
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cardiovasc. Med. , 07 July 2023
Sec. Aortic Surgery and Endovascular Repair Archive
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1205373
This article is part of the Research Topic Case Reports in Aortic Surgery and Endovascular Repair: 2023 View all 10 articles
Background: Aortic dissection (AD) is a severe cardiovascular disease characterized by aortic rupture, aortic valve insufficiency, aortic branch lumen stenosis, and occlusion. Acute ST-segment elevation myocardial infarction may be the primary manifestation when aortic dissection affects the coronary artery, leading to delayed or missed diagnosis of aortic dissection, and preventing patients from receiving timely and comprehensive treatment. Simultaneous aortic repair and coronary artery bypass grafting surgery are controversial because of their high mortality rates. Personalized and optimal treatment plans for patients should be taken seriously based on their different conditions and treatment options.
Case presentation: A 42-year-old man who experienced 1 h of persistent precordialgia was admitted to a local second-level hospital for emergency treatment. Electrocardiogram (ECG) showed evidence of ST-segment elevation, and myocardial enzyme levels were CK-MB 18.35 ng/ml and troponin 0.42 ng/ml. The patient was treated for acute myocardial infarction (AMI) and urgently sent to the interventional catheter room. Coronary angiography showed stenosis of the starting part of the right coronary artery trunk. Thus, stent implantation was performed, and the stenosis section recovered patency; however, postoperative precordialgia was not alleviated. Computed tomography angiography (CTA) revealed a type A AD. The patient was immediately transferred to a higher-level hospital, underwent emergency surgery with cardiopulmonary bypass (CPB) ascending aorta replacement, SUN's procedure (total arch replacement and stented elephant trunk implantation), and simultaneous implantation of extracorporeal membrane oxygenation (ECMO), and regained consciousness within intensive care unit care. ECMO was discontinued when hemodynamics stabilized. The patient ultimately recovered well and was discharged.
Conclusion: This case demonstrated that precordialgia is not limited to myocardial infarction but may also be accompanied by aortic dissection. Percutaneous coronary intervention (PCI) can timely and effectively restore coronary artery perfusion, strive for the opportunity of aortic repair surgery, and can overcome pump failure caused by myocardial infarction, cardiopulmonary bypass, heart block time, and myocardial ischemia-reperfusion injury. Personalized treatment is crucial for patients with complex type A aortic dissection.
Acute type A AD is a severe cardiovascular disease that may present with aortic rupture, aortic branch lumen stenosis, or occlusion. Hypoperfusion of the aortic branches caused by dissection of the false lumen can occur in all branches from the coronary artery opening to the abdominal aortic bifurcation (1). If the coronary artery is affected by acute type A AD, AMI may be the primary manifestation, and AD may be diagnosed late or go undetected if not fully considered (2).
Therapeutic methods for patients with AD complicated by AMI remain controversial. If a patient undergoes a one-stage surgery, including aortic repair and coronary artery bypass grafting (CABG), the mortality rate is often extremely high because of a severe setback of cardiac function, including existing myocardial infarction, CPB, heart block time, and myocardial ischemia-reperfusion injury. Owing to the high mortality rate, some scholars believe that implementing PCI treatment first is beneficial for such patients because it allows them to undergo second-stage aortic repair surgery and can reduce the mortality rate (3–5).
The etiology of acute chest pain in patients is not only limited to AMI but also includes the possibility of AD leading to coronary artery involvement and AMI (6). Early diagnosis can provide comprehensive treatment guidance. Notably, myocardial infarction may lead to heart pump failure. If a patient needs to undergo CPB aortic repair surgery again, the myocardial damage will undoubtedly worsen, leading to the inability to detach from the CPB. ECMO should be an indispensable treatment to maintain effective circulation and allow time for myocardial recovery.
In this article, we report a rare case of acute chest pain in a patient who was initially diagnosed with AMI and underwent PCI; however, the diagnosis of AD was delayed. After AD was confirmed, emergency aortic repair surgery was performed, and ECMO was implanted owing to heart pump failure. Based on relevant treatment experiences and a comprehensive literature review, we aimed to elucidate the clinical diagnosis and treatment of this unique and complex combined disease.
A 42-year-old man (height 170 cm, weight 75 kg) who experienced severe precordialgia, which persisted for 1 h, was admitted to a local second-level hospital for emergency treatment. Although he had a history of hypertension for 5 years, he did not take antihypertensive drugs according to the advice of doctors. He had no trauma, surgery, or family history. There was no apparent cause of the sudden precordialgia. His blood pressure was 115/76 mmHg, and his heart rate was 105 bpm at admission. ECG showed ST-segment elevation (II/III/aVF) (Figure 1A), and myocardial enzyme levels were CK-MB 18.35 ng/ml and troponin 0.42 ng/ml. No scheduled cardiac ultrasound examination was performed, and the patient was treated for acute ST-segment elevation myocardial infarction, received 300 mg of aspirin and 180 mg of ticagrelor, and was immediately sent to the interventional catheter room. Coronary angiography showed severe stenosis of the starting part of the right coronary artery trunk (Figure 1B). Subsequently, PCI and coronary stent implantation were performed, as well as stenosis section recovery patency (Figure 1C). However, precordialgia was not alleviated after PCI, and no improvement was observed after the tirofiban injection. Moreover, hemodynamics became unstable, oxygenation function decreased, blood pressure dropped to 80/50 mmHg, peripheral blood oxygen saturation was 85%, and the heart rate was130 bpm, leading to mechanical ventilation 22 h after PCI and vasoactive drugs to maintain blood pressure. At this point, clinical physicians began to consider the possibility of AD, and an aortic CTA examination confirmed the diagnosis of type A AD (Figures 2A–D). The rupture of the aortic intima was located in the descending thoracic aorta, aortic dissection was reverse-tearing to the ascending aorta, a false lumen was extending to the upper edge of the right coronary artery opening, the hematoma inside the false lumen was very thick, and the true lumen of the ascending aorta became smaller due to compression by the false lumen. Local hospital staff urgently transferred the patient to a higher-level hospital, and emergency surgery was performed, including CPB (right axillary artery perfusion), ascending aorta replacement, and SUN's procedure (total arch replacement and stented elephant trunk implantation). It was difficult to detach the CPB during surgery because of right ventricular failure caused by preoperative myocardial ischemia. Therefore, VA-ECMO (left femoral artery and vein) was simultaneously implanted. The CPB time was 337 min, cardiac arrest time was 132 min, circulatory arrest time was 21 min, and intraoperative minimum temperature was 28°C. Owing to preoperative antiplatelet therapy, coagulation function had been severely affected, posing significant difficulties for surgical hemostasis. Platelets, cryoprecipitate, and fibrinogen were also infused. Fortunately, there was no severe progressive bleeding, and thoracic drainage on POD1 after the surgery was 500 ml. To prevent coronary stent thrombosis after PCI, platelet inhibitors on POD2 were re-administered. The patient regained consciousness on POD4 in the intensive care unit. Cardiac troponin and myocardial enzyme levels continued to decrease after surgery, circulation gradually stabilized, and the dosage of vasoactive drugs gradually decreased. ECMO was discontinued on POD5 when the hemodynamics returned to stability. Tracheotomy was performed on POD10 because of moderate pulmonary infection, and the postoperative mechanical ventilation time was 276 h. The tracheostomy was closed on POD20. Postoperative aortic CTA revealed that the artificial vessels were unobstructed (Figure 3A). The thoracic stent was well dilated without internal leakage (Figure 3B). The opening of the right coronary artery was unobstructed, the coronary stent implantation was visible (Figure 3C), and smooth morphology of the thoracic aorta was observed (Figures 3D, 4). The heart rate and blood pressure of the patient were stable, and heart function was normal through rehabilitation training after 6 months. Moreover, a cardiac ultrasound examination indicated normal cardiac structure and valve function, with a left ventricular ejection fraction of 55%.
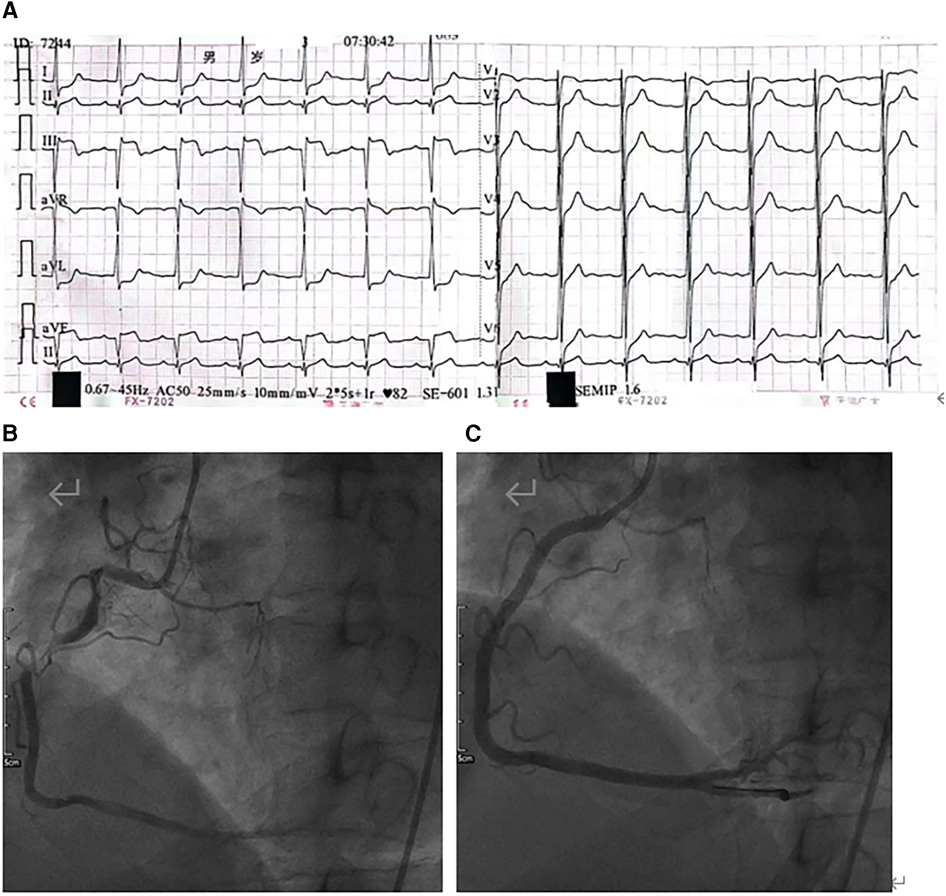
Figure 1. (A) Electrocardiogram (ECG) showing ST-segment elevation (II/III/aVF) and inferior acute myocardial infarction. (B) Coronary angiography showing irregular long-segment stenosis of the right coronary artery from the opening to the middle segment. (C) After stent implantation, the right coronary artery shows good morphology and an unobstructed vascular lumen.
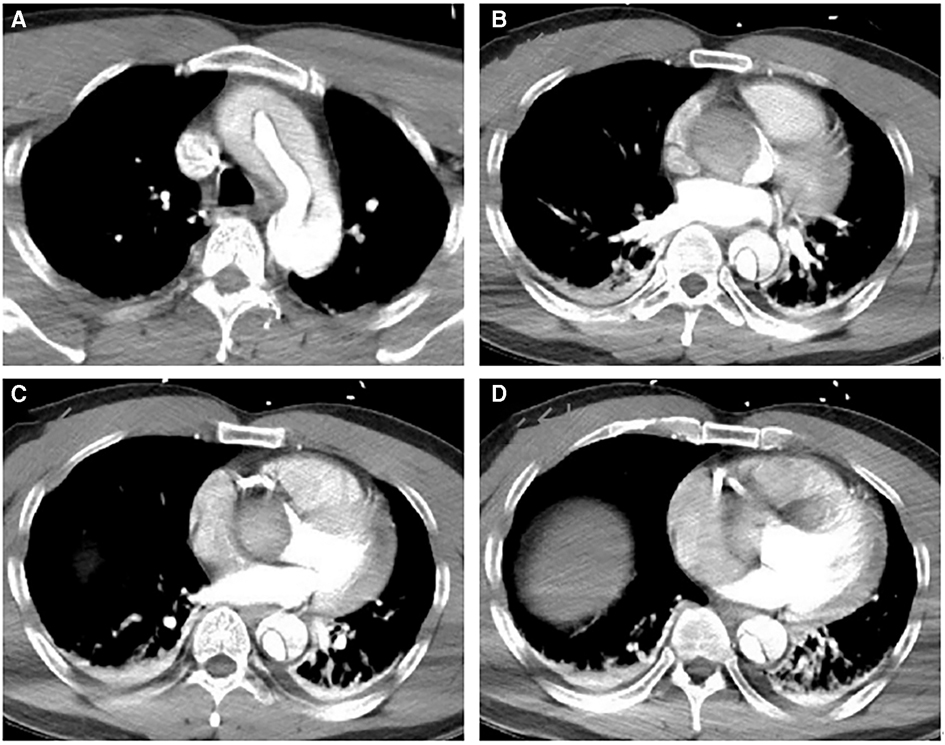
Figure 2. Preoperative aortic CTA image. (A–D) CTA shows the false lumen of the ascending aorta compressing the true lumen, extending above the opening of the right coronary artery. They indicate a thick hematoma in the false lumen compressing the right coronary artery.
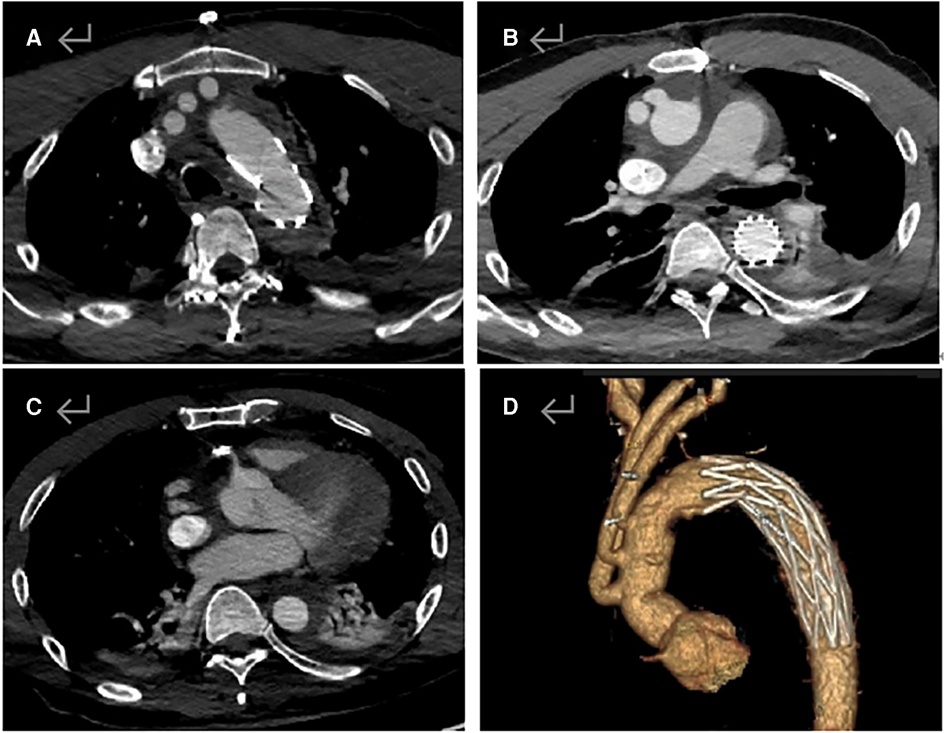
Figure 3. Postoperative aortic CTA image. (A) Artificial vessels in the aortic arch and the cephalobrachial artery are unobstructed. (B) Ascending aorta is unobstructed, and the stent is well dilated without internal leakage. (C) Opening of the right coronary artery is unobstructed, and stent implantation is visible. (D) Smooth morphology of the thoracic aorta.
Acute type A AD can be multifactorial and lead to rupture of the aortic intima and entry of circulating blood into the aortic wall, resulting in the formation of a false lumen. Acute type A AD is the most critical complication of cardiovascular surgery. The mortality rate of acute type A AD increases by 1% every hour within 48 h of onset. Conservative treatment has a high mortality rate of 50% within 2 days. Surgery is the preferred treatment for acute type A AD (7), whereas SUN's procedure is the standard procedure for Stanford type A AD (8).
The most common causes of death are dissection rupture, aortic valve insufficiency, ischemia of vital organs caused by aortic branch blood supply occlusion, and organ necrosis (9). The low perfusion of branch vessels is primarily due to the false lumen of the branch vessel intimal tear, which receives most of the blood; therefore, the false lumen expands and compresses the true lumen, resulting in insufficient blood supply to the organs. Acute type A AD can affect almost all aortic branches, from the coronary artery to the femoral artery. The incidence and severity of hypoperfusion differ among the various organs. Poor preoperative organ perfusion is a vital factor that significantly affects the prognosis of patients with acute type A AD (10). Accurate AD classification provides precise guidance on surgical treatment and evidence for disease prognosis (11). However, surgical treatment strategies are controversial when complex AD involves organs, such as the brain or other important branch arteries (12). In patients with AD complicated by AMI, preoperative myocardial ischemia, intraoperative cardiac blockade time, and ischemia-reperfusion injury have multiple impacts on cardiac function. The 5-year mortality rate in patients with AD and myocardial infarction is high, and surgical treatment can help reduce this mortality rate (13). However, concomitant CABG performed in acute type A AD repair surgery is associated with high in-hospital mortality (14–16).
The primary clinical manifestations of AD are persistent chest and back pain, and if complicated with AMI, precordium pain may persist. Acute type A AD is misdiagnosed or delayed because only acute coronary syndrome is considered when chest pain occurs. Therefore, the possibility of patients with AMI having AD should be considered (17–19). The acute coronary syndrome is not limited to simple coronary artery disease. AMI may also be the first manifestation of acute type A AD, which can be rapidly diagnosed through cardiac ultrasound and requires aortic CTA examination if necessary. Point-of-care ultrasound (POCUS) can quickly diagnose type A AD and change treatment strategies in patients with ST-segment elevation myocardial infarction (20). AD with concomitant AMI is prone to misdiagnosis, and transesophageal echocardiography (TTE) and D-dimer examination can help detect AD (21–23). Moreover, surveys have shown that for such patients, if a TTE examination is only performed after antithrombotic treatment, there is a correlation with a high surgery mortality rate (24). During the diagnosis and treatment of this case in a local secondary hospital, a cardiac ultrasound examination was overlooked. Therefore, we believe that even if a patient is diagnosed with simple AMI, a cardiac ultrasound examination should be performed before emergency intervention. In addition, aortic CTA can provide detailed information on the morphology of AD, including the location of intimal rupture and the involvement of branching vessels (25).
If AD diagnosis is missed, coronary angiography and PCI for patients with AMI are understandable, although interventional catheterization carries the risk of aortic rupture. However, if AD complicated by AMI can be accurately diagnosed simultaneously, PCI can be considered a priority treatment without urgent surgical procedures; some studies have been conducted accordingly (26, 27). The hospitalization mortality rate of patients with AD complicated by coronary artery involvement is as high as 40%. The first-stage PCI treatment strategy is of great help to patients undergoing aortic repair surgery and can reduce the mortality rate of such patients (28, 29). Cho et al. believed that using only guidewires to restore coronary artery perfusion could be an innovative interventional method as the first-stage treatment for coronary malperfusion caused by acute type A AD (30). In this case, although the diagnosis of AD was initially missed at a local secondary hospital, PCI did benefit the patient as it restored the right coronary artery supply and secured opportunities for referral and aortic repair surgery. However, it is equally difficult to determine the interval from PCI to aortic repair because patients with type A AD face the risk of aortic rupture at any time, even if first-stage PCI is successful. Therefore, we believe that aortic repair should be performed immediately after successful PCI. Similarly, not all types of coronary artery involvement caused by type A AD can be corrected using PCI. Some types can only be resolved through surgical repair of both the aorta and coronary arteries or through simultaneous CABG. Kreibich et al. recommended surgical strategies based on different coronary artery lesions, such as CABG for type C lesions and repair of coronary artery openings for type A and B lesions (31–33). Tong et al. reported that repair surgery is still possible even if the opening of the coronary artery intima is torn (34).
Due to the anatomical location of the coronary artery, right coronary artery involvement is more common than left coronary artery involvement in acute type A AD (35). Before undergoing AD center repair surgery, whether PCI is required in patients with concomitant right coronary artery hypoperfusion should be comprehensively evaluated, including concomitant cardiac tamponade, left and right ventricular function, and surgical team efficiency (36). Intraoperative coronary angiography is feasible if hospital conditions permit (37).
Intra-aortic balloon pump treatment is inappropriate because of aortic dissection etiology. ECMO should be used as an essential treatment for cardiac dysfunction to ensure that patients can be weaned from CPB and transferred to the intensive care unit (38, 39). Before VA-ECMO implantation, it is necessary to fully evaluate whether the artery to be intubated is affected by dissection, ensuring that the artery is supplied by the true lumen. Using the axillary artery for arterial catheterization ensures anterograde blood flow to the aorta (40–46). However, in this case, the right axillary artery was intubated for CPB, and the right femoral artery had just undergone a puncture for PCI. Fortunately, the left femoral artery of the patient was not affected by the dissection and was supplied by the true lumen of the aorta. Of course, it was also possible to consider intubating the branch of the aortic artificial vessel, which has the advantage of obtaining anterograde blood flow to the aorta. However, based on previous experience, in patients who had been treated with platelet inhibitors, if ECMO was established through branch intubation of artificial vessels, the impact of ECMO blood flow would lead to severe leakage of blood vessels. Therefore, left femoral artery catheterization was performed to establish ECMO. ECMO can significantly improve the survival rate of patients with cardiogenic shock caused by myocardial infarction or aortic repair surgery (47–53). With improvements in surgical technology and team efficiency, personalized and optimal treatment plans should be provided for patients based on their different conditions and treatment options.
Our case demonstrated that caution regarding possible aortic dissection is required in patients with AMI. It also indicated that although PCI can serve as a transitional treatment if emergency aortic repair surgery cannot be performed, the aortic repair is fundamental, and ECMO must be prepared to overcome pump failure.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the patient for the publication of this case report and accompanying images.
JY and WY reviewed the literature and drafted the manuscript. HZ provided the case resources. JG processed the data. JX edited and revised the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the Open Project of the Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases Ministry of Education (No. XN201930).
The authors thank Editage (www.editage.cn) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AD, aortic dissection; AMI, acute myocardial infarction; CTA, computed tomography angiography; CPB, cardiopulmonary bypass; SUN's procedure, total arch replacement and stented elephant trunk implantation; ECMO, extracorporeal membrane oxygenation; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting.
1. Bayamin K, Power A, Chu MWA, Dubois L, Valdis M. Malperfusion syndrome in acute type A aortic dissection: thinking beyond the proximal repair. J Card Surg. (2022) 37(11):3827–34. doi: 10.1111/jocs.16872
2. Hussain A, Rossi A, Smith A, Lopez-Marco A, Khalil A, Roberts N. Type A aortic dissection masquerading as an Inferior myocardial infarction. Aorta (Stamford). (2021) 9(5):184–5. doi: 10.1055/s-0041-1732396
3. Hashimoto O, Saito Y, Sasaki H, Yumoto K, Oshima S, Tobaru T, et al. Treatment strategies and in-hospital mortality in patients with type A acute aortic dissection and coronary artery involvement. J Thorac Cardiovasc Surg. (2022):S0022-5223(22)00354-3. doi: 10.1016/j.jtcvs.2022.03.016
4. Chung K, Huang P, Lee K. Percutaneous coronary intervention as a bridge therapy for aortic dissection related bilateral coronary malperfusion: a case report. Heart Surg Forum. (2023) 26(1):E040–2. doi: 10.1532/hsf.5091
5. Saito Y, Hashimoto O, Nakayama T, Sasaki H, Yumoto K, Oshima S, et al. Right versus left coronary artery involvement in patients with type A acute aortic dissection. Int J Cardiol. (2023) 371:49–53. doi: 10.1016/j.ijcard.2022.10.021
6. McConaghy JR, Sharma M, Patel H. Acute chest pain in adults: outpatient evaluation. Am Fam Physician. (2020) 102(12):721–7.33320506
7. Wei J, Chen Z, Zhang H, Sun X, Qian X, Yu C. In-hospital major adverse outcomes of acute type A aortic dissection. Eur J Cardiothorac Surg. (2019) 55(2):345–50. doi: 10.1093/ejcts/ezy269
8. Sun L, Li J. Progress and challenges in diagnosis and treatment of Stanford type A aortic dissection in China. Chin J Surg. (2017) 55:241–4.28355758
9. Wang C, Zhang L, Li T, Xi Z, Wu H, Li D. Surgical treatment of type A acute aortic dissection with cerebral malperfusion: a systematic review. J Cardiothorac Surg. (2022) 17(1):140. doi: 10.1186/s13019-022-01894-8
10. Munir W, Chong JH, Harky A, Bashir M, Adams B. Type A aortic dissection: involvement of carotid artery and impact on cerebral malperfusion. Asian Cardiovasc Thorac Ann. (2021) 29(7):635–42. doi: 10.1177/0218492320984329
11. Sievers HH, Rylski B, Czerny M, Baier ALM, Kreibich M, Siepe M, et al. Aortic dissection reconsidered: type, entry site, malperfusion classification adding clarity and enabling outcome prediction. Interact Cardiovasc Thorac Surg. (2020) 30(3):451–7. doi: 10.1093/icvts/ivz281
12. Grimm JC, Magruder JT, Crawford TC, Sciortino CM, Zehr KJ, Mandal K, et al. Differential outcomes of type A dissection with malperfusion according to affected organ system. Ann Cardiothorac Surg. (2016) 5(3):202–8. doi: 10.21037/acs.2016.03.11
13. Liu F, Qian SC, Jing S, Wang Z, Yang XC, Chen ML. Incidence and outcome of acute myocardial infarction in patients with aortic dissection and risk factor control. Front Surg. (2021) 8:678806. doi: 10.3389/fsurg.2021.678806
14. Morjan M, Reser D, Savic V, Sromicki J, Maisano F, Mestres CA. Concomitant coronary artery bypass in patients with acute type A aortic dissection. Semin Thorac Cardiovasc Surg. (2022) 34(2):410–6. doi: 10.1053/j.semtcvs.2021.03.043
15. Zhang K, Dong SB, Pan XD, Lin Y, Zhu K, Zheng J, et al. Concomitant coronary artery bypass grafting during surgical repair of acute type A aortic dissection affects operative mortality rather than midterm mortality. Asian J Surg. (2021) 44(7):945–51. doi: 10.1016/j.asjsur.2021.01.031
16. Cecchini A, Qureshi MH, Peshin S, Othman A, Gajjar B. Type A aortic dissection presenting as acute coronary syndrome in a young male patient: a case report. Cureus. (2022) 14(11):e31578. doi: 10.7759/cureus.31578
17. Wang W, Wu J, Zhao X, You B, Li C. Type-A aortic dissection manifesting as acute inferior myocardial infarction: 2 case reports. Medicine (Baltimore). (2019) 98(43):e17662. doi: 10.1097/MD.0000000000017662
18. Abdelwahed YS, Weber AC, Siegrist PT. Left main ischemia as first manifestation of aortic dissection. JACC Cardiovasc Interv. (2021) 14(4):476–80. doi: 10.1016/j.jcin.2020.11.016
19. Nallamothu BK, Eagle KA. When zebras run with horses: the diagnostic dilemma of acute aortic dissection complicated by myocardial infarction. J Interv Cardiol. (2002) 15(4):297–9. doi: 10.1111/j.1540-8183.2002.tb01107.x
20. Abrams E, Allen A, Lahham S. Aortic dissection with subsequent hemorrhagic tamponade diagnosed with point-of-care ultrasound in a patient presenting with STEMI. Clin Pract Cases Emerg Med. (2019) 3(2):103–6. doi: 10.5811/cpcem.2019.1.40869
21. Luo JL, Wu CK, Lin YH, Kao HL, Lin MS, Ho YL, et al. Type A aortic dissection manifesting as acute myocardial infarction: still a lesson to learn. Acta Cardiol. (2009) 64(4):499–504. doi: 10.2143/AC.64.4.2041615
22. Kaito D, Yamamoto R, Nakama R, Hashizume K, Ueno K, Sasaki J. D-dimer for screening of aortic dissection in patients with ST-elevation myocardial infarction. Am J Emerg Med. (2022) 59:146–51. doi: 10.1016/j.ajem.2022.07.024
23. Hui L, Wang D, Liu T, Liu B, Wang Y, Liu B. Diagnostic performance of transthoracic echocardiography in screening acute type A aortic dissection from ST-segment elevated myocardial infarction. Cardiovasc Diagn Ther. (2022) 12(5):603–13. doi: 10.21037/cdt-22-59
24. Liu B, Cai LD, Wang Y. Association between delayed transthoracic echocardiography and in-hospital mortality in type A acute aortic dissection-associated ST-segment elevated myocardial infarction. J Thorac Dis. (2021) 13(5):2923–32. doi: 10.21037/jtd-20-3470
25. Hashimoto O, Saito Y, Nakayama T, Okino S, Sakai Y, Nakamura Y, et al. Prognostic impact of branch vessel involvement on computed tomography versus clinical presentation of malperfusion in patients with type a acute aortic dissection. Am J Cardiol. (2021) 152:158–63. doi: 10.1016/j.amjcard.2021.05.005
26. Fu D, Beccarino N, Ali I, Xu J, Pena I, Meraj P. A case of complex percutaneous coronary intervention for ST elevation myocardial infarction caused by extension of type A aortic dissection into the left main coronary artery. Cardiovasc Revasc Med. (2022):S1553-8389(22)00614-5. doi: 10.1016/j.carrev.2022.06.260
27. Taguchi Y, Kubo S, Ikuta A, Osakada K, Takamatsu M, Takahashi K, et al. Percutaneous coronary intervention for left main coronary artery malperfusion in acute type A aortic dissection. Cardiovasc Interv Ther. (2022) 37(2):333–42. doi: 10.1007/s12928-021-00793-4
28. Shigihara S, Shirakabe A, Okazaki H, Asai K. Successful treatment of a patient with type-A acute aortic dissection with emergent percutaneous coronary intervention and thoracic endovascular aortic repair under percutaneous cardiopulmonary support: a case report. Eur Heart J Case Rep. (2022) 6(9):ytac344. doi: 10.1093/ehjcr/ytac344
29. Noda K, Inoue Y, Matsuo J, Yokawa K, Uehara K, Sasaki H, et al. Type A aortic dissection with left coronary malperfusion. Gen Thorac Cardiovasc Surg. (2022) 70(2):178–80. doi: 10.1007/s11748-021-01728-9
30. Cho T, Uchida K, Yasuda S, Matsuzawa Y, Kobayashi Y. Early coronary reperfusion using only guidewires for acute type A aortic dissection. Gen Thorac Cardiovasc Surg. (2021) 69(9):1344–6. doi: 10.1007/s11748-021-01662-w
31. Kreibich M, Bavaria JE, Branchetti E, Brown CR, Chen Z, Khurshan F, et al. Management of patients with coronary artery malperfusion secondary to type A aortic dissection. Ann Thorac Surg. (2019) 107(4):1174–80. doi: 10.1016/j.athoracsur.2018.09.065
32. Benbouchta K, Berrajaa M, Ofkire M, Ouafi NE, Bazid Z. An uncommon acute type A aortic dissection mimicking an inferior STEMI. Pan Afr Med J. (2020) 36:247. doi: 10.11604/pamj.2020.36.247.23821
33. Fujiyoshi T, Koizumi N, Nishibe T, Sugiyama K, Ogino H. Direct repair of localized aortic dissection with critical malperfusion of the left main trunk. Ann Thorac Cardiovasc Surg. (2018) 24(6):320–3. doi: 10.5761/atcs.cr.17-00140
34. Tong G, Wu J, Chen Z, Zhuang D, Zhao S, Liu Y, et al. Coronary malperfusion secondary to acute type A aortic dissection: surgical management based on a modified neri classification. J Clin Med. (2022) 11(6):1693. doi: 10.3390/jcm11061693
35. Kayali F, Jubouri M, Al-Tawil M, Tan S, Williams IM, Mohammed I, et al. Coronary artery involvement in type A aortic dissection: fate of the coronaries. J Card Surg. (2022) 37(12):5233–42. doi: 10.1111/jocs.16981
36. Uchida K, Karube N, Minami T, Cho T, Matsuki Y, Nemoto H, et al. Treatment of coronary malperfusion in type A acute aortic dissection. Gen Thorac Cardiovasc Surg. (2018) 66(11):621–5. doi: 10.1007/s11748-018-1014-y
37. Yang J, Li X, Chen Z, Sun T, Fan R, Yu C. Case report: intraoperative open-heart coronary angiography in acute type A aortic dissection. Front Cardiovasc Med. (2021) 8:731581. doi: 10.3389/fcvm.2021.731581
38. Nguyen SN, Moroi MK, Takayama H, Takeda K. Salvage extracorporeal membrane oxygenation for acute type A aortic dissection with coronary malperfusion. J Card Surg. (2020) 35(12):3614–6. doi: 10.1111/jocs.15058
39. Wang Y, Zhu Z, Xu R, Li D, Wang T, Liu K. A complete occlusion of right coronary artery due to Stanford type A aortic dissection—successful treatment with extracorporeal membrane oxygenation (ECMO). Braz J Cardiovasc Surg. (2019) 34(4):491–4. doi: 10.21470/1678-9741-2018-0060
40. Ohira S, Malekan R, Goldberg JB, Lansman SL, Spielvogel D, Kai M, et al. Axillary artery cannulation for veno-arterial extracorporeal membrane oxygenation support in cardiogenic shock. JTCVS Tech. (2021) 5:62–71. doi: 10.1016/j.xjtc.2020.10.035
41. Tayal R, Hirst CS, Garg A, Kapur NK. Deployment of acute mechanical circulatory support devices via the axillary artery. Expert Rev Cardiovasc Ther. (2019) 17(5):353–60. doi: 10.1080/14779072.2019.1606712
42. Pisani A, Braham W, Brega C, Lajmi M, Provenchere S, Danial P, et al. Right axillary artery cannulation for venoarterial extracorporeal membrane oxygenation: a retrospective single centre observational study. Eur J Cardiothorac Surg. (2021) 59(3):601–9. doi: 10.1093/ejcts/ezaa397
43. Yang C, Peng G, Xu X, Wei B, Yang H, He J. The technique of intraoperative axillary artery cannulation for extracorporeal membrane oxygenation in lung transplantation. J Thorac Dis. (2019) 11(7):2939–44. doi: 10.21037/jtd.2019.07.32
44. Nishikawa M, Willey J, Takayama H, Kaku Y, Ning Y, Kurlansky PA, et al. Stroke patterns and cannulation strategy during veno-arterial extracorporeal membrane support. J Artif Organs. (2022) 25(3):231–7. doi: 10.1007/s10047-021-01300-5
45. Omer S, Rajagopal K. Commentary: when preserving life, do not neglect the limb: the role of axillary artery cannulation in venoarterial extracorporeal membrane oxygenation. JTCVS Tech. (2021) 5:72–3. doi: 10.1016/j.xjtc.2020.11.026
46. Shah A, Madathil RJ, Griffith BP, Kaczorowski DJ. Venoarterial extracorporeal membrane oxygenation via direct axillary artery cannulation. Innovations (Phila). (2021) 16(3):297–9. doi: 10.1177/15569845211012655
47. Wagner JF, Abfalterer H, Ozpeker C, Tiller C, Brenner C, Kilo J, et al. Benefit of extracorporeal membrane oxygenation in myocardial infarction-induced cardiogenic shock. J Cardiovasc Surg (Torino). (2023) 64(2):207–14. doi: 10.23736/S0021-9509.22.12270-6
48. Hou JY, Wang CS, Lai H, Sun YX, Li X, Zheng JL, et al. Veno-arterial extracorporeal membrane oxygenation for patients undergoing acute type A aortic dissection surgery: a six-year experience. Front Cardiovasc Med. (2021) 8:652527. doi: 10.3389/fcvm.2021.652527
49. Mariscalco G, Fiore A, Ragnarsson S, El-Dean Z, Jonsson K, Dalen M, et al. Venoarterial extracorporeal membrane oxygenation after surgical repair of type A aortic dissection. Am J Cardiol. (2020) 125(12):1901–5. doi: 10.1016/j.amjcard.2020.03.012
50. Wang Y, Piao H, Li B, Wang W, Huang M, Zhu Z, et al. Extracorporeal membrane oxygenation in Stanford type A aortic dissection. Int Heart J. (2019) 60(4):845–8. doi: 10.1536/ihj.18-496
51. Fan F, Zhou Q, Pan J, Cao H, Li K, Xue Y, et al. Clinical outcomes of postoperative extracorporeal membrane oxygenation support in Stanford type a aortic dissection. BMC Anesthesiol. (2021) 21(1):35. doi: 10.1186/s12871-021-01252-6
52. Maybauer MO, El Banayosy A, Koerner MM, Hooker RL, Swant LV, Mihu MR, et al. Mechanical cardiopulmonary resuscitation for venoarterial ECMO implantation in pulmonary embolism complicated by type B aortic dissection and retroperitoneal hemorrhage. J Card Surg. (2020) 35(10):2821–4. doi: 10.1111/jocs.14782
Keywords: case report, aortic dissection, acute myocardial infarction, percutaneous coronary intervention, SUN’s procedure, extracorporeal membrane oxygenation
Citation: Yu J, Yu W, Zeng H, Gao J and Xiong J (2023) Case report: Surgery combined with extracorporeal membrane oxygenation for a patient with type A aortic dissection complicated with myocardial infarction after percutaneous coronary intervention. Front. Cardiovasc. Med. 10:1205373. doi: 10.3389/fcvm.2023.1205373
Received: 13 April 2023; Accepted: 22 June 2023;
Published: 7 July 2023.
Edited by:
Antonio Miceli, Istituto Clinico Sant'Ambrogio, ItalyReviewed by:
Savvas Lampridis, Hammersmith Hospital, United Kingdom© 2023 Yu, Yu, Zeng, Gao and Xiong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianxian Xiong eGlvbmdqaWFueGlhbkAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.