
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Cardiovasc. Med. , 28 June 2023
Sec. Aortic Surgery and Endovascular Repair Archive
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1203003
This article is part of the Research Topic Case Reports in Atherosclerosis and Vascular Medicine: 2023 View all 7 articles
 Fuzheng Guo1
Fuzheng Guo1 Zhibin He2*
Zhibin He2*
This report presents a patient with rheumatoid arthritis and COVID-19 infection one month earlier who experienced embolic episodes resulting in acute lower-limb ischemia from an unusual source. The blood flow was successfully restored by femoropopliteal thromboembolectomy. In determining the source of the embolism, the patient underwent electrocardiogram, transthoracic echocardiogram, and aortic CTA. The latter revealed a large, pedunculated, and mobile thrombus arising from the aortic arch and the descending thoracic aorta. Considering the patient's general health condition, we performed anticoagulation of the floating thrombus in the aortic lumen. The mechanism of aortic floating thrombosis exhibits considerable complexity. There are no standardized treatment protocols or clinical guidelines, and its treatment mainly includes open surgery, aortic endoluminal stent -graft insertion and pharmacological anticoagulation. Treatment strategy should be based on the cause of the disease and the patient's physical condition.
With China's deregulation of COVID-19 epidemic control, there is increasing evidence of the nonrespiratory effects of the coronavirus, including the development of coagulopathy-related manifestations such as arterial thromboembolism. The above has increased the vigilance of vascular surgeons. Multiple risk factors are associated with the formation and development of arterial thrombosis. Age, sex, history of coronary artery disease, and prior myocardial infarction are associated with thrombotic events following COVID-19 infection (1). Most arterial thromboses are acute myocardial infarction or ischemic stroke (2). Reports of aortic thrombosis are less frequent in cases of arterial thrombosis complications (3). Stroke and peripheral arterial embolism are commonly suspected to originate from the heart. Because of the large diameter of the aorta, high flow, and absence of significant mural atherosclerosis, thrombosis is difficult to form and persist.
With the increasing use of imaging techniques in recent years, floating aortic thrombi have become increasingly recognized as systemic emboli, creating a life-threatening risk of ischemic stroke or peripheral emboli. The pathophysiological mechanisms underlying this disease are not fully understood (4). Several authors have reported mobile thrombi arising from aneurysms and protruding atheromas that are significantly related to aging (5). However, in patients without severe atherosclerosis, floating aortic thrombi are also observed. We report a case of a pedunculated thrombus within the aorta, resulting in acute lower limb ischemia.
A 62-year-old woman was admitted with acute pain, coldness, and anesthesia in the right lower limb. No palpable pulses were observed in the popliteal, posterior tibial, or dorsalis pedis. The pulses in the left lower limb were normal. The patient had a history of rheumatoid arthritis (RA) but did not receive regular treatment. She had a history of cigarette smoking (36 pack/years). One month ago, the patient was infected with COVID-19 and was treated at home due to mild symptoms. The result of patient's nucleic acid test for COVID-19 was negative on admission. The patient received serological analysis to detect hypercoagulable and vasculitis disorders, including antiphospholipid antibodies. Laboratory test results showed no apparent abnormalities. Computed tomographic angiography (CTA) of the limb revealed no contrast agent over the entire course of the right common iliac artery, external iliac artery, or proximal segment of the internal iliac artery (Figure 1). Electrocardiography revealed sinus tachycardia (Supplementary Figures S1, S2). Echocardiography and cervical artery duplex ultrasonography were non-contributory. The chest x-ray was normal, and aortic CTA was performed before surgery, demonstrating a large filling defect caused by a floating thrombus in the aortic arch and the descending thoracic aorta (Figure 2, Supplementary Figure S3).
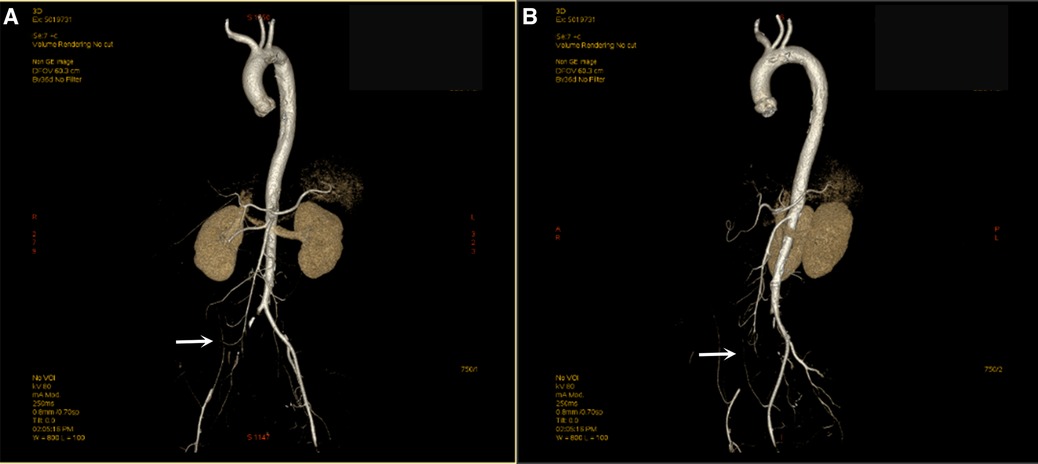
Figure 1. Computed tomography angiography (CTA) shows embolization of the right common iliac artery. The right common iliac artery was not filled with contrast, and the distal vessels were slimmer than the left (A,B).
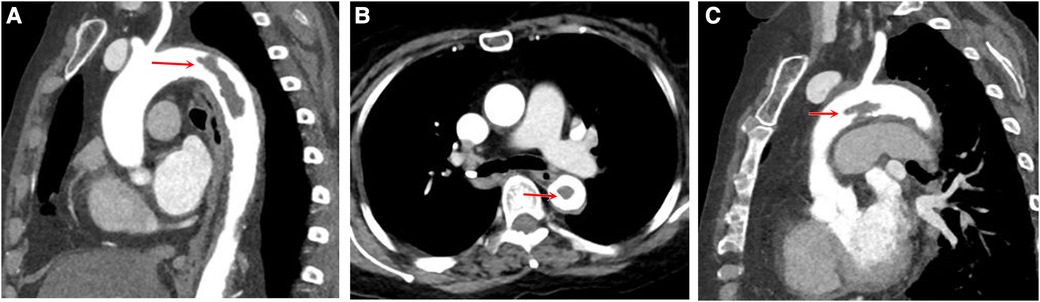
Figure 2. Aortic computed tomography scan displaying floating thrombus in the aortic lumen. The multiplanar reconstruction of CTA shows a filling defect in the descending aorta (A) and in the aortic arch (C), with the long axis in line with the direction of blood flow, proximally attached to the wall and distally free. The cross-sectional view shows the floating thrombus in the descending aorta (B).
The diagnosis of acute lower extremity ischemia due to a floating aortic thrombus was clear based on the patient's symptoms, signs, and imaging data. Figure 3 was showcasing a timeline with relevant data from the episode of care. A femoropopliteal thromboembolectomy was performed, which removed the embolus and restored limb perfusion (Supplementary Figure S4). After surgery, she received low-molecular-weight heparin treatment (Enoxaparin, 6000I U/12 h). We administered adequate anticoagulation using rivaroxaban at a daily dosage of 20 mg to prevent thrombotic progression post-discharge. Additionally, cilostazol, a phosphodiesterase inhibitor, was prescribed to enhance blood supply to the lower extremities and prevent platelet aggregation. Concurrently, patients were advised to stop smoking. The patient was apprised of the potential for thrombus re-dislodgement and advised to undergo anticoagulation therapy for a minimum of 3–6 months, with subsequent treatment contingent upon the management of rheumatoid arthritis.
Patients with severe COVID-19 infections may experience arterial thrombotic events such as acute coronary infarction, stroke, and limb ischemia (1). Although aortic thrombosis is uncommon, it has been reported in COVID-19 patients (3), and its pathophysiology is not fully defined. A procoagulant nature may develop aortic thrombosis due to various diseases, including sepsis, disseminated intravascular coagulation (DIC), autoimmune diseases, pregnancy, and cancer. The most common location was the descending thoracic aorta and the aortic arch (6). Protruding atheromas and aneurysms seem to be closely associated with the thrombosis in aorta (7). However, the pathogenic mechanism was unsuitable for younger patients because the aortic wall of these patients was normal or the atherosclerosis was not severe. Consequently, there may be a separate clinical entity from atheromatous disease in relatively young individuals. An adherent thrombus in the non-atherosclerotic aortic wall could represent an endothelial arterial disorder. Many studies have revealed that steroids can damage the endothelium directly and cause thickening of the intima (8). Oral contraceptives or pregnancy have been reported to be closely associated with intimal hyperplasia (9). Also, it has been reported that viral infections are related to thrombotic events (3). Inflammation, endothelial cell injury, platelet activation, and hypercoagulability contribute to viruses associated coagulopathy.
In this case, we believe that the floating aortic thrombus was caused by multiple factors.
First, although the patient denied a history of cardiovascular disease, she was at a high risk of atherosclerotic cardiovascular disease because of tobacco use, elevated blood pressure, and obesity (10). Second, the patient had a history of rheumatoid arthritis and oral steroid hormones. Studies have pointed out that patients with rheumatoid arthritis have an increased risk of cardiovascular disease compared to the general population, with a 68% higher risk of myocardial infarction and a 41% higher risk of stroke (11). Because of premature atherosclerotic development, high endothelial dysfunction rates, and rapid progression of atherosclerosis, RA patients are more likely to have cardiovascular events (12). It is accepted that atherosclerosis is a chronic inflammatory disease like RA. There are significant similarities between these two diseases in terms of their pathogenic and genetic factors. Inflammation associated with rheumatoid arthritis increases the incidence of vascular insults and the progression of atherosclerosis (13). Glucocorticoids can effectively suppress RA-related inflammation. However, there are numerous adverse cardiovascular effects associated with their use, including stroke, myocardial infarction, and heart failure. The adverse effects on lipid metabolism may account in part for their detrimental cardiovascular effects (11). Third, the patient was infected with COVID-19 one month ago. Similar to other coronaviruses, a significant association has been found between COVID-19 and the risk of thrombosis. A meta-analysis found that arterial thrombosis occurs in 4.4% of cases (14). The pathophysiology of thromboembolism in COVID-19 involves several aspects. Thrombotic and thromboembolic events are predisposed to COVID-19 because of excessive inflammation, endothelial injury, platelet activation, and hypercoagulability in the blood.
No standard protocol is available for treating floating aortic thrombi (15). Treatment options include anticoagulation, surgical thrombectomy, and endovascular treatment (7). Under comprehensive consideration, anticoagulation therapy was selected for aortic floating thrombus.
In the context of the novel coronavirus pandemic, COVID-19 could predispose patients to both venous and arterial thrombotic events. Although arterial thrombosis is a rare complication of this disease compared to venous thromboembolism, it cannot be underestimated. Aortic thrombosis is infrequent in arterial thrombosis, and some cases are diagnosed during screening for other diseases. Larger case series studies are required to determine whether aortic thrombosis is an uncommon complication of thrombotic events linked to COVID-19. Meanwhile, the procoagulant nature of RA is correlated with thrombosis in atypical locations and should be considered in patients with cardiovascular events. Evaluating the aorta is warranted in all patients with peripheral emboli of uncertain pathogenesis.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
FG: designed the study, analyzed the data and wrote and revised the manuscript. ZH: designed the study, performed the investigation and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by Peking University People's Hospital Scientific Research Development Funds (Grant/Award Number: RDJP2022-06).
The authors would like to acknowledge the contribution of the survey team members.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1203003/full#supplementary-material
Supplementary Figure 1
The patient's bedside electrocardiogram at the time of admission. ECG results showed sinus rhythm with a heart rate of 112 beats per minute.
Supplementary Figure 2
ECG repeated the next day manifests a heart rate of 91 beats per minute with sinus rhythm.
Supplementary Figure 3
Revascularization imaging of aortic CTA; thick arrows indicate floating thrombus.
Supplementary Figure 4
(A) Dislodged thrombus in the iliac artery. (B) The femoral artery is dissected out, and the proximal and distal ends are banded separately.
1. Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID-19 in a New York city health system. JAMA. (2020) 324(8):799–801. doi: 10.1001/jama.2020.13372
2. Lo Re V 3rd, Dutcher SK, Connolly JG, Perez-Vilar S, Carbonari DM, DeFor TA, et al. Association of COVID-19 vs influenza with risk of arterial and venous thrombotic events among hospitalized patients. JAMA. (2022) 328(7):637–51. doi: 10.1001/jama.2022.13072
3. de Carranza M, Salazar DE, Troya J, Alcázar R, Peña C, Aragón E, et al. Aortic thrombus in patients with severe COVID-19: review of three cases. J Thromb Thrombolysis. (2021) 51(1):237–42. doi: 10.1007/s11239-020-02219-z
4. Piazza G, Morrow DA. Diagnosis, management, and pathophysiology of arterial and venous thrombosis in COVID-19. JAMA. (2020) 324(24):2548–9. doi: 10.1001/jama.2020.23422
5. Zavala JA, Amarrenco P, Davis SM, Jones EF, Young D, Macleod MR, et al. Aortic arch atheroma. Int J Stroke. (2006) 1:74–80. doi: 10.1111/j.1747-4949.2006.00026.x
6. Fayad ZY, Semaan E, Fahoum B, Briggs M, Tortolani A, D'Ayala M. Aortic mural thrombus in the normal or minimally atherosclerotic aorta. Ann Vasc Surg. (2013) 27:282–90. doi: 10.1016/j.avsg.2012.03.011
7. Weiss S, Bühlmann R, von Allmen RS, Makaloski V, Carrel TP, Schmidli J, et al. Management of floating thrombus in the aortic arch. J Thorac Cardiovasc Surg. (2016) 152:810–7. doi: 10.1016/j.jtcvs.2016.03.078
8. Nashel DJ. Is atherosclerosis a complication of long-term corticosteroid treatment? Am J Med. (1986) 80:925–9. doi: 10.1016/0002-9343(86)90639-x
9. Lamy AL, Roy PH, Morissette JJ, Cantin R. Intimal hyperplasia and thrombosis of the visceral arteries in a young woman: possible relation with oral contraceptives and smoking. Surgery. (1988) 103(6):706–10.3375998
10. Li X, Wu C, Lu J, Chen B, Li Y, Yang Y, et al. Cardiovascular risk factors in China: a nationwide population-based cohort study. Lancet Public Health. (2020) 5(12):e672–81. doi: 10.1016/s2468-2667(20)30191-2
11. Atzeni F, Rodríguez-Carrio J, Popa CD, Nurmohamed MT, Szűcs G, Szekanecz Z. Cardiovascular effects of approved drugs for rheumatoid arthritis. Nat Rev Rheumatol. (2021) 17(5):270–90. doi: 10.1038/s41584-021-00593-3
12. Mason JC, Libby P. Cardiovascular disease in patients with chronic inflammation: mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur Heart J. (2015) 36(8):482–9c. doi: 10.1093/eurheartj/ehu403
13. Libby P. Role of inflammation in atherosclerosis associated with rheumatoid arthritis. Am J Med. (2008) 121(10 Suppl 1):S21–31. doi: 10.1016/j.amjmed.2008.06.014
14. Goudarzi E, Yousefimoghaddam F, Ramandi A, Khaheshi I. COVID-19 and peripheral artery thrombosis: a mini review. Curr Probl Cardiol. (2022) 47(10):100992. doi: 10.1016/j.cpcardiol.2021.100992
Keywords: aortic arch, floating thrombus, embolism, atherosclerosis, COVID-19
Citation: Guo F and He Z (2023) Acute limb ischemia caused by floating thrombus in the aorta: a case report and literature review. Front. Cardiovasc. Med. 10:1203003. doi: 10.3389/fcvm.2023.1203003
Received: 10 April 2023; Accepted: 5 June 2023;
Published: 28 June 2023.
Edited by:
Mark G. Davies, The University of Texas Health Science Center at San Antonio, United StatesReviewed by:
Joseph Hart, Medical College of Wisconsin, United States© 2023 Guo and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhibin He ZHJjbHcwMUAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.