principles of hydrogel-based tissue transformation technologies and their applications. Cell. (2021) 184(16):4115–36. doi: 10.1016/j.cell.2021.07.009
50. Oliveira FC, Carvalho JO, Magalhaes L, da Silva JM, Pereira SR, Gomes Junior AL, et al. Biomineralization inspired engineering of nanobiomaterials promoting bone repair. Mater Sci Eng C Mater Biol Appl. (2021) 120:111776. doi: 10.1016/j.msec.2020.111776
51. Loebel C, Burdick JA. Engineering stem and stromal cell therapies for musculoskeletal tissue repair. Cell Stem Cell. (2018) 22(3):325–39. doi: 10.1016/j.stem.2018.01.014
52. Griffin DR, Weaver WM, Scumpia PO, Di Carlo D, Segura T. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat Mater. (2015) 14(7):737–44. doi: 10.1038/nmat4294
53. Binder BY, Williams PA, Silva EA, Leach JK. Lysophosphatidic acid and sphingosine-1-phosphate: a concise review of biological function and applications for tissue engineering. Tissue Eng Part B Rev. (2015) 21(6):531–42. doi: 10.1089/ten.teb.2015.0107
54. Wang X, Schroder HC, Wiens M, Schlossmacher U, Muller WE. Biosilica: molecular biology, biochemistry and function in demosponges as well as its applied aspects for tissue engineering. Adv Mar Biol. (2012) 62:231–71. doi: 10.1016/B978-0-12-394283-8.00005-9
55. Zhao X, Lin Y, Wang Q. Virus-based scaffolds for tissue engineering applications. Wiley Interdiscip Rev Nanomed Nanobiotechnol. (2015) 7(4):534–47. doi: 10.1002/wnan.1327
56. Elisseeff J, Badylak SF, Boeke JD. Immune and genome engineering as the future of transplantable tissue. N Engl J Med. (2021) 385(26):2451–62. doi: 10.1056/NEJMra1913421
57. Karbassi E, Fenix A, Marchiano S, Muraoka N, Nakamura K, Yang X, et al. Cardiomyocyte maturation: advances in knowledge and implications for regenerative medicine. Nat Rev Cardiol. (2020) 17(6):341–59. doi: 10.1038/s41569-019-0331-x
58. Feric NT, Radisic M. Maturing human pluripotent stem cell-derived cardiomyocytes in human engineered cardiac tissues. Adv Drug Deliv Rev. (2016) 96:110–34. doi: 10.1016/j.addr.2015.04.019
59. Zhao Y, Rafatian N, Feric NT, Cox BJ, Aschar-Sobbi R, Wang EY, et al. A platform for generation of chamber-specific cardiac tissues and disease modeling. Cell. (2019) 176(4):913–27. e918. doi: 10.1016/j.cell.2018.11.042
60. Zhang YS, Arneri A, Bersini S, Shin SR, Zhu K, Goli-Malekabadi Z, et al. Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip. Biomaterials. (2016) 110:45–59. doi: 10.1016/j.biomaterials.2016.09.003
61. Hong H, Seo YB, Kim DY, Lee JS, Lee YJ, Lee H, et al. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials. (2020) 232:119679. doi: 10.1016/j.biomaterials.2019.119679
Keywords: cardiovascular tissue engineering, potential research direction, biomaterial, bibliometric analysis, VOSviewer
Citation: Lai P, Sheng M, Ye J-h, Tang Z-x, Hu S, Wang B, Yuan J-l, Yang Y-h, Zhong Y-m and Liao Y-l (2023) Research trends in cardiovascular tissue engineering from 1992 to 2022: a bibliometric analysis. Front. Cardiovasc. Med. 10:1208227. doi: 10.3389/fcvm.2023.1208227
Received: 18 April 2023; Accepted: 18 July 2023;
Published: 1 August 2023.
Edited by:
Clotilde Castaldo, University of Naples Federico II, Italy© 2023 Lai, Sheng, Ye, Tang, Hu, Wang, Yuan, Yang, Zhong and Liao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi-ming Zhong eWltaW5nemhvbmcxMjNAMTYzLmNvbQ== Yong-ling Liao bGlhb3lvbmdsaW5nOTE5NkAxNjMuY29t
†These authors have contributed equally to this work
Abbreviations CTE, cardiovascular tissue engineering; WoSCC, Web of Science Core Collection; Np, the number of publications; Nc, the number of citations excludes self-citations; ACN, average cited number; and APY, average published year; NA, not applicable.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.