- 1Department of Cardiac Surgery, Bluhm Cardiovascular Institute, Northwestern University Feinberg School of Medicine and Northwestern Memorial Hospital, Chicago, IL, United States
- 2Department of Radiology, Northwestern University Feinberg School of Medicine and Northwestern Memorial Hospital, Chicago, IL, United States
- 3Department of Pulmonary and Critical Care, Northwestern University Feinberg School of Medicine and Northwestern Memorial Hospital, Chicago, IL, United States
- 4Department of Cardiology, Bluhm Cardiovascular Institute, Northwestern University Feinberg School of Medicine and Northwestern Memorial Hospital, Chicago, IL, United States
We present unusual coronary-pulmonary collaterals in a 65-year-old CTEPH patient. Perfusion mapping of a dual-energy computed tomography (DECT) study revealed areas of right lung that were minimally perfused despite unilateral occlusion of the right pulmonary artery, leading to the discovery of coronary-pulmonary collaterals via invasive coronary angiography. Pulmonary thromboendarterectomy removed the clot en-bloc. Post-surgery DECT and catheterization confirmed restoration of pulmonary arterial circulation and excellent hemodynamic response. Here, suggestion of perfusion to a proximally obstructed lung with DECT helped to document the presence of rarely documented coronary-pulmonary artery collaterals.
Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is classified as group 4 pulmonary hypertension where unresolved thromboembolic disease obstructs the pulmonary artery. The resultant lung ischemia commonly leads to bronchial arterial collateralization thought to lower pulmonary vascular resistance and reduce mortality (1). While CTEPH is frequently studied with CT-PA and ventilation-perfusion scans, dual-energy CT (DECT) scans are an emerging evaluation tool among others including photon counting CT or rapid kV switching dual-energy CT (2–4). DECT depends on dual sensor-based simultaneous computed tomography scans at two different tube voltages with contrast iodine. This allows for subsequent image processing based on material decomposition detected as attenuation differences at different energy levels to calculate perfusion blood volume (PBV) at each voxel and accordingly visualize contrast uptake based on a given color scale. Depending on phase of contrast uptake, perfusion defects are visualized such as in V/Q imaging during the arterial phase, while imaging timed later can reveal perfusion due to collaterals (5). A DECT study is dose neutral when compared to a standard CTA, though some studies have shown some differences depending on employed technique and differences in normalization of image quality, signal to noise ratio and dose length product (6–8). Here, images were acquired with a dual energy-dual source scanner with 2 separate 64-row detectors and image postprocessing was completed using Syngo DE Lung PBV, Siemens Healthcare software, which uses the three-material decomposition theory of iodine, air and soft-tissue to calculate PBV. Ultimately, patients with CTEPH may be candidates for surgical cure with pulmonary thromboendarterectomy based on image-guided assessment and clinical status (9). The purpose of this case study is to describe utility of DECT-PBV to evaluate pulmonary embolism and its functional consequences as well as illustrate its role for the first time in characterizing the rarely documented coronary-pulmonary collaterals in a unilateral CTEPH patient.
Case presentation
We present a 65-year-old former 40-pack year smoker with a medical history of chronic obstructive pulmonary disease requiring supplemental oxygen, leading to atrial fibrillation, diastolic heart failure, and chronic thromboembolic pulmonary hypertension (CTEPH) who was referred to our institution for advanced surgical management of CTEPH. On admission, the patient was experiencing increased dyspnea and significant oxygen desaturation, necessitating transfer to our institution for further evaluation and diagnostics.
The patient was in his usual state of health until 4 years prior when he developed right lower extremity swelling and dyspnea and was diagnosed with a deep vein thrombosis (DVT) and pulmonary embolism (PE). Review of these historical records demonstrated extensive pulmonary embolic material beginning in the right main pulmonary artery extending into segmental and subsegmental arteries. He was initiated on warfarin and remained adherent with therapeutic INR's.
Transthoracic echocardiography revealed a normal sized left ventricle with an ejection fraction of 64%. The right ventricle was moderately dilated with decreased systolic function, there was interventricular flattening in both systole and diastole, the right atrium was severely enlarged, and estimated right ventricular systolic pressures were elevated. RV basal diameter was 4.45 cm, RA volume was 94.3 ml, TAPSE was 1.71 cm, and S’ prime was 9.79 cm/s.
Dual-energy computed tomography (DECT) redemonstrated unilateral complete occlusion of right pulmonary arterial circulation with thrombus propagation into the proximal right pulmonary artery. No filling defects in the left pulmonary arterial system were visualized. Despite the apparent complete obstruction of the right pulmonary artery, perfused blood volume (PBV) mapping of the dual energy CT study revealed areas of the right lung that were still minimally perfused (Figure 1 A,B).
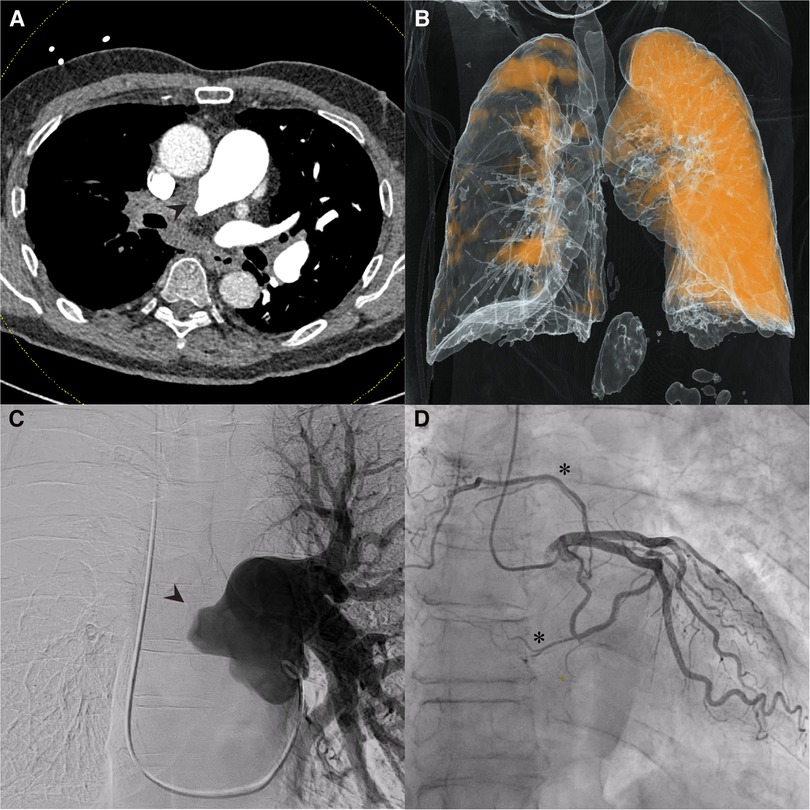
Figure 1. Dual-Energy computed tomography (DECT), pulmonary and coronary angiography. (A) Coronal DECT image demonstrating obstruction of right pulmonary artery (arrowhead); (B) Perfusion mapping demonstrates limited perfusion of right lung from unknown systemic supply at time of imaging. (C) Pulmonary angiography confirmed complete obstruction of right pulmonary circulation (arrowhead); (D) Coronary angiography revealed multiple systemic coronary-pulmonary collaterals from the left circumflex artery (asterisks).
Pulmonary angiography with left and right heart catheterization was undertaken for surgical candidacy and planning. Right heart catheterization revealed a right atrial pressure of 4mmHg, mean pulmonary artery pressure of 40mmHg, pulmonary wedge pressure of 4mmHg, cardiac output of 3.5, cardiac index of 1.8 and pulmonary vascular resistance of 10.3 wood units. Pulmonary angiography clearly demonstrated complete obstruction of the right pulmonary artery (Figure 1C). Coronary angiography revealed the presence of coronary to pulmonary collaterals traveling to the pulmonary circulation from the left circumflex artery (Figure 1D). An aortogram was not clinically indicated as systemic collateral flow has been classically described and is not routinely investigated in CTEPH. With the delayed timing of the DECT images, it is likely that the perfusion mapping in the right hemithorax represents collateral flow through the coronary-pulmonary collaterals seen on the coronary angiography, bronchial collaterals, or both. There was no evidence the patient experienced coronary steal syndrome or angina given the presence of these coronary-pulmonary collaterals.
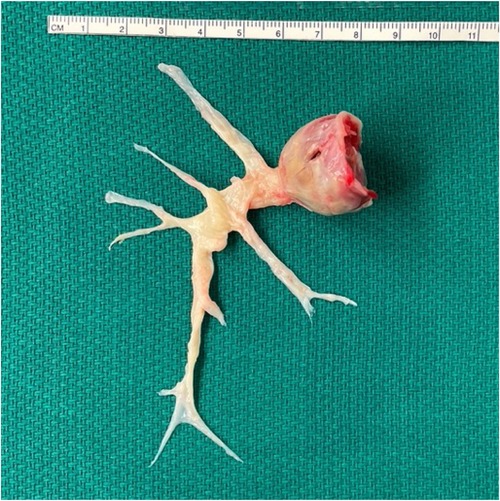
The patient underwent successful pulmonary thromboendarterectomy, Cox-Maze 3, and left atrial appendage occlusion without complication. Briefly, a primary median sternotomy was performed, bypass was initiated, and aortic cross clamp was applied. The right pulmonary artery was opened, and the entire clot was removed en bloc from all 10 segments of the right pulmonary artery under hypothermic circulatory arrest (Figure 2). The left pulmonary artery was inspected and found to have no significant clot consistent with pre-operative imaging. After bypass flows were resumed, an atrial clip was placed at the base left atrial appendage externally and atriotomies were performed to complete the Cox-Maze 3 procedure.
Post-surgery DECT confirmed patency of the right pulmonary artery and restoration of segmental and subsegmental pulmonary arterial circulation (Figure 3A,B). Pathology from the cast was negative for malignancy. Catheterization revealed an excellent hemodynamic response to surgery with a right atrial pressure of 3 mmHg (delta −1 mmHg), mean pulmonary artery pressure of 22 mmHg (delta −18 mmHg), cardiac output of 3.9 (delta +0.4), cardiac index of 2.0 (delta +0.2) and pulmonary vascular resistance of 4.9 wood units (delta −5.4 wood units). Repeat echocardiography revealed RV basal diameter of 3.84 cm (delta −0.61 cm), RA volume of 52.5 ml (delta −41.8 ml), TAPSE of 0.84 cm (delta −0.87 cm), and S’ of 7.87 cm/s (delta −1.92 cm/s).
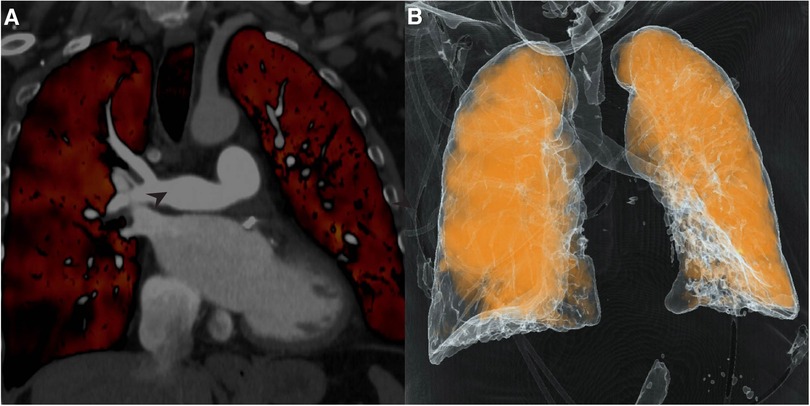
Figure 3. Dual-Energy computed tomography (DECT). (A) Repeat DECT demonstrates patent pulmonary artery post-thromboendarterectomy; (B) Perfusion mapping reveals significantly improved perfusion of right lung.
Discussion
We highlight that DECT-derived PBV mapping is a uniquely powerful tool to characterize the pulmonary vasculature, and to some extent the systemic vasculature, of patients and should receive more widespread consideration for evaluation of CTEPH because it allows for simultaneous assessment of pulmonary vessel obstruction and arterial perfusion to confirm diagnoses and guide clinical decision-making (Figure 4). First, significant focal perfusion to a proximally obstructed lung was revealed, eventually leading to a diagnosis of unilateral CTEPH. Unilateral CTEPH is independently a rarely discovered entity, seemingly documented only in case reports in patients with underlying thrombophilic disorders (10, 11). Whether the unilateral nature of this patient's disease contributed in some manner to the development of the coronary-pulmonary collaterals in this patient without a thrombophilia is unknown. Second, the DECT study revealed greater than expected perfusion to the obstructed lung. As a result, when invasive angiography was pursued, special attention was paid to elucidate the source of collateral flow, leading to the discovery of the rarely documented coronary-pulmonary artery collaterals. The physiological and molecular mechanisms underpinning angiogenesis and development of coronary-pulmonary collaterals are unclear. The contribution of systemic to pulmonary collaterals and the two-compartment model of pathophysiology in CTEPH remains to be elucidated. Thus far, while documentation of these collaterals in CTEPH patients has remained limited, research has suggested that the prevalence of coronary-pulmonary collaterals in CTEPH may be higher than previously thought (12–15). Bronchopulmonary collaterals in CTEPH have been more commonly documented and are suggested to promote bronchial dilation, lower post-operative pulmonary vascular resistance, and ultimately reduce post-operative mortality (1). Ultimately, it may be that bronchial collateralization contributed to some of the perfusion findings; however, the location of the discovered coronary-pulmonary collaterals vs. the PBV/perfusion map suggests they were a significant contributor as well. These coronary-pulmonary collaterals may share pathophysiology similar to bronchial-pulmonary collaterals, decrease pulmonary arterial resistance, and may also develop due to hypoxic factors. Our institution has adopted a delayed phase imaging protocol with the use of DECT to help understand and identify collateral flow.
Ethics statement
Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Author contributions
AG made substantial contributions to the conception/design, data analysis/interpretation of data, and drafting of article. RM made substantial contributions to the conception/design, data analysis/interpretation of data, critical revision of article. RA, MJC, JDF and SCM made substantial contributions to conception/design and critical revision of article. All authors approve the final version of this manuscript to be published and agree to be accountable for their contributions of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Delcroix M, Vonk Noordegraaf A, Fadel E, Lang I, Simonneau G, Naeije R. Vascular and right ventricular remodelling in chronic thromboembolic pulmonary hypertension. Eur Respir J. (2013) 41(1):224–32. doi: 10.1183/09031936.00047712
2. Heinrich M, Uder M, Tscholl D, Grgic A, Kramann B, Schäfers HJ. CT Scan findings in chronic thromboembolic pulmonary hypertension: predictors of hemodynamic improvement after pulmonary thromboendarterectomy. Chest. (2005) 127(5):1606–13. doi: 10.1378/chest.127.5.1606
3. Hong YJ, Shim J, Lee SM, Im DJ, Hur J. Dual-Energy CT for pulmonary embolism: current and evolving clinical applications. Korean J Radiol. (2021) 22(9):1555–68. doi: 10.3348/kjr.2020.1512
4. Abdellatif W, Ebada MA, Alkanj S, Negida A, Murray N, Khosa F, et al. Diagnostic accuracy of dual-energy CT in detection of acute pulmonary embolism: a systematic review and meta-analysis. Can Assoc Radiol J. (2021) 72(2):285–92. doi: 10.1177/0846537120902062
5. Petritsch B, Kosmala A, Gassenmaier T, Weng AM, Veldhoen S, Kunz AS, et al. Diagnosis of pulmonary artery embolism: comparison of single-source CT and 3rd generation dual-source CT using a dual-energy protocol regarding image quality and radiation dose. Rofo. (2017) 189(6):527–36. Diagnostik der akuten Lungenarterienembolie: Vergleich von Single-Source CT und Dritt-Generation Dual-Source CT unter Einsatz eines Dual-Energy Protokolls—Bildqualität und Strahlenexposition. doi: 10.1055/s-0043-103089
6. Krauss B, Grant KL, Schmidt BT, Flohr TG. The importance of spectral separation: an assessment of dual-energy spectral separation for quantitative ability and dose efficiency. Invest Radiol. (2015) 50(2):114–8. doi: 10.1097/rli.0000000000000109
7. Faby S, Kuchenbecker S, Sawall S, Simons D, Schlemmer HP, Lell M, et al. Performance of today's Dual energy CT and future multi energy CT in virtual non-contrast imaging and in iodine quantification: a simulation study. Med Phys. (2015) 42(7):4349–66. doi: 10.1118/1.4922654
8. Henzler T, Fink C, Schoenberg SO, Schoepf UJ. Dual-energy CT: radiation dose aspects. AJR Am J Roentgenol. (2012) 199(5 Suppl):S16–25. doi: 10.2214/ajr.12.9210
9. Kim NH. Assessment of operability in chronic thromboembolic pulmonary hypertension. Proc Am Thorac Soc. (2006) 3(7):584–8. doi: 10.1513/pats.200605-106LR
10. Hon S, Channick RN, Farber HW. Unilateral chronic thromboembolic pulmonary disease: a mimic of pulmonary artery agenesis. Am J Respir Crit Care Med. (2020) 201(10):e74–5. doi: 10.1164/rccm.201905-0997IM
11. Laczika K, Lang IM, Quehenberger P, Mannhalter C, Muhm M, Klepetko W, et al. Unilateral chronic thromboembolic pulmonary disease associated with combined inherited thrombophilia. Chest. (2002) 121(1):286–9. doi: 10.1378/chest.121.1.286
12. Lee NS, Auger WR, Pretorius V, Blanchard DG, Daniels LB. Coronary artery-to-pulmonary artery collaterals in chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Imaging. (2014) 7(6):962–6. doi: 10.1161/circimaging.114.002368
13. Lee NS, Blanchard DG, Knowlton KU, McDivit AM, Pretorius V, Madani MM, et al. Prevalence of coronary artery-pulmonary artery collaterals in patients with chronic thromboembolic pulmonary hypertension. Pulm Circ. (2015) 5(2):313–21. doi: 10.1086/681225
14. Haghbayan H, Coomes EA, Gunaratne K. A man in his 40s with coronary-to-pulmonary artery collateralizations. JAMA Cardiol. (2020) 5(3):356. doi: 10.1001/jamacardio.2019.5289
Keywords: CTEPH, DECT, pulmonary endarterectomy, coronary angiogram, pulmonary angiography, collaterals, chronic thromboembolic pulmonary hypertension
Citation: Goyal A, Avery R, Cuttica MJ, Flaherty JD, Malaisrie SC and Mylvaganam R (2023) Case report: Multimodality imaging of unusual coronary to pulmonary collaterals in chronic thromboembolic pulmonary hypertension. Front. Cardiovasc. Med. 10:1204736. doi: 10.3389/fcvm.2023.1204736
Received: 12 April 2023; Accepted: 19 May 2023;
Published: 8 June 2023.
Edited by:
Julian A. Luetkens, University Hospital Bonn, GermanyReviewed by:
Daniel Kuetting, University Hospital Bonn, GermanyRodrigo Salgado, Antwerp University Hospital & Holy Heart Lier, Belgium
© 2023 Goyal, Avery, Cuttica, Flaherty, Malaisrie and Mylvaganam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: S. Chris Malaisrie Y2hyaXMubWFsYWlzcmllQG5tLm9yZw==
 Ansh Goyal
Ansh Goyal Ryan Avery
Ryan Avery Michael J. Cuttica3
Michael J. Cuttica3 S. Chris Malaisrie
S. Chris Malaisrie