- 1Division of Cardiology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital, College of Medicine, Chang Gung University, Kaohsiung, Taiwan
- 2Division of Cardiovascular Medicine, Chi-Mei Medical Center, Tainan, Taiwan
Background: Left bundle branch pacing (LBBP) is an emerging physiological pacing modality. Left ventricular (LV) myocardial work (MW) incorporates afterload and LV global longitudinal strain to estimate global and segmental myocardial contractility. However, the effect of LBBP on LV MW remains unknown. This study aimed to evaluate the impact of LBBP on LV MW in patients receiving pacemaker for bradyarrhythmia.
Methods: We prospectively enrolled 70 bradycardia patients with normal LV systolic function receiving LBBP (n = 46) and non-selective His-bundle pacing (NS-HBP) (n = 24). For comparative analysis, patients receiving right ventricular pacing (RVP) (n = 16) and control subjects (n = 10) were enrolled. Two-dimensional speckle tracking echocardiography was performed. The LV pressure-strain loop was non-invasively constructed to assess global LV MW.
Results: After 6-month follow-up, LBBP group (with >40% ventricular pacing during 6 months) had shorter peak strain dispersion (PSD) compared with RVP group, and higher LV global longitudinal strain compared with RVP group and NS-HBP group, but had no difference in left intraventricular mechanical dyssynchrony, including septal-to-posterior wall motion delay and PSD, compared with NS-HBP group. During ventricular pacing, LBBP group had higher global MW index (GWI) (2,189 ± 527 vs. 1,493 ± 799 mmHg%, P = 0.002), higher global constructive work (GCW) (2,921 ± 771 vs. 2,203 ± 866 mmHg%, P = 0.009), lower global wasted work (GWW) (211 ± 161 vs. 484 ± 281 mmHg%, P < 0.001) and higher global MW efficiency (GWE) (91.4 ± 5.0 vs. 80.9 ± 8.3%, P < 0.001) compared with RVP group, and had lower GWW (211 ± 161 vs. 406 ± 234 mmHg%, P < 0.001) and higher GWE (91.4 ± 5.0 vs. 86.4 ± 8.1%, P < 0.001) compared with NS-HBP group.
Conclusions: In this study we found that in patients with mid-term (6-month) high ventricular pacing burden (>40%), LBBP preserved more LV MW compared with NS-HBP and RVP. Further studies are warranted to assess the association between LV MW and long-term clinical outcomes in LBBP with high ventricular pacing burden.
1. Introduction
Permanent pacemaker is an effective therapy for patients with symptomatic bradyarrhythmia, including sinus nodal dysfunction and atrioventricular block. Right ventricular pacing (RVP) over the right ventricular (RV) apex or the RV septum has been reported to increase the risks of atrial fibrillation, heart failure (HF) hospitalization, or mortality (1, 2). Previous studies have demonstrated that acute RVP leads to electrical and mechanical dyssynchrony of left ventricle (LV) (3, 4), and long-term pacing also results in histopathological changes and LV remodeling (5, 6). Recently, conduction system pacing, such as His-bundle pacing (HBP) and left bundle branch pacing (LBBP), are emerging physiological pacing strategies (7, 8). In clinical practice, LBBP is more feasible than HBP, owing to more stable threshold during follow-up (8). Theoretically, LBBP could directly stimulate left bundle branch area to synchronize LV contraction in patients with left bundle branch block. However, myocardial deformation imaging studies with LV global longitudinal strain (GLS) for intraventricular or interventricular synchrony for LBBP compared with HBP and RVP are limited (9–12). Moreover, LV myocardial work (MW), derived from non-invasive LV pressure-strain loop estimations that incorporates both information on afterload and LV GLS, can overcome the limitations and shortcomings of load dependency of speckle tracking echocardiography (STE) and may provide additional information regarding dyssynchronous contraction, segmental work, and myocardial contractility (13, 14). Furthermore, global LV MW efficiency has been reported to be associated with long-term survival in patients after ST-segment elevation myocardial infarction (15). However, study to compare the difference in the impact on the LV MW among LBBP, RVP and HBP is lacking (13, 14). Accordingly, we conducted this prospective study to evaluate the immediate and mid-term effects of LBBP on the intraventricular and interventricular mechanical synchronies, and LV MW compared with HBP and RVP in patients receiving transvenous permanent pacemaker implantation for symptomatic bradyarrhythmia.
2. Materials and methods
2.1 Study subjects
Patients with symptomatic sinus nodal dysfunction and atrioventricular block receiving conduction system pacing were prospectively enrolled in our hospital between July, 2020 and December, 2021. For comparative analysis of case-control study, gender- and comorbidity-matched patients receiving transvenous RVP for symptomatic bradyarrhythmia and gender- and comorbidity-matched control subjects without pacemaker between July, 2020 and December, 2021 were also enrolled in this study. The exclusion criteria included patients with: (1) any history of HF with left ventricular ejection fraction (LVEF) <50% due to severe valvular heart disease or cardiomyopathy, (2) permanent atrial fibrillation, (3) left bundle branch block, (4) end-stage renal disease, and (5) malignancy with expected lifespan <1 year.
2.2 Procedures of conduction system pacing
The detailed procedures are provided in Supplementary Methods: Procedures of conduction system pacing.
2.3 Echocardiographic data acquisition
Echocardiography was performed within 7 days and at 6-month follow-up after the pacemaker implantation. All echocardiographic measurements were obtained initially during sinus or intrinsic rhythm for patients with normal atrioventricular conduction at the time of echocardiography, and then obtained during the DDD pacing mode with an atrioventricular delay of 100–120 ms to ensure complete capture by pacing without fusion in patients with normal atrioventricular conduction, at 2-fold output of pacing threshold, and a pacing rate between 80 and 110 beats/min. Because of inconsistent pacing threshold of HBP group during follow-up, echocardiographic images were only acquired during non-selective HBP (NS-HBP). Transthoracic echocardiographic images were recorded using a Vivid E9 ultrasound system (GE Healthcare, Milwaukee, WI, US) with M5S 3.5 MHz transducers. All echocardiographic measurements were obtained for at least 3 consecutive beats for sinus and pacing rhythm, and 5 consecutive beats for atrial fibrillation rhythm, and were stored with digital loops for offline analysis with EchoPAC (Version 202, GE Healthcare, Chicago, IL, US).
Based on the recommendations for cardiac chamber quantification by echocardiography (16), left atrial diameters, and LV dimensions and volumes were measured using two-dimensional echocardiography, and LVEF was calculated by biplane Simpson's method from the apical 4-chamber and 2-chamber views. The mean E/e' ratio (the average value of septal E/e' and lateral E/e'), tricuspid annular plane systolic excursion (TAPSE), and S' wave of the RV free wall (FW) were also obtained.
2.4 Evaluations of intraventricular and interventricular synchronies
The intraventricular and interventricular synchronies were evaluated by pulsed-wave Doppler imaging echocardiography, tissue Doppler (Ts) imaging and STE. The regional durations of time measured for the basal segments in the LVFW, RVFW and interventricular septum (IVS) were from the start of QRS to the peak velocity of S' wave of Ts in the LVFW (Ts-LVFW), RVFW (Ts-RVFW) and septum (Ts-IVS), respectively (17).
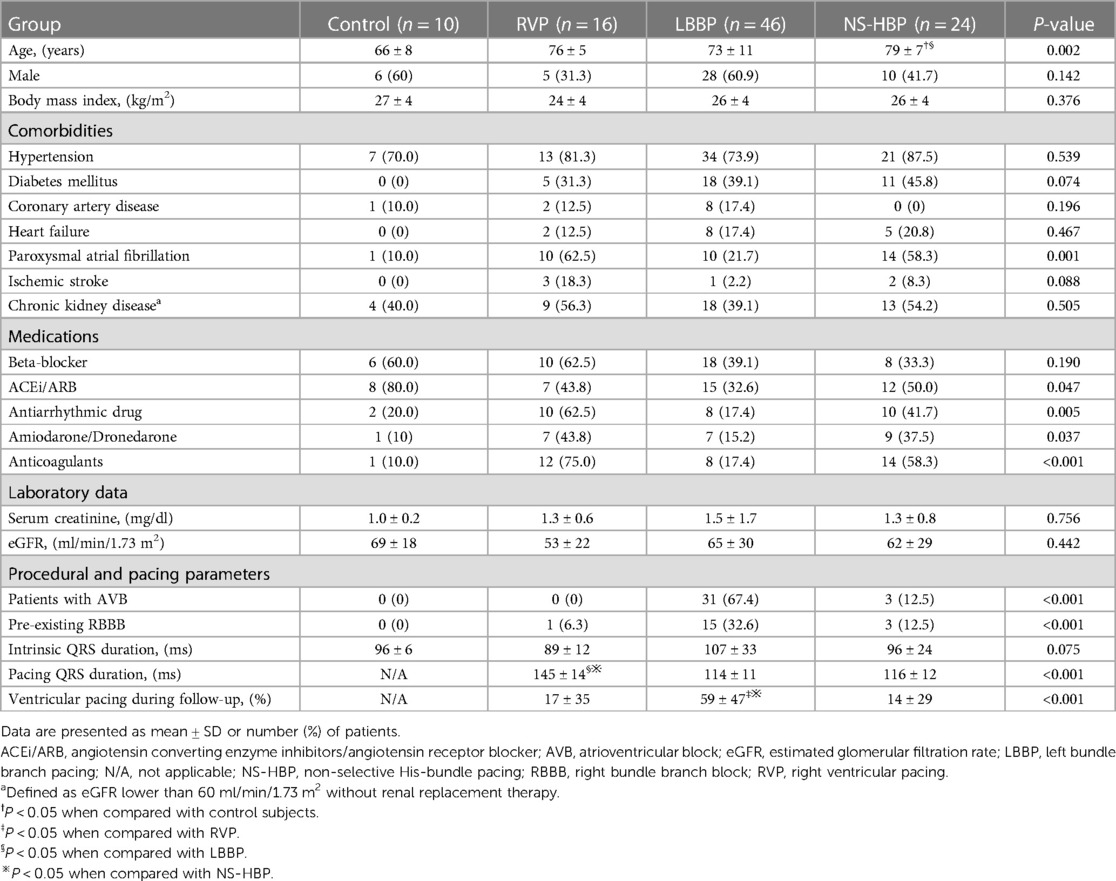
The LV GLS, peak strain dispersion (PSD), septal-to-posterior wall motion delay (SPWMD), and difference of Ts between LVFW and IVS (Ts-LVFW-IVS) were used as LV mechanical synchrony parameters (3, 4, 9, 10, 17, 18). The value of Ts-LVFW-IVS means the time of S' in the LVFW minus the time of S' in the septum. The LV GLS measured by STE at a frame rate of 50–80 frames/s, and the apical four-chamber (A4C) view, apical three-chamber (LAX) view, and apical two-chamber (A2C) view of STE were recorded as well as electrocardiography. Peak LV GLS was then performed using echocardiography software that divided the myocardium into 6 segments in each view, creating graphs of shortening over the cardiac cycle. A bull's-eye plot was created of peak LV GLS for each myocardial segment (Figure 1). That absolute values of GLS were used when comparison among different study groups. PSD is defined as the standard deviation of time to peak longitudinal systolic strain of LV segments (Figure 1). The difference of Ts between Ts-RVFW and Ts-IVS (Ts-RVFW-IVS) was used as RV intraventricular mechanical synchrony parameter (10). For interventricular synchrony, the value of LV pre-ejection period (LV PEP), interventricular mechanical delay (IVMD), and different of Ts between LVFW and RVFW (Ts-LVFW-RVFW) were calculated (10, 17). Pulsed-wave Doppler flow velocity signal was recorded from the LV outflow tract, and LV PEP were measured as the time intervals between the Q-wave on the surface ECG and the onset of Doppler flow. IVMD was defined as the time difference between LV PEP and RV PEP, which were the periods from the start of QRS wave to the start of pulsed-wave Doppler flow of LV and RV outflow tracts, respectively. The values of LV PEP >140 ms and IVMD >40 ms were both considered indicative of interventricular dyssynchrony (17, 19).
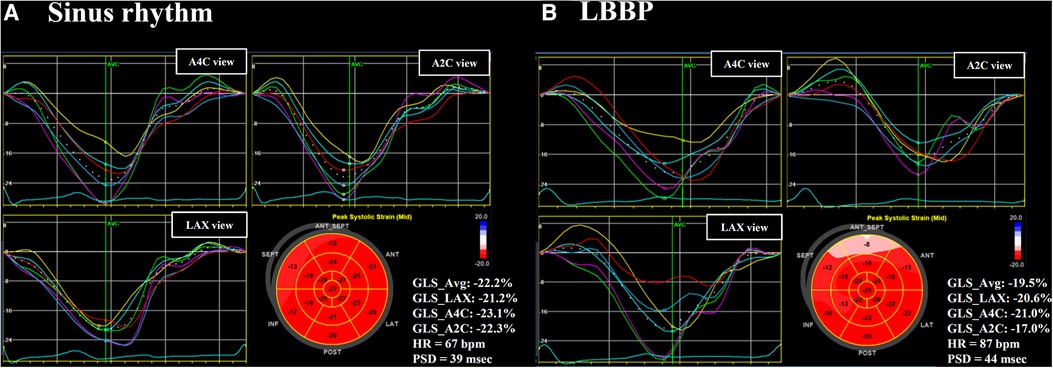
Figure 1. Left ventricular GLS and PSD. Left ventricular (LV) GLS and PSD assessed in a patient during sinus rhythm (A) and during LBBP (B). The value of GLS in apical three-chamber (LAX) view, apical four-chamber (A4C) view, and apical two-chamber (A2C) view were calculated individually. A bull's-eye plot was created of peak LV GLS for each myocardial segment. GLS, global longitudinal strain; HR, heart rate; LBBP, left bundle branch pacing; PSD, peak strain dispersion.
2.5 Quantification of LV myocardial work
Global LV MW data were quantified using non-invasive LV pressure-strain loop estimations and the area within the LV pressure–strain loop represents MW (13, 14). Non-invasive blood pressure recordings to estimate LV pressure were measured by a sphygmomanometer to obtain the brachial cuff systolic blood pressure immediately prior to the echocardiographic study (14). The quantification of MW was conducted by EchoPAC (Version 202, GE Healthcare, Chicago, IL, US) according to the following steps. First, the duration of isovolumic and ejection phases was defined by valvular timing (the opening and closing time of mitral and aortic valves) according to pulse-wave Doppler imaging. Then, the LV pressure-strain loop was constructed automatically with the combination of LV strain and non-invasive blood pressure measurements adjusted by the duration of the isovolumic and ejection phases (Figure 2) (14). Global myocardial work index (GWI) was calculated as the average of MW from the three apical views using GLS pressure loop from mitral valve closure to mitral valve opening. Global constructive work (GCW) was defined as the MW performed by LV segmental shortening during systole or by LV segmental lengthening during isovolumic relaxation. Global wasted work (GWW) was defined as the MW performed by LV segmental lengthening during systole or MW performed by LV segmental shortening during isovolumic relaxation. Global myocardial work efficiency (GWE) was defined as the ratio of GCW to total work (GCW plus GWW), expressed as a percentage (Figure 2).
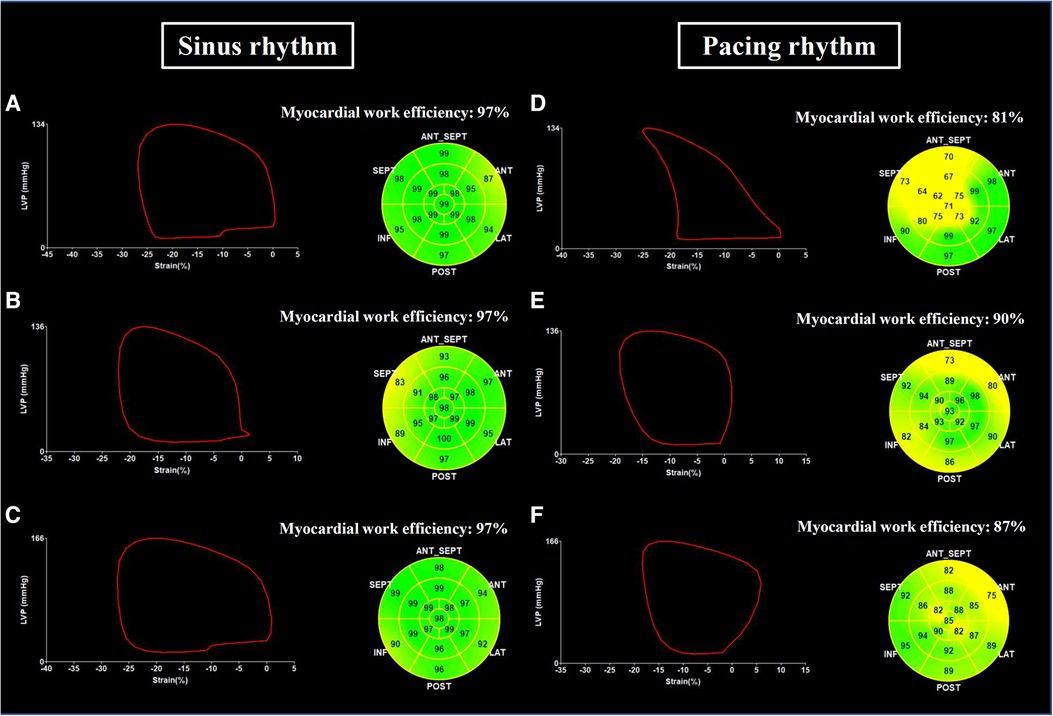
Figure 2. Examples of LV pressure-strain loop and myocardial work efficiency. Global left ventricular pressure-strain loop and myocardial work efficiency assessed in a patient with RVP during sinus rhythm (A) and during ventricular pacing (D), in a patient with LBBP during sinus rhythm (B) and during ventricular pacing (E), and in a patient with NS-HBP during sinus rhythm (C) and during ventricular pacing (F) NS-HBP, non-selective His-bundle pacing; LBBP, left bundle branch pacing; RVP, right ventricular pacing.
2.6 Intra- and inter-observer variability
Intra- and inter-observer variabilities were calculated by intraclass correlation coefficient and the standard error of measurement. For intra-observer analysis of the LV synchrony data, 18 random measurements were reanalyzed, with a period of 2–4 weeks between the first and second analyses. Intraclass correlation coefficients for intra-observer agreement were 0.933 (P < 0.001) for GLS and 0.948 (P < 0.001) for GWI. Inter-observer variability was performed by a different cardiologist repeating measurements from the same images. For interobserver analysis of the LV synchrony data, 18 random measurements were analyzed. The intraclass correlation coefficients for interobserver agreement were 0.986 (P < 0.001) for GLS and 0.910 (P < 0.001) for GWI.
2.7 Statistical analysis
Continuous variables are expressed as mean ± standard deviation, and categorical variables were expressed as numbers and percentages. Continuous variables were compared using unpaired Student's t-test or the Kruskal–Wallis test in case of a non-normal distribution. Categorical variables were compared using Chi-square test or Fisher exact test as appropriate. Paired sample t-test or Wilcoxon test in case of a non-normal distribution was performed to assess differences of paired samples. Comparison of the continuous variables among groups was performed by one-way analysis of variance (ANOVA), with Bonferroni correction when significant events were detected. A two-sided P-value <0.05 was considered statistically significant. All statistical analyses were performed using the SPSS software (version 22.0; SPSS Inc., Chicago, IL, USA).
3. Result
3.1 Study enrollment
This study recruited 109 consecutive patients receiving transvenous permanent conduction system pacing. Thirty-nine patients were excluded, including 6 patients with history of HF due to severe valvular heart disease or cardiomyopathy, 7 patients with end-stage renal disease, 6 patients with permanent atrial fibrillation, 3 patients that refused to join the study and 17 patients with left ventricular septal pacing (LVSP) according to the reported criteria (20–22). Finally, 70 patients with physiological pacing, including 24 patients with NS-HBP and 46 patients with LBBP, were enrolled. For comparative analysis of case-control study, 16 patients with RVP and 10 subjects without pacemaker (control group) were also enrolled.
3.2 Patient characteristics
Table 1 lists the clinical characteristics of the study patients. The mean age of the entire study subjects was 74 ± 9 years and 51.0% of the study subjects were male. The NS-HBP group was older than the LBBP and control groups (Table 1). The NS-HBP and RVP groups had a higher prevalence of atrial fibrillation, and a higher prescription rate of antiarrhythmic and anticoagulants compared with the control and LBBP groups (Table 1). The control group had a higher prescription rate of angiotensin converting enzyme inhibitors/angiotensin receptor blockers compared with the other 3 groups (Table 1).
3.3 Procedural and pacing parameters
The LBBP group had a higher prevalence of atrioventricular block and pre-existing RBBB, and a higher burden of ventricular pacing during follow-up compared with the other 3 groups (Table 1). In the LBBP group, the stimulus to left ventricular activation time was 69 ± 8 ms, and the LBB potential to QRS was 23 ± 6 ms. The pacing QRS duration was wider in the RVP group compared with the LBBP and NS-HBP groups (145 ± 14 vs. 114 ± 11 vs. 116 ± 12 ms, respectively, P < 0.001) (Table 1). About pacing parameters, LBBP group had higher R wave amplitude at implant and follow-up, lower pacing threshold at follow-up, and higher pacing impedance at implant and follow-up compared with NS-HBP group (Figure 3).
Figure 3. Pacing parameters of pacemakers. Comparison of pacing parameters including R-wave amplitude (A), pacing threshold (B), and pacing impedance (C) among RVP, LBBP and NS-HBP groups at implant, 6-month and 12-month follow-ups. Abbreviations as in Figure 2.
3.4 Echocardiographic parameters and cardiac functions during sinus or intrinsic rhythm and atrial synchronized ventricular pacing
Conventional echocardiographic findings at implant and at 6-month follow-up are summarized in Table 2. The left atrial size significantly increased during ventricular pacing at 6-month follow-up in the RVP group compared with the LBBP and NS-HBP groups (46 ± 15 vs. 39 ± 8 vs. 38 ± 8 mm, P = 0.031) (Table 2). The LV end-diastolic and end-systolic volumes during ventricular pacing did not differ among the RVP, LBBP and NS-HBP groups at implant and at 6-month follow-up. Likewise, LVEF during ventricular pacing did not differ among the RVP, LBBP and NS-HBP groups at implant and at 6-month follow-up. The mean E/e', TAPSE, and S' wave of RVFW during ventricular pacing also did not differ among the RVP, LBBP and NS-HBP groups at implant and at 6-month follow-up (Table 2).
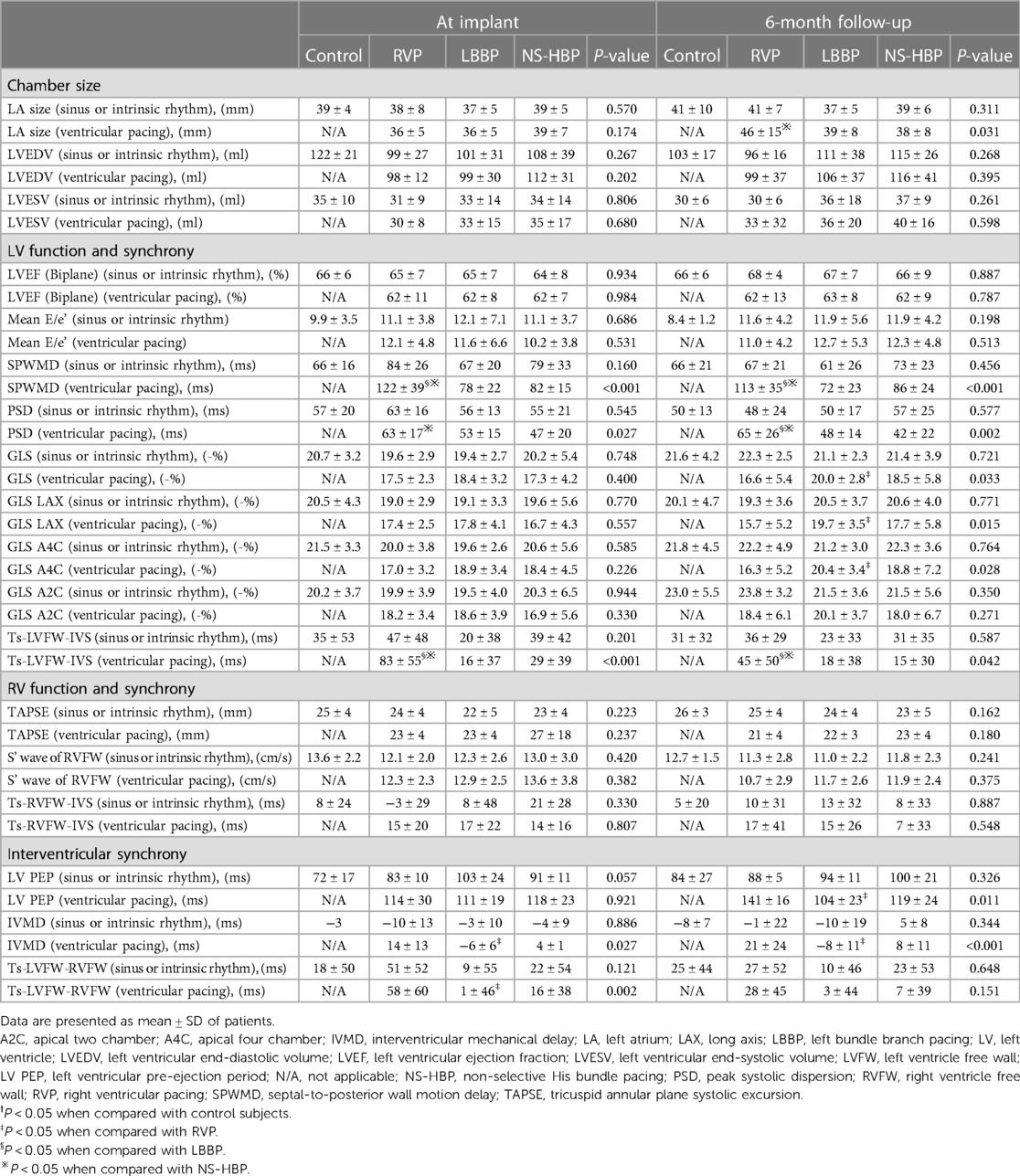
Table 2. Echocardiographic parameters, cardiac function and intraventricular and interventricular mechanical synchronies during sinus or intrinsic and synchronized ventricular pacing in the control and different pacing groups at implant and 6-month follow-up.
3.5 Left intraventricular mechanical synchrony
During sinus or intrinsic rhythm, parameters of LV mechanical synchrony, in terms of SPWMD, PSD, LV GLS, and Ts-LVFW-IVS, did not differ among the 4 groups at implant and at 6-month follow-up. However, during ventricular pacing, RVP group had longer SPWMD compared with LBBP and NS-HBP groups (122 ± 39 vs. 78 ± 22 vs. 82 ± 15 ms, respectively, P < 0.001) at implant and (113 ± 35 vs. 72 ± 23 vs. 86 ± 24 ms, respectively, P < 0.001) at 6-month follow-up, longer PSD compared with LBBP and NS-HBP groups (63 ± 17 vs. 53 ± 15 vs. 47 ± 20 ms, respectively, P = 0.027) at implant and (65 ± 26 vs. 48 ± 14 vs. 42 ± 22 ms, respectively, P = 0.002) at 6-month follow-up, lower LV GLS, especially over segments of anteroseptal and septal walls, compared with LBBP and NS-HBP groups (16.6 ± 5.4 vs. 20.0 ± 2.8 vs. 18.5 ± 5.8%, respectively, P = 0.033) at 6-month follow-up, and longer Ts-LVFW-IVS compared with LBBP and NS-HBP groups (83 ± 55 vs. 16 ± 37 vs. 29 ± 39 ms, P < 0.001) at implant and (45 ± 50 vs. 18 ± 38 vs. 15 ± 30 ms, respectively, P = 0.042) at 6-month follow-up (Table 2).
3.6 Right intraventricular mechanical synchrony
During sinus or intrinsic rhythm and ventricular pacing, the parameter of RV mechanical synchrony, in terms of Ts-RVFW-IVS, did not differ among the RVP, LBBP and NS-HBP groups at implant and at 6-month follow-up (Table 2).
3.7 Interventricular mechanical synchrony
During sinus or intrinsic rhythm, parameters of interventricular mechanical synchrony, in terms of LV PEP, IVMD and Ts-LVFW-RVFW, did not differ among the 4 groups at implant and at 6-month follow-up. However, during ventricular pacing, RVP group had longer LV PEP (141 ± 16 vs. 104 ± 23 ms, P = 0.014) compared with LBBP group at 6-month follow-up, longer IVMD compared with the LBBP group (14 ± 13 vs. −6 ± 6 ms, P = 0.032) at implant and (21 ± 24 vs. −8 ± 11 ms, P = 0.002) at 6-month follow-up, and longer Ts-LVFW-RVFW compared with the LBBP group (58 ± 60 vs. 1 ± 46 ms, P = 0.001) at implant (Table 2).
3.8 LV myocardial work analysis
There were no significant differences in all the parameters of MW (GWI, GCW, GWW and GWE) during sinus or intrinsic rhythm among the 4 groups at implant and at the 6-month follow-up (Table 3 and Figure 4). However, during ventricular pacing, LBBP group had higher GWI compared with RVP group (2,174 ± 529 vs. 1,689 ± 562 mmHg%, P = 0.023) at implant and (2,189 ± 527 vs. 1,493 ± 799 mmHg%, P = 0.002) at 6-month follow-up, and NS-HBP group had higher GWI compared to RVP group only at the 6-month follow-up (2,146 ± 703 vs. 1,493 ± 799 mmHg%, P = 0.010) (Table 3 and Figure 4). During ventricular pacing, NS-HBP group had higher GCW compared with RVP group (2,921 ± 771 vs. 2,203 ± 866 mmHg%, P = 0.009) at 6-month follow-up (Table 3 and Figure 4). During ventricular pacing, LBBP group had lower GWW compared with RVP and NS-HBP groups (211 ± 161 vs. 484 ± 281 vs. 406 ± 234 mmHg%, respectively, P < 0.001) at 6-month follow-up, and consequently, LBBP group had higher GWE compared with RVP and NS-HBP groups (89.5 ± 4.3 vs. 83.4 ± 7.2 vs. 85.0 ± 9.5%, respectively, P = 0.004) at implant and (91.4 ± 5.0 vs. 80.9 ± 8.3 vs. 86.4 ± 8.1%, respectively, P < 0.001) at 6-month follow-up (Table 3 and Figure 4).
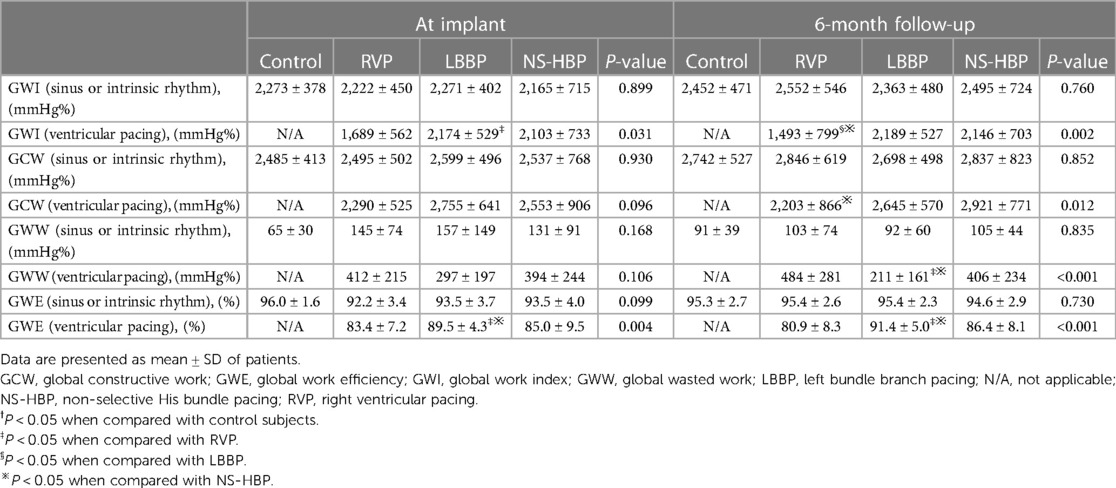
Table 3. Myocardial work parameters during sinus or intrinsic rhythm and synchronized ventricular pacing rhythm in the control and different pacing groups at implant and 6-month follow-up.
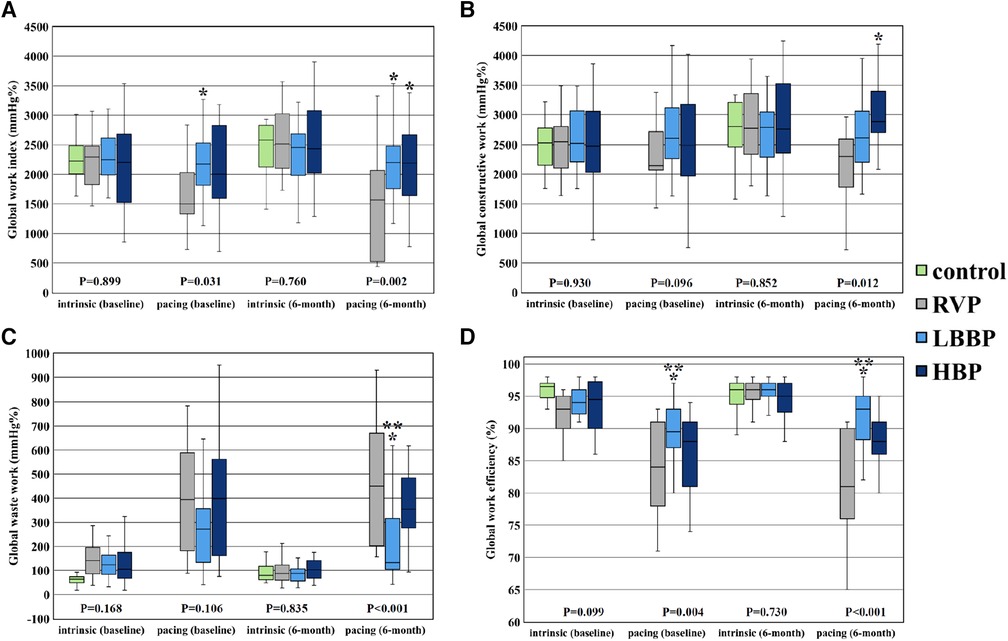
Figure 4. Parameters of global LV myocardial work. Comparison of global LV myocardial work parameters including global work index (A), global constructive work (B), global waste work (C), and global work efficiency (D) among RVP, LBBP and NS-HBP groups at implant, and 6-month follow-ups. Asterisk (*) means P-value <0.05 compared with RVP group; double asterisk (**) means P-value <0.05 compared with NS-HBP group. Abbreviations as in Figure 2.
4. Discussion
The main findings of this study were (1) LBBP group had less left intraventricular mechanical dyssynchrony during ventricular pacing compared with RVP group but had no difference in left intraventricular mechanical dyssynchrony compared to NS-HBP group at 6-month follow-up; (2) right intraventricular mechanical synchrony during ventricular pacing of LBBP group did not differ from that of NS-HBP and RVP groups; (3) LBBP group had less interventricular mechanical dyssynchrony during ventricular pacing compared with RVP group at 6-month follow-up; (4) LBBP group had better LV MW parameters (GWI, GWW and GWE) during ventricular pacing compared with RVP group and better LV MW parameters (GWW and GWE) during ventricular pacing compared with NS-HBP group at 6-month follow-up (Figure 4).
4.1 The left atrial size and LV systolic and diastolic function of LBBP
Long-term RVP can cause LV systolic dysfunction, pacing-induced cardiomyopathy and HF (1–4, 23). The mechanism of adverse effects is due to asynchronous electrical activation of LV caused by RVP-induced left bundle branch block pattern (3, 4, 23). Our recent study revealed that delta increment in pacing QRS duration, similar to electrical dyssynchrony, increased the risk of cardiovascular mortality in patients with pre-existing bundle branch block (24). Nahlawi et al. reported that RVP caused deterioration of LVEF after one-week continuous pacing, that persisted even after cessation of pacing 24 h later (25).
The left atrial size significantly increased during ventricular pacing at 6-month follow-up in the RVP group compared with the LBBP and NS-HBP groups (Table 2). Consequently, RVP increased the risks of atrial fibrillation, HF hospitalization and stroke (2).
4.2 The intraventricular and interventricular mechanical synchrony of LBBP
Interventricular and intraventricular mechanical dyssynchronies play a crucial role on ventricular pump function (3, 4, 17, 23). RVP can induce significant left intraventricular mechanical dyssynchrony, especially between the anteroseptal wall (with early activation) and the posterolateral wall (with late activation) (4, 9, 23). Recently, several studies reported that NS-HBP with high output (mean threshold 1.8 ± 1.2 V at 0.42 ms) and LBBP could prevent LV mechanical dyssynchrony, but NS-HBP with low output (mean threshold 1.0 ± 0.4 V at 0.42 ms) could not (9, 26). In this study, LBBP had better left intraventricular mechanical synchrony at implant and 6-month follow-up and better LV GLS, particularly over the anterior wall (LAX view) and septum (A4C view), at 6-month follow-up compared with RVP (Table 2). Moreover, LBBP provided stable threshold at long-term follow-ups compared with NS-HBP (Figure 3).
Interventricular dyssynchrony indicates a delay in activation between LV and RV, resulting in a lack of coordinated contraction between LV and RV. In HF patients, interventricular dyssynchrony was associated with higher risk of cardiac events, independent of the QRS duration and LVEF (18). In this study, LBBP had less interventricular mechanical dyssynchrony during ventricular pacing compared with RVP. An intra-patient-controlled study (10) reported that LBBP resulted in modest delay in RV activation and less interventricular synchrony measured by IVMD and Ts-LVFW-RVFW compared with NS-HBP in patients with bradycardia. However, in this study, LBBP, including 15 patients (32.6%) with pre-existing right bundle branch block, did not differ in interventricular synchrony compared to NS-HBP (Tables 1, 2).
4.3 LV myocardial work efficiency of LBBP
Global LV MW is a novel method to estimate LV systolic function, derived from computed LV pressure-strain loops incorporating both brachial cuff systolic blood pressure recordings and STE data (13, 14). Previous studies have demonstrated that there is a good correlation between LVEF and GWE in the general population, and GWE can be applied to assess the outcomes in patients with cardiac diseases (14, 15, 27, 28). Moreover, lower resting values of LV GLS and GWE in HF patients with preserved LVEF suggest an early subclinical myocardial damage, seemly associated with lower exercise capacity, greater pulmonary congestion, and blunted LV contractile reserve during effort (28). Furthermore, in the Geisinger-Rush Conduction System Pacing Registry, Sharma et al. (29) reported that LBBP patients with a ventricular pacing burden >20% had a lower risk of all-cause mortality, HF hospitalization, or upgrade to biventricular pacing compared to RVP. In an animal study, RVP caused significant redistribution of work away from the septum toward the LV lateral wall and significant redistribution of perfusion toward the LV anterolateral regions and a 30%–40% decreased myocardial efficiency (stroke work/myocardial oxygen consumption), whereas LV apical and septal pacing (via transventricular septal approach) did not significantly change myocardial efficiency (30). In this study, we first reported that during atrial synchronized ventricular pacing, the LBBP group had better GWE compared with RVP and NS-HBP groups (Table 3 and Figure 4). Further studies are warranted to assess that the better GWE achieved with LBBP compared with RVP in this study translates into better long-term clinical outcomes in different patient population with high burden ventricular pacing.
5. Limitation
There are several limitations in this study. First, this was a single-center prospective study with small sample size, and STE study only at implant and 6-month follow-up. Second, although several studies reported how to differentiate between LBBP and LVSP (20–22), it is still an inconclusive issue. In this study, we excluded 17 patients with LVSP using the algorithm reported by Chen et al. (20). Third, the echocardiographic images during non-selective LBBP with or without anodal capture were not distinguished in this study. Fourth, the worse GWW and GWE estimations during NS-HBP compared with LBBP might be attributed to partial right ventricular myocardial capture. Finally, individually regional LV myocardial work was not further evaluated during LBBP.
6. Conclusion
After 6-month of >40% ventricular pacing burden, LBBP resulted in less left intraventricular mechanical dyssynchrony and better LV MW parameters (GWI, GWW and GWE) compared with RVP, and had better MW parameters (GWW and GWE) compared with NS-HBP. Further studies are warranted to assess the association between GWE and long-term clinical outcomes in LBBP with high burden ventricular pacing.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The study protocol was approved by the Institutional Review Board of Chang Gung Medical Foundation (permit number: 202000970B0). The patients provided written informed consent to participate in this study.
Author contributions
H-CC contributed to the analysis and interpretation of data and wrote the manuscript. W-HL and Y-LC contributed to discussion and reviewed and edited the manuscript. W-CL, Y-NF, and S-ZC contributed to collection of data. M-CC contributed to study design and revised the manuscript critically for important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the research program of Chang Gung Memorial Hospital (grant number CMRPG8K1491).
Acknowledgments
We are indebted to Kyoko Soejima and Toshiaki Sato (Kyorin University Hospital, Tokyo, Japan) for their help in the technical support of conduction system pacing. Also, we appreciated the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital for their statistical work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1201841/full#supplementary-material
References
1. Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, et al. Dual chamber and VVI implantable defibrillator trial investigators. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the dual chamber and VVI implantable defibrillator (DAVID) trial. JAMA. (2002) 288:3115–23. doi: 10.1001/jama.288.24.3115
2. Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KL, et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. (2003) 107:2932–7. doi: 10.1161/01.CIR.0000072769.17295.B1
3. Tops LF, Suffoletto MS, Bleeker GB, Boersma E, van der Wall EE, Gorcsan J 3rd, et al. Speckle-tracking radial strain reveals left ventricular dyssynchrony in patients with permanent right ventricular pacing. J Am Coll Cardiol. (2007) 50:1180–8. doi: 10.1016/j.jacc.2007.06.011
4. Liu WH, Guo BF, Chen YL, Tsai TH, Fu M, Chua S, et al. Right ventricular outflow tract pacing causes intraventricular dyssynchrony in patients with sick sinus syndrome: a real-time three-dimensional echocardiographic study. J Am Soc Echocardiogr. (2010) 23:599–607. doi: 10.1016/j.echo.2010.03.006
5. Karpawich PP, Rabah R, Haas JE. Altered cardiac histology following apical right ventricular pacing in patients with congenital atrioventricular block. Pacing Clin Electrophysiol. (1999) 22:1372–7. doi: 10.1111/j.1540-8159.1999.tb00631.x
6. Vernooy K, Dijkman B, Cheriex EC, Prinzen FW, Crijns HJ. Ventricular remodeling during long-term right ventricular pacing following his bundle ablation. Am J Cardiol. (2006) 97:1223–7. doi: 10.1016/j.amjcard.2005.11.044
7. Dandamudi G, Vijayaraman P. How to perform permanent his bundle pacing in routine clinical practice. Heart Rhythm. (2016) 13:1362–6. doi: 10.1016/j.hrthm.2016.03.040
8. Huang W, Chen X, Su L, Wu S, Xia X, Vijayaraman P. A beginner’s guide to permanent left bundle branch pacing. Heart Rhythm. (2019) 16:1791–6. doi: 10.1016/j.hrthm.2019.06.016
9. Sun Z, Di B, Gao H, Lan D, Peng H. Assessment of ventricular mechanical synchronization after left bundle branch pacing using 2-D speckle tracking echocardiography. Clin Cardiol. (2020) 43:1562–72. doi: 10.1002/clc.23481
10. Sheng X, Pan YW, Yu C, Wang B, Zhang P, Li J, et al. Comparison of synchronization between left bundle branch and his bundle pacing in atrial fibrillation patients: an intra-patient-controlled study. Pacing Clin Electrophysiol. (2021) 44:1523–31. doi: 10.1111/pace.14331
11. Liu W, Hu C, Wang Y, Cheng Y, Zhao Y, Liu Y, et al. Mechanical synchrony and myocardial work in heart failure patients with left bundle branch area pacing and comparison with biventricular pacing. Front Cardiovasc Med. (2021) 20:727611. doi: 10.3389/fcvm.2021.727611
12. Wang P, Yang L, Zheng S, Mai J, Wei Y, Liu Y, et al. Left bundle branch pacing on mechanical synchrony and myocardial work in bradycardia patients. Int J Cardiovasc Imaging. (2023) 39:369–78. doi: 10.1007/s10554-022-02742-5
13. Russell K, Eriksen M, Aaberge L, Wilhelmsen N, Skulstad H, Remme EW, et al. A novel clinical method for quantification of regional left ventricular pressure-strain loop area: a non-invasive index of myocardial work. Eur Heart J. (2012) 33:724–33. doi: 10.1093/eurheartj/ehs016
14. Chan J, Edwards NFA, Khandheria BK, Shiino K, Sabapathy S, Anderson B, et al. A new approach to assess myocardial work by non-invasive left ventricular pressure-strain relations in hypertension and dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging. (2019) 20:31–9. doi: 10.1093/ehjci/jey131
15. Lustosa RP, Butcher SC, van der Bijl P, El Mahdiui M, Montero-Cabezas JM, Kostyukevich MV, et al. Global left ventricular myocardial work efficiency and long-term prognosis in patients after ST-segment-elevation myocardial infarction. Circ Cardiovasc Imaging. (2021) 14:e012072. doi: 10.1161/CIRCIMAGING.120.012072
16. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2015) 28:1–39.e14. doi: 10.1016/j.echo.2014.10.003
17. Ghio S, Constantin C, Klersy C, Serio A, Fontana A, Campana C, et al. Interventricular and intraventricular dyssynchrony are common in heart failure patients, regardless of QRS duration. Eur Heart J. (2004) 25:571–8. doi: 10.1016/j.ehj.2003.09.030
18. Bader H, Garrigue S, Lafitte S, Reuter S, Jaïs P, Haïssaguerre M, et al. Intra-left ventricular electromechanical asynchrony. A new independent predictor of severe cardiac events in heart failure patients. J Am Coll Cardiol. (2004) 43:248–56. doi: 10.1016/j.jacc.2003.08.038
19. Ghio S, Freemantle N, Serio A, Magrini G, Scelsi L, Pasotti M, et al. Baseline echocardiographic characteristics of heart failure patients enrolled in a large European multicentre trial (CArdiac REsynchronisation Heart Failure study). Eur J Echocardiogr. (2006) 7:373–8. doi: 10.1016/j.euje.2005.10.006
20. Chen X, Qian Z, Zou F, Wang Y, Zhang X, Qiu Y, et al. Differentiating left bundle branch pacing and left ventricular septal pacing: an algorithm based on intracardiac electrophysiology. J Cardiovasc Electrophysiol. (2022) 33:448–57. doi: 10.1111/jce.15350
21. Wu S, Chen X, Wang S, Xu L, Xiao F, Huang Z, et al. Evaluation of the criteria to distinguish left bundle branch pacing from left ventricular septal pacing. JACC Clin Electrophysiol. (2021) 7:1166–77. doi: 10.1016/j.jacep.2021.02.018
22. Jastrzębski M, Burri H, Kiełbasa G, Curila K, Moskal P, Bednarek A, et al. The V6-V1 interpeak interval: a novel criterion for the diagnosis of left bundle branch capture. Europace. (2022) 24:40–7. doi: 10.1093/europace/euab164
23. Tops LF, Schalij MJ, Bax JJ. The effects of right ventricular apical pacing on ventricular function and dyssynchrony implications for therapy. J Am Coll Cardiol. (2009) 54:764–76. doi: 10.1016/j.jacc.2009.06.006
24. Chen HC, Liu WH, Tseng CH, Chen YL, Lee WC, Fang YN, et al. Delta increment in pacing QRS duration predicts cardiovascular mortality in patients with pre-existing bundle branch block receiving permanent pacemakers. Acta Cardiol Sin. (2022) 38:504–15. doi: 10.6515/ACS.202207_38(4).20220115A
25. Nahlawi M, Waligora M, Spies SM, Bonow RO, Kadish AH, Goldberger JJ. Left ventricular function during and after right ventricular pacing. J Am Coll Cardiol. (2004) 44:1883–8. doi: 10.1016/j.jacc.2004.06.074
26. Fang F, Zhang Q, Chan JY, Xie JM, Fung JW, Yip GW, et al. Deleterious effect of right ventricular apical pacing on left ventricular diastolic function and the impact of pre-existing diastolic disease. Eur Heart J. (2011) 32:1891–9. doi: 10.1093/eurheartj/ehr118
27. El Mahdiui M, van der Bijl P, Abou R, Ajmone Marsan N, Delgado V, Bax JJ. Global left ventricular myocardial work efficiency in healthy individuals and patients with cardiovascular disease. J Am Soc Echocardiogr. (2019) 32:1120–7. doi: 10.1016/j.echo.2019.05.002
28. D’Andrea A, Ilardi F, D’Ascenzi F, Bandera F, Benfari G, Esposito R, et al. Impaired myocardial work efficiency in heart failure with preserved ejection fraction. Eur Heart J Cardiovasc Imaging. (2021) 22:1312–20. doi: 10.1093/ehjci/jeab153
29. Sharma PS, Patel NR, Ravi V, Zalavadia DV, Dommaraju S, Garg V, et al. Clinical outcomes of left bundle branch area pacing compared to right ventricular pacing: results from the Geisinger-Rush Conduction System Pacing Registry. Heart Rhythm. (2022) 19:3–11. doi: 10.1016/j.hrthm.2021.08.033
30. Mills RW, Cornelussen RN, Mulligan LJ, Strik M, Rademakers LM, Skadsberg ND, et al. Left ventricular septal and left ventricular apical pacing chronically maintain cardiac contractile coordination, pump function and efficiency. Circ Arrhythm Electrophysiol. (2009) 2:571–9. doi: 10.1161/CIRCEP.109.882910
Keywords: global longitudinal strain, left bundle branch pacing, mechanical synchrony, myocardial work efficiency, his bundle pacing
Citation: Chen H-C, Liu W-H, Chen Y-L, Lee W-C, Fang Y-N, Chong S-Z and Chen M-C (2023) Left bundle branch pacing preserved left ventricular myocardial work in patients with bradycardia. Front. Cardiovasc. Med. 10:1201841. doi: 10.3389/fcvm.2023.1201841
Received: 7 April 2023; Accepted: 5 September 2023;
Published: 14 September 2023.
Edited by:
Simon Pecha, University Heart and Vascular Center, University Medical Center Hamburg-Eppendorf, GermanyReviewed by:
Teresa Strisciuglio, University of Naples Federico II, ItalyEspen W. Remme, Oslo University Hospital, Norway
© 2023 Chen, Liu, Chen, Lee, Fang, Chong and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mien-Cheng Chen Y2hlbm1pZW5AbXM3Ni5oaW5ldC5uZXQ=
Abbreviations FW, free wall; GCW, global constructive work; GLS, global longitudinal strain; GWE, global myocardial work efficiency; GWI, global myocardial work index; GWW, global wasted work; NS-HBP, non-selective His-bundle pacing; HF, heart failure; IVMD, interventricular mechanical delay; IVS, interventricular septum; LBBP, left bundle branch pacing; LV, left ventricle; LVEF, left ventricular ejection fraction; LV PEP, left ventricle pre-ejection period; LVSP, left ventricular septal pacing; MW, myocardial work; PSD, peak strain dispersion; RBBB, right bundle branch block; RV, right ventricle; RVP, right ventricular pacing; SPWMD, septal-to-posterior wall motion delay; STE, speckle tracking echocardiography; TAPSE, tricuspid annular plane systolic excursion; Ts, tissue Doppler.
 Huang-Chung Chen
Huang-Chung Chen Wen-Hao Liu1
Wen-Hao Liu1 Wei-Chieh Lee
Wei-Chieh Lee Mien-Cheng Chen
Mien-Cheng Chen