- 1Department of Anesthesiology, Rhode Island Hospital, Providence, RI, United States
- 2Division of Cardiac Surgery, Department of Surgery, Massachusetts General Hospital, Boston, MA, United States
- 3Department of Anesthesiology, Perioperative and Pain Medicine, Brigham and Women’s Hospital, Boston, MA, United States
- 4Department of Medicine, Brigham and Women’s Hospital, Boston, MA, United States
- 5Department of Anesthesia, Critical Care and Pain Medicine, Beth Israel Deaconess Medical Center, Boston, MA, United States
- 6Division of Cardiac Surgery, Department of Surgery, Brigham and Women’s Hospital, Boston, MA, United States
- 7Cardio-Thoracic-Vascular Department, University Hospital—San Giovanni di Dio e Ruggi d’Aragona, Salerno, Italy
- 8Department of Anesthesiology, Boston University School of Medicine, Boston, MA, United States
Objectives: It is uncertain whether concurrent mitral valve repair or replacement for moderate or greater secondary mitral regurgitation at the time of coronary artery bypass graft or aortic valve replacement surgery improves long-term survival.
Methods: Patients undergoing coronary artery bypass graft and/or aortic valve replacement surgery with moderate or greater secondary mitral regurgitation were reviewed. The effect of concurrent mitral valve repair or replacement upon long-term mortality was assessed while accounting for patient and operative characteristics and mitral regurgitation severity.
Results: Of 1,515 patients, 938 underwent coronary artery bypass graft or aortic valve replacement surgery alone and 577 underwent concurrent mitral valve repair or replacement. Concurrent mitral valve repair or replacement did not alter the risk of postoperative mortality for patients with moderate mitral regurgitation (hazard ratio = 0.93; 0.75–1.17) or more-than-moderate mitral regurgitation (hazard ratio = 1.09; 0.74–1.60) in multivariable regression. Patients with more-than-moderate mitral regurgitation undergoing coronary artery bypass graft-only surgery had a survival advantage from concurrent mitral valve repair or replacement in the first two postoperative years (P = 0.028) that did not persist beyond that time. Patients who underwent concurrent mitral valve repair or replacement had a higher rate of later mitral valve operation or reoperation over the five subsequent years (1.9% vs. 0.2%; P = 0.0014) than those who did not.
Conclusions: These observations suggest that mitral valve repair or replacement for more-than-moderate mitral regurgitation at the time of coronary artery bypass grafting may be reasonable in a suitably selected coronary artery bypass graft population but not for aortic valve replacement, with or without coronary artery bypass grafting. Our findings are supportive of 2021 European guidelines that severe secondary mitral regurgitation “should” or be “reasonabl[y]” intervened upon at the time of coronary artery bypass grafting but do not support 2020 American guidelines for performing mitral valve repair or replacement concurrent with aortic valve replacement, with or without coronary artery bypass grafting.
Introduction
Secondary mitral regurgitation (MR) results from a progressive increase in left ventricular volume and mitral annular circumference with decreased regional or global myocardial function, commonly due to ischemic heart disease or aortic valve dysfunction (1). The underlying mechanisms of MR from left ventricular dilation are mitral annular enlargement, distortion of the mitral annulus and subvalvular apparatus, and an increase in interpapillary muscle distance (2). MR is commonly observed intraoperatively in patients undergoing aortic valve replacement (AVR) and/or coronary artery bypass grafting (CABG).
Left ventricular remodeling after repair of coronary arterial or aortic valve disease may reduce the severity of secondary MR, thus potentially reducing the need for concurrent mitral valve repair or replacement (MVR/P) (3, 4). However, when moderate or worse MR is observed prior to coronary revascularization or aortic valve surgery, it is presently debated whether MVR/P should be performed at the time of surgery and whether the correction of MR impacts survival (5, 6). The risks of not performing concurrent MVR/P, including persistent secondary MR and the need for cardiac reoperation, loss of potential long-term benefits in survival, and functional status, need to be balanced against the operative and postoperative morbidity and mortality of concomitant MVR/P (3, 5–9). Further, concomitant MVR/P may significantly improve the risk–benefit profile for later transcatheter aortic valve replacement (TAVR) compared to surgical AVR. However, well-conducted trials have failed to demonstrate improved mortality after concomitant MVR/P for moderate MR (7, 8, 10), and thus, the value of concomitant MVR/P for secondary MR during CABG surgery is still debated. Current Guidelines of the American Association for Thoracic Surgery state that “In patients with moderate IMR undergoing CABG, MV repair with an undersized complete rigid annuloplasty ring may be considered” with Class of Recommendation (COR) IIb, Level of Evidence (LOR) B (11). These guidelines differ slightly from those of the American College of Cardiology and American Heart Association (12) and the European Society of Cardiology and the European Association for Cardio-Thoracic Surgery (13).
This study aimed to examine the effect of concomitant MVR/P upon long-term mortality in patients with moderate or greater MR undergoing surgical CABG and/or AVR. We performed a single-center retrospective study to examine the hypothesis that concomitant MVR/P would decrease mortality after AVR and/or CABG while accounting for other risk factors for mortality. Secondary outcome analyses were performed to examine the effect of MVR/P upon subsequent operation on the mitral valve and readmission for heart failure.
Patients and methods
Patient selection
Medical records of 15,068 patients undergoing CABG, AVR, or MVR/P surgery between January 1, 2002, and January 2, 2016, at Brigham and Women's Hospital (BWH) were reviewed. Patients aged <18 or ≥90 years, who did not underwent AVR or CABG surgery, who had mitral stenosis, who underwent prior cardiac surgery, surgery on the tricuspid or pulmonic valve or the ascending aorta, and surgery for endocarditis, who had a missing assessment of MR severity by either preoperative transthoracic echocardiogram (pTTE) or intraoperative transesophageal echocardiogram (iTEE), or with missing mortality information were excluded. Patients with pTTE or iTEE reports describing primary myxomatous disease, chordal rupture, cleft, and perforated, flail, or prolapsing leaflet were also excluded from the analysis. To examine clinically significant MR defined as moderate or greater by either pTTE or iTEE, patients who had mild or less MR by both pTTE and iTEE were excluded. This yielded 1,515 patients available for analysis (PRISMA diagram; Figure 1).
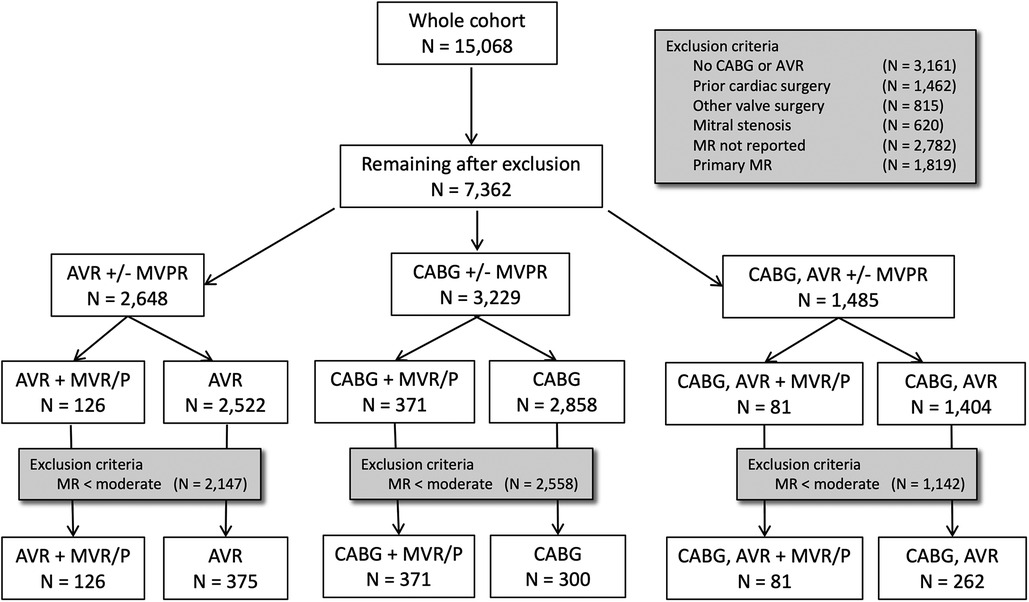
Figure 1. PRISMA diagram. There are two patient exclusion steps. The first step excludes patients not eligible by operative, valvular, or surgical criteria. The second step excludes patients with less-than-moderate severity of mitral regurgitation on both preoperative TTE and intraoperative TEE.
Data collection
Patient demographics and hospital outcomes were obtained from medical records and defined according to the specifications of the Society for Thoracic Surgeons Adult Cardiac Surgery database (14). The primary outcome of all-cause mortality was obtained from institutional follow-up protocols and the U.S. Social Security Death Index. Time to event was calculated from the date of surgery to the date of death or to January 8, 2016. Patients were followed for a median of 8.2 years (IQR 7.93 years). Performance of delayed MVR/P was identified by individual chart review, while subsequent readmission for heart failure was defined as primary admission diagnostic codes (ICD9: 428 and subcodes; ICD10: I05.1, I08.0, I08.1, I08.3, I09.81, I50.1, I50.2, I50.3, I50.4, I50.9, I97.13) occurring after discharge from initial AVR and/or CABG surgery.
Echocardiographic evaluation
Echocardiographic assessments of the MR grade were recorded from routinely obtained pTTE obtained within 180 days prior to surgery and read by an institutional or community cardiologist. iTEE was obtained intraoperatively prior to cardiopulmonary bypass. Over-reading of images was not performed as the reports constituted the information available to the surgeon. Defined pre hoc, we grouped each of the reported pTTE and iTEE patient MR assessments into less-than-moderate, moderate, or more-than-moderate MR. The most severe grade of MR assessed by pTTE or iTEE was used for analysis as it has been previously shown to provide the most predictive value for mortality (15).
Analysis plan
To test the principal hypothesis that concurrent mitral intervention for moderate or severe MR in patients undergoing CABG and/or AVR substantially improves mortality outcomes, we performed separate analyses on each of the three patient populations—patients who underwent CABG ± MV intervention, patients who underwent CABG and AVR ± MV intervention, and patients who underwent AVR ± MV intervention, while accounting for measured MR severity and other patient and operative characteristics. For each operative cohort, we examined the association with mortality, readmission for heart failure, and reoperation (15).
Statistical analysis
Statistical analysis was performed using R (https://cran.r-project.org/) and SAS (SAS Institute Inc., Cary, NC, United States) software. Continuous variables are presented as mean (SD) and compared using the unpaired Student’s t-test for normally distributed continuous variables. Non-normally distributed variables are described as median and 10–90th percentiles of range and compared using a Kruskal–Wallis test. Categorical variables were compared using the Fisher exact test. Missing data were excluded from the analysis.
Predictors of mortality outcome were described by Kaplan–Meier estimates and analyzed by Cox proportional hazards regression. For these analyses, we selected variables based on their clinical significance, variation between the study cohorts, and known contributions to life expectancy. These included age, gender, chronic obstructive pulmonary disease (COPD), preoperative dialysis, peripheral vascular disease, cerebrovascular disease, history of myocardial infarction, heart failure, preoperative atrial fibrillation, and an LVEF <40%. For exposure and each potential confounder, we first performed univariate analyses of mortality, followed by a stepwise multivariable analysis of potential confounders with a Cox proportional hazards univariate P-value <0.20. The year of operation, patient gender, the primary surgical operation, and concurrent mitral valve surgery were forced into the multivariate model. Model overfitting was tested with Cox–Snell residuals. Results are presented as hazard ratios (HRs) with 95% confidence intervals (CIs).
Results
Cohort characteristics
Across the three classes of surgery, 1,515 patients with clinically significant MR, graded as moderate or greater by either pTTE or iTEE, were followed over the median 8.2-year follow-up period (Table 1). Overall, patients were more frequently older than 60 years (85%), male (57%), and Caucasian (95%). There were significant differences in cardiac risk factors between operative cohorts (Supplementary Table S3). Comparison of MR severity assessed by pTTE and iTEE showed a preponderance of iTEE assessment of MR severity being less than pTTE severity (Supplementary Table S2). A total of 938 patients underwent AVR and/or CABG surgery alone, while 577 patients underwent concurrent MVR/P. Patients who underwent concurrent MVR/P were younger, more likely to be male, with coronary artery disease, with reduced ejection fraction, without aortic valve disease, and with more severe MR when assessed by either pTTE or iTEE (Table 1). Patients undergoing CABG surgery were more likely to have concurrent MVR/P. Few patients underwent subsequent mitral valve operation or reoperation (0.85%) after the initial surgery.
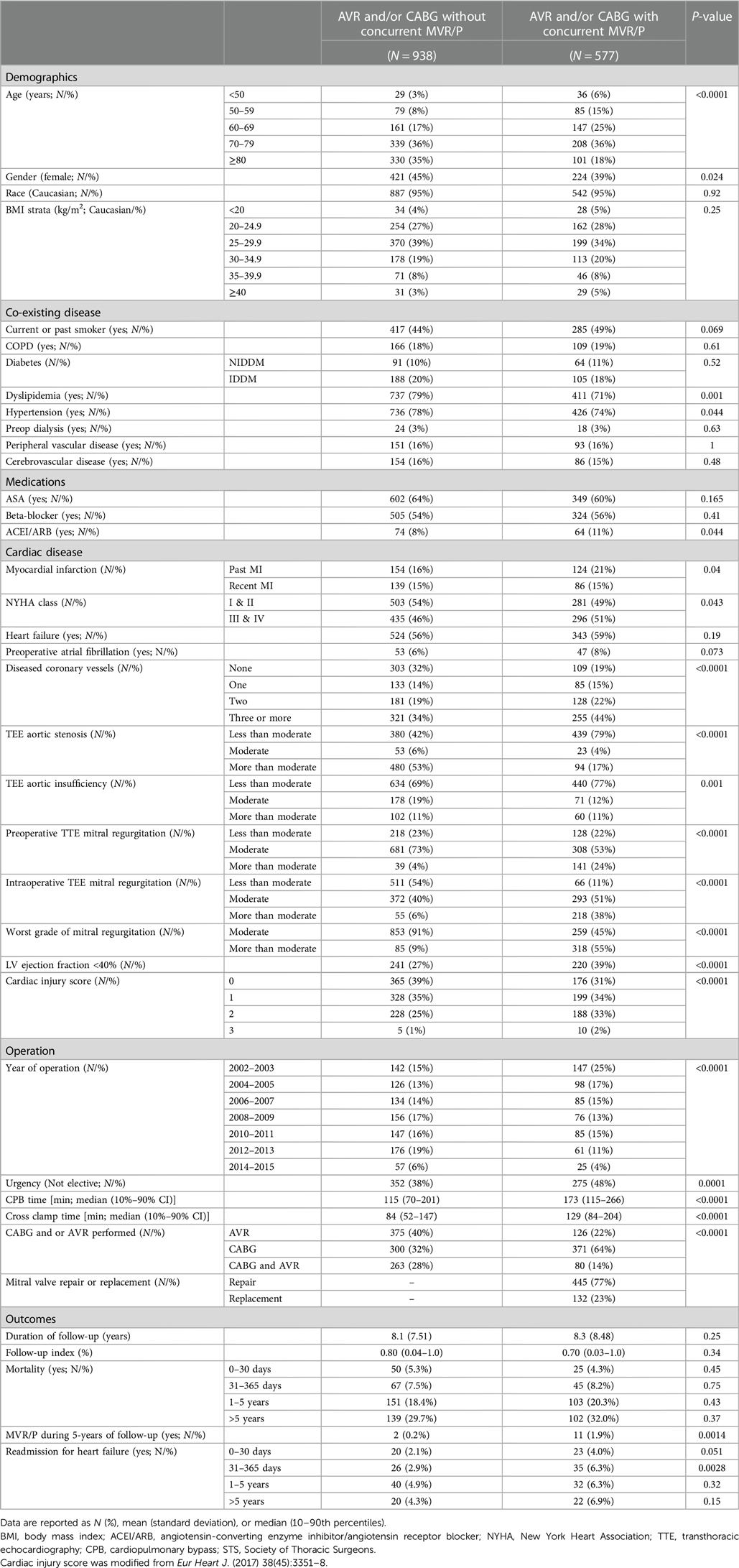
Table 1. Characteristics of the study cohort of 1,515 patients stratified by whether or not a concurrent MVR/P operation was performed.
Univariate predictors of mortality
Across the cohort, there was an increased risk of mortality associated with older age, female gender, medical comorbidities, and urgent surgery when assessed using a univariate Cox proportional hazards mortality model (Supplementary Table S4). Patients having CABG surgery alone (HR = 1.23; 1.02–1.47) were at increased risk of mortality compared to AVR (HR = 1.00), while patients having combined AVR/CABG were at further increased risk (HR = 1.47; 1.19–1.82). Concurrent mitral valve surgery did not alter the risk of postoperative mortality in the study cohort (HR = 1.00; 0.86–1.17) nor when stratified by moderate MR (HR = 0.94; 0.76–1.15) or severe MR (HR = 0.81; 0.57–1.13). In all operative cohorts, patients with moderate MR, defined as the worst observed severity of MR, had no improvement in survival from concurrent performance of MVR/P (see Figures 2–4 for AVR, CABG, and AVR/CABG, respectively). In patients undergoing CABG-only surgery with the worst MR severity by either pTTE or iTEE being more-than-moderate MR, there was a survival advantage from concurrent MVR/P in the first two postoperative years (P = 0.028; Figure 3) but not beyond that period. This was not observed in patients undergoing AVR or AVR/CABG surgery.
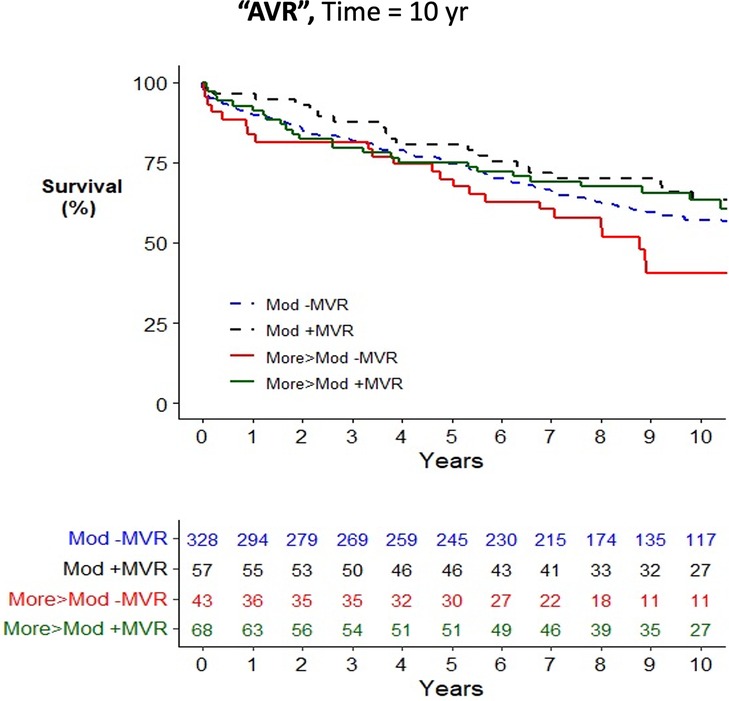
Figure 2. Kaplan–Meier plot of survival of 501 patients undergoing AVR who underwent MVR/P or not, stratified by the worst measured severity of MR. Observed mortality is stratified by the source of the most severe grade of MR (moderate, >moderate) and whether or not MVR/P was concurrently performed. Pairwise comparison of survival between AVR patients with moderate MR who underwent MVR/P or did not showed no statistical significance (P = 0.31) after adjustment for two comparisons. Pairwise comparison of survival between patients with more-than-moderate MR who underwent MVR/P or did not showed no statistical significance (P = 0.063) after adjustment for two comparisons.
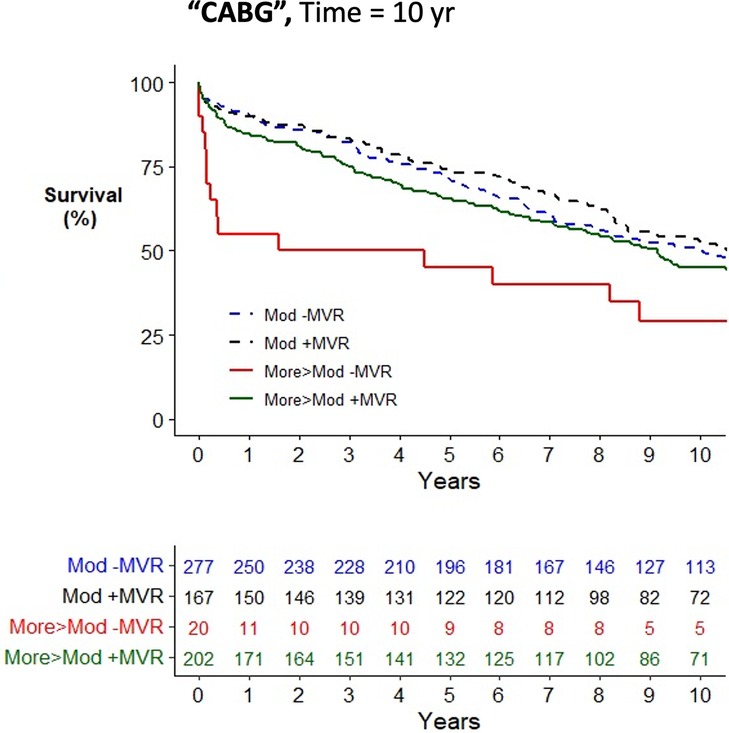
Figure 3. Kaplan–Meier plot of survival of 672 patients undergoing CABG who underwent MVR/P or not stratified by the worst measured severity of MR. Observed mortality is stratified by the source of the most severe grade of MR (moderate, >moderate) and whether or not MVR/P was concurrently performed. Pairwise comparison of survival between CABG patients with moderate MR who underwent MVR/P or did not showed no statistical significance (P = 0.49) after adjustment for two comparisons. Pairwise comparison of survival between patients with more-than-moderate MR who underwent MVR/P or did not showed statistical significance when adjusted for two comparisons (P = 0.028), which was explained by mortality in the first 2 postoperative years.
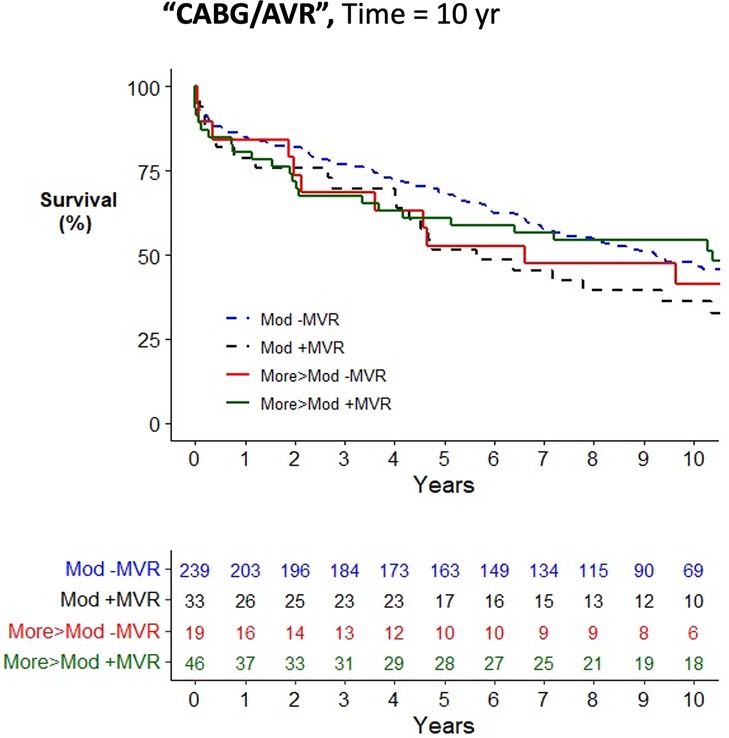
Figure 4. Kaplan–Meier plot of survival of 345 patients undergoing AVR/CABG who underwent MVR/P or not stratified by the worst severity of MR. Observed mortality is stratified by the source of the most severe grade of MR (moderate, >moderate) and whether or not MVR/P was concurrently performed. Pairwise comparison of survival between AVR/CABG patients with moderate MR who underwent MVR/P or did not showed no statistical significance (P = 0.15) after adjustment for two comparisons. Pairwise comparison of survival between patients with more-than-moderate MR who underwent MVR/P or did not showed no statistical significance (P = 0.55) after adjustment for two comparisons.
Mortality risk from concurrent MVR/P
To determine whether concurrent MVR/P at the time of CABG and/or AVR improved long-term survival, we used multivariate Cox proportional hazard modeling, stratified by the most severe grade of MR severity observed by either pTTE or iTEE (Supplementary Table S3). Hazards models for each operative class (AVR, CABG, and AVR/CABG; Supplementary Table S5) did not show a survival advantage from concurrent MVR/P after adjustment for other covariates. For patients with moderate MR, there was no observed survival advantage (HR = 0.93; 0.75–1.17) after accounting for other predictors of mortality. Similar findings were observed for patients with more-than-moderate MR (HR = 1.09; 0.74–1.60). There was no evidence of model overfitting (Supplementary Table S6 and Figure S1).
Readmission for heart failure and mitral valve reoperation
To determine whether concurrent MVR/P at the time of CABG and/or AVR reduced the rate of readmission for heart failure or subsequent MVR/P during the following 5 years, we compared these outcomes by whether concurrent MVR/P was performed (Table 1). Patients who underwent concurrent MVR/P had a higher rate of later mitral valve operation or reoperation over the five subsequent years (1.9% vs. 0.2%; P = 0.0014) than those who did not. Similarly, patients who underwent concurrent MVR/P had a higher incidence of readmission with a primary diagnosis of heart failure over the first postoperative year (10.3% vs. 5.0%; Table 1).
Discussion
In this retrospective study, we compared patients with moderate or greater secondary MR when undergoing CABG and/or AVR and assessed whether concurrent MVR/P improved survival and other outcomes. We observed improved survival in the first 2 years after surgery in a subgroup of patients undergoing CABG surgery with concomitant MVR/P who had more-than-moderate MR. This improvement in survival was not observed in patients undergoing AVR or CABG/AVR surgery nor in patients with moderate MR undergoing any operation. The low frequency of subsequent mitral surgery or admission for heart failure was not significantly reduced by concurrent mitral valve surgery.
Well-conducted randomized trials for the treatment of moderate or severe MR by MVR/P have been performed (10, 16), yielding consensus on the value of MVR/P alone in secondary MR (11–13). These studies have not demonstrated significant improvement in short to intermediate-term mortality after MVR/P but have yielded important insights into the value of mitral valve replacement vs. repair. In contrast, the potential mortality benefit of concurrent MVR/P during primary surgery for CABG and/or AVR has not been demonstrated. This determination is important because expected or unexpected findings of moderate or greater MR during CABG and/or AVR occur intraoperatively, and surgical guidance is lacking.
The benefit of MVR/P concomitant with CABG for moderate MR has been examined in randomized trials (7, 8, 10), several observational studies (17–19), and meta-analyses (22–22). Consensus indicates no survival benefit from concomitant MVR/P with CABG, as we also observed, but studies of survival after concomitant AVR or AVR/CABG are few (23) and limited in scope. Although there is evidence that concomitant MVR/P decreases echocardiographic measures of MR after both AVR and AVR/CABG, survival comparison is generally lacking, but when present, it indicates no survival benefit from concomitant MVR/P (24, 25).
Our results did not reveal a significant survival advantage of performing concomitant MVR/P for either moderate or more-than-moderate MR at the time of AVR or AVR/CABG surgery but may have been limited by the small number of patients with unrepaired severe MR. Our findings are not yet supportive of current European guidelines that severe secondary MR “should” be intervened upon at the time of CABG and/or AVR (Class 1C) (13). Current American guidelines call an intervention in this scenario “reasonable” (Class 2A) (12). Our observations suggest that MVR/P for more than moderate MR at the time of CABG may be reasonable in a suitably selected CABG population but not for AVR with or without CABG. The decision to surgically treat secondary MR has depended on severity, patient comorbidity, and technical complexity. The addition of a second valve surgery to an AVR has been previously shown to increase operative risk (26), which we did not observe. We interpret these findings as not yet justifying performing concurrent MVR/P for moderate or more severe MR in all situations. Unrepaired, moderate, or severe MR has been variably reported to be an independent risk factor for mortality after surgical AVR (SAVR) (6, 27) and after TAVR (27, 28). TAVR allows individual assessment of the reduction of MR severity after treatment of aortic stenosis while not adding the risk of concurrent MVR/P in all patients. Based on the findings of this study and TAVR's ability to provide a window into an assessment of improvement in MR, the role of SAVR and concurrent MVR/P seems weak. The absence of large-scale studies examining the effect of MVR/P concurrent with AVR/CABG upon mortality and our observed lack of benefit seems to also not support its use.
Limitations
The single-center, retrospective design of this study had inherent limitations, but it allowed for the assessment of long-term postoperative outcomes. Patients were not randomly assigned to undergo concomitant MVR/P; therefore, there is a potential for bias by clinical presentation or surgical practices that are unaccounted for in this study. We could not differentiate between outcomes of mitral valve repair vs. mitral valve replacement nor the severity of MR in follow-up. The statistical techniques used accounted for a number of variables but are unable to account for unmeasured confounders, which can occur in retrospective studies. We used all-cause mortality rather than cardiac-specific mortality as our primary outcome. While this would include mortality not due to primary disease, it allows for a more complete accounting of mortality. We are unable to identify specific indications for reoperations and/or readmissions, as well as those that may have occurred at other institutions, as a cause of mortality. We are also unable to comment on postoperative medical management or postoperative severity of MR.
Conclusions
In this retrospective study comparing survival in patients with secondary MR undergoing CABG and/or AVR, we assessed whether concurrent MVR/P improved survival. Improved survival was only observed in a small cohort of patients with more-than-moderate MR undergoing CABG surgery and only in the first 2 years after surgery. This improvement in survival was not observed in patients undergoing AVR or CABG/AVR surgery nor in patients with moderate MR undergoing any operation. Our findings suggest that MVR/P for more-than-moderate MR at the time of CABG is reasonable in a suitably selected population but is not indicated when undergoing AVR, with or without CABG.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Mass General Brigham Institutional Review Board (IRB: 2011P001259, approved June 14, 2011). The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because the authors were unable to obtain consent from deceased individuals and anonymized medical records.
Author contributions
SA, JM, and SB contributed to conception and design of the study. SA, RM, GM, and JS worked on data collection, organized the data, and collated any missing data. CO and MH performed the statistical analysis. SA and SB wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1202174/full#supplementary-material
References
1. Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgitation. Lancet. (2009) 373:1382–94. doi: 10.1016/s0140-6736(09)60692-9
2. Nappi F, Cristiano S, Nenna A, Chello M. Ischemic mitral valve prolapse. J Thorac Dis. (2016) 8:3752–61. doi: 10.21037/jtd.2016.12.33
3. Alghamdi AA, Elmistekawy EM, Singh SK, Latter DA. Is concomitant surgery for moderate functional mitral regurgitation indicated during aortic valve replacement for aortic stenosis? A systematic review and evidence-based recommendations. J Card Surg. (2010) 25:182–7. doi: 10.1111/j.1540-8191.2009.00965.x
4. Penicka M, Linkova H, Lang O, Fojt R, Kocka V, Vanderheyden M, et al. Predictors of improvement of unrepaired moderate ischemic mitral regurgitation in patients undergoing elective isolated coronary artery bypass graft surgery. Circulation. (2009) 120:1474–81. doi: 10.1161/CIRCULATIONAHA.108.842104
5. Chatterjee S, Tripathi B, Virk HU, Ahmed M, Bavishi C, Krishnamoorthy P, et al. Does surgical repair of moderate ischemic mitral regurgitation improve survival? A systematic review. Curr Cardiol Rep. (2016) 18:22. doi: 10.1007/s11886-016-0701-5
6. Harling L, Saso S, Jarral OA, Kourliouros A, Kidher E, Athanasiou T. Aortic valve replacement for aortic stenosis in patients with concomitant mitral regurgitation: should the mitral valve be dealt with? Eur J Cardiothorac Surg. (2011) 40:1087–96. doi: 10.1016/j.ejcts.2011.03.036
7. Chan KM, Punjabi PP, Flather M, Wage R, Symmonds K, Roussin I, et al. Coronary artery bypass surgery with or without mitral valve annuloplasty in moderate functional ischemic mitral regurgitation: final results of the randomized ischemic mitral evaluation (RIME) trial. Circulation. (2012) 126:2502–10. doi: 10.1161/CIRCULATIONAHA.112.143818
8. Fattouch K, Guccione F, Sampognaro R, Panzarella G, Corrado E, Navarra E, et al. POINT: efficacy of adding mitral valve restrictive annuloplasty to coronary artery bypass grafting in patients with moderate ischemic mitral valve regurgitation: a randomized trial. J Thorac Cardiovasc Surg. (2009) 138:278–85. doi: 10.1016/j.jtcvs.2008.11.010
9. Harris KM, Sundt TM 3rd, Aeppli D, Sharma R, Barzilai B. Can late survival of patients with moderate ischemic mitral regurgitation be impacted by intervention on the valve? Ann Thoracic Surg. (2002) 74:1468–75. doi: 10.1016/S0003-4975(02)03920-6
10. Michler RE, Smith PK, Parides MK, Ailawadi G, Thourani V, Moskowitz AJ, et al. Two-year outcomes of surgical treatment of moderate ischemic mitral regurgitation. New Engl J Med. (2016) 374:1932–41. doi: 10.1056/NEJMoa1602003
11. American Association For Thoracic Surgery Ischemic Mitral Regurgitation Consensus Guidelines Writing Committee, Kron IL, LaPar DJ, Acker MA, Adams DH, Ailawadi G, et al. 2016 update to the American association for thoracic surgery consensus guidelines: ischemic mitral valve regurgitation. J Thorac Cardiovasc Surg. (2017) 153:1076–9. doi: 10.1016/j.jtcvs.2016.11.068
12. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. (2021) 143:e35–71. doi: 10.1161/CIR.0000000000000932
13. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. (2022) 43:561–632. doi: 10.1093/eurheartj/ehab395
14. Shahian DM, Jacobs JP, Edwards FH, Brennan JM, Dokholyan RS, Prager RL, et al. The society of thoracic surgeons national database. Heart. (2013) 99:1494–501. doi: 10.1136/heartjnl-2012-303456
15. Asher SR, Malzberg GW, Ong CS, Malapero RJ, Wang H, Shekar P, et al. Joint preoperative transthoracic and intraoperative transoesophageal echocardiographic assessment of functional mitral regurgitation severity provides better association with long-term mortality. Interact Cardiovasc Thorac Surg. (2021) 32:9–19. doi: 10.1093/icvts/ivaa230
16. Goldstein D, Moskowitz AJ, Gelijns AC, Ailawadi G, Parides MK, Perrault LP, et al. Two-year outcomes of surgical treatment of severe ischemic mitral regurgitation. New Engl J Med. (2016) 374:344–53. doi: 10.1056/NEJMoa1512913
17. Prifti E, Bonacchi M, Frati G, Giunti IG, Leacche M, Proietti P, et al. Should mild-to-moderate and moderate ischemic mitral regurgitation be corrected in patients with impaired left ventricular function undergoing simultaneous coronary revascularization? J Card Surg. (2001) 16:473–83. doi: 10.1111/j.1540-8191.2001.tb00552.x
18. Trichon BH, Felker GM, Shaw LK, Cabell CH, O’Connor CM. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Cardiol. (2003) 91:538–43. doi: 10.1016/s0002-9149(02)03301-5
19. Kang DH, Kim MJ, Kang SJ, Song JM, Song H, Hong MK, et al. Mitral valve repair versus revascularization alone in the treatment of ischemic mitral regurgitation. Circulation. (2006) 114:I499–503. doi: 10.1161/CIRCULATIONAHA.105.000398
20. Teng Z, Ma X, Zhang Q, Yun Y, Ma C, Hu S, et al. Additional mitral valve procedure and coronary artery bypass grafting versus isolated coronary artery bypass grafting in the management of significant functional ischemic mitral regurgitation: a meta-analysis. J Cardiovasc Surg. (2017) 58:121–30. doi: 10.23736/S0021-9509.16.08852-2
21. Anantha Narayanan M, Aggarwal S, Reddy YNV, Alla VM, Baskaran J, Kanmanthareddy A, et al. Surgical repair of moderate ischemic mitral regurgitation-A systematic review and meta-analysis. Thorac Cardiovasc Surgeon. (2017) 65:447–56. doi: 10.1055/s-0036-1598012
22. Wang L, Li B, Liu C, Rong T, Yu Y, Gu C. Short- and medium-term effects of combined mitral valve surgery and coronary artery bypass grafting versus coronary artery bypass grafting alone for patients with moderate ischemic mitral regurgitation: a meta-analysis. J Cardiothorac Vasc Anesth. (2016) 30:1578–86. doi: 10.1053/j.jvca.2016.06.032
23. Schubert SA, Yarboro LT, Madala S, Ayunipudi K, Kron IL, Kern JA, et al. Natural history of coexistent mitral regurgitation after aortic valve replacement. J Thorac Cardiovasc Surg. (2016) 151:1032–9, 1042.e1. doi: 10.1016/j.jtcvs.2015.12.006
24. Coutinho GF, Correia PM, Pancas R, Antunes MJ. Management of moderate secondary mitral regurgitation at the time of aortic valve surgery. Eur J Cardiothorac Surg. (2013) 44:32–40. doi: 10.1093/ejcts/ezs676
25. Kim GS, Kim JB, Han S, Choo SJ, Chung CH, Lee JW, et al. Mitral valve repair versus replacement for moderate-to-severe mitral regurgitation in patients undergoing concomitant aortic valve replacement. Interact Cardiovasc Thorac Surg. (2014) 18:73–9. doi: 10.1093/icvts/ivt402
26. Litmathe J, Boeken U, Kurt M, Feindt P, Gams E. Predictive risk factors in double-valve replacement (AVR and MVR) compared to isolated aortic valve replacement. Thorac Cardiovasc Surg. (2006) 54:459–63. doi: 10.1055/s-2006-924247
27. Nombela-Franco L, Ribeiro HB, Urena M, Allende R, Amat-Santos I, DeLarochelliere R, et al. Significant mitral regurgitation left untreated at the time of aortic valve replacement: a comprehensive review of a frequent entity in the transcatheter aortic valve replacement era. J Am Coll Cardiol. (2014) 63:2643–58. doi: 10.1016/j.jacc.2014.02.573
Keywords: mitral valve surgery, aortic valve surgery, coronary artery bypass surgery, mortality, survival, outcomes, mitral regurgitation, guidelines
Citation: Asher SR, Ong CS, Malapero RJ, Heydarpour M, Malzberg GW, Shahram JT, Nguyen TB, Shook DC, Shernan SK, Shekar P, Kaneko T, Citro R, Muehlschlegel JD and Body SC (2023) Effect of concurrent mitral valve surgery for secondary mitral regurgitation upon mortality after aortic valve replacement or coronary artery bypass surgery. Front. Cardiovasc. Med. 10:1202174. doi: 10.3389/fcvm.2023.1202174
Received: 7 April 2023; Accepted: 8 September 2023;
Published: 29 September 2023.
Edited by:
Antonio Miceli, Istituto Clinico Sant'Ambrogio, ItalyReviewed by:
Flavio Tarasoutchi, University of São Paulo, BrazilKatharina Huenges, University Medical Center Schleswig-Holstein, Germany
Keti Vitanova, Technical University Munich, Germany
© 2023 Asher, Ong, Malapero, Heydarpour, Malzberg, Shahram, Nguyen, Shook, Shernan, Shekar, Kaneko, Citro, Muehlschlegel and Body. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shyamal R. Asher c2Nib2R5QGJ1LmVkdQ==
 Shyamal R. Asher
Shyamal R. Asher Chin Siang Ong2
Chin Siang Ong2 Raymond J. Malapero
Raymond J. Malapero Jasmine T. Shahram
Jasmine T. Shahram Rodolfo Citro
Rodolfo Citro Simon C. Body
Simon C. Body