- Department of Cardiology, Wuxi People’s Hospital Affiliated to Nanjing Medical University, Wuxi, China
Background: Accumulated clinical studies utilized intracardiac echocardiography (ICE) to guide percutaneous left atrial appendage occlusion (LAAO). However, its procedural success and safety compared to traditional transesophageal echocardiography (TEE) remained elusive. Therefore, we performed a meta-analysis to compare efficacy and safety of ICE and TEE for LAAO.
Methods: We screened studies from four online databases (including the Cochrane Library, Embase, PubMed, and Web of Science) from their inception to 1 December 2022. We used a random or fixed-effect model to synthesize the clinical outcomes and conducted a subgroup analysis to identify the potential confounding factors.
Results: A total of twenty eligible studies with 3,610 atrial fibrillation (AF) patients (1,564 patients for ICE and 2,046 patients for TEE) were enrolled. Compared with TEE group, there was no significant difference in procedural success rate [risk ratio (RR) = 1.01; P = 0.171], total procedural time [weighted mean difference (WMD) = −5.58; P = 0.292], contrast volume (WMD = −2.61; P = 0.595), fluoroscopic time (WMD = −0.34; P = 0.705; I2 = 82.80%), procedural complications (RR = 0.82; P = 0.261), and long-term adverse events (RR = 0.86; P = 0.329) in the ICE group. Subgroup analysis revealed that ICE group might be associated with the reduction of contrast use and fluoroscopic time in the hypertension proportion <90 subgroup, with lower total procedure time, contrast volume, and the fluoroscopic time in device type subgroup with multi-seal mechanism, and with the lower contrast use in paroxysmal AF (PAF) proportion ≤50 subgroup. Whereas, ICE group might increase the total procedure time in PAF proportion >50 subgroup and contrast use in multi-center subgroup, respectively.
Conclusion: Our study suggests that ICE may have comparable efficacy and safety compared to TEE for LAAO.
1. Introduction
Atrial fibrillation (AF) is the most common persistent atrial arrhythmia worldwide, with a prevalence estimated to be between 2% and 4% in adults. An expected 2.3-fold increase in prevalence is anticipated due to extended life expectancy in the general population and increased detection of undiagnosed AF (1). Cardioembolic stroke is the most concerning complication of AF, as abnormal blood flow in the left atrium increases the likelihood of thrombus rupture from the left atrial appendage (LAA), subsequently leading to thromboembolisms in the peripheral and cerebral arteries (2).
The primary prevention strategy of thromboembolism for AF is the use of oral anticoagulants (OACs). However, challenge remains due to the limitation of adherence and bleeding risk for safety and efficacy of OACs. Since most thrombus in nonvalvular AF originates from the LAA, left atrial appendage occlusion (LAAO) is an emerging alternative for OACs. Transesophageal echocardiography (TEE) is the standard imaging modality to guide LAAO and is the most widely used imaging modality. However, it has some significant limitations, including increased pain with local or conscious anesthesia, prolonged procedure time and hospitalization burden with general anesthesia, aggravated risk of possible esophageal injury under “one-stop” ablation, and high dependence on a dedicated echocardiography operator.
Recently, an expert consensus suggested that intracardiac echocardiography (ICE) might be considered as an alternative imaging modality to guide LAAO, especially with the progress of the “one-stop” ablation therapy for AF (3). However, studies comparing TEE with ICE for LAAO were limited, leading to the related outcomes (e.g., efficacy and safety outcomes) remaining elusive. Therefore, we evaluated the clinical outcomes of TEE and ICE guidance for LAAO to further assess the safety and efficacy outcomes between two imaging modalities.
2. Methods
2.1. Study design
This systematic review was carried on according to the PRISMA guidelines. The registered protocol is displayed in the PROSPERO database (CRD42022368692).
2.2. Search strategy
Two independent reviewers (ZYZ and FL) conducted comprehensive searches of four online databases (Cochrane Library, Embase, PubMed and Web of Science) from inception to 1 December 2022. Search keywords were “ICE”, “Intracardiac echocardiography”, “TEE “, “transesophageal echocardiography”, “atrial fibrillation”, “left atrial appendage closure”, “LAAC”, “left atrial appendage occlusion”, and “LAAO”. Clinical studies related to the outcomes of ICE or outcomes comparing TEE vs. ICE for LAAO were included. Reference lists of review articles were hand searched, and eligible articles were searched for potential publications not previously identified.
2.3. Search design
Two reviewers (ZYZ and JZ) independently searched the literature and screen the titles, abstracts, and full texts to select all relevant studies that met the inclusion criteria. A study would be included if the following criteria were met: (1) randomized controlled trials and cohort, observational studies, and single-arm studies; (2) studies comparing clinical outcomes comparing TEE vs. ICE for endocardial LAAO, including efficacy outcome (e.g., procedural success) and safety outcomes (e.g., short-term complications and long-term complications); (3) studies with full text published in peer-reviewed journals; and studies containing the most data for multiple publications of the same study. Case reports, editorial, review articles, studies without original data letters and studies reporting clinical outcomes with hybrid LAAO procedures were excluded. Meanwhile, a third reviewer (R.X.W) resolved any disagreements about eligibility.
2.4. Data extraction and quality assessment
Data from eligible studies included in the analysis were extracted by two independent researchers (ZYZ and FL), and any potential disagreements were resolved by a third researcher (RXW). The extracted data mainly included: title, first author, publication year, study design, sample size, follow-up time, LAAO device, pre-procedure imaging and ICE location. Meanwhile, we also extracted relevant clinical outcomes, including: acute procedural success, total procedural time, fluoroscopic time, contrast volume, short-term complications, and long-term complications.
Two independent researchers (ZYZ and JZ) evaluated study quality by two appraisal tools. The Newcastle-Ottawa Quality Assessment Scale (NOS) was used to evaluated the two-arm observation (4). The Institute of Health Economics checklist was used for the single-arm study (5). Any disagreements were discussed and resolved by consulting a third researcher (RXW).
2.5. Statistical analysis
Stata version 16.0 was used for statistical analyses. Continuous variables were displayed as means ± SD, and categorical variables were presented as frequencies and percentages. For observational studies with two arms, we calculated the relative risk (RR) and corresponding 95% confidence intervals (CI) for each outcome. For single-arm analysis, we calculated the incidence of events (number of events divided by number of patients) and 95% confidence intervals. P < 0.05 was considered statistically significant.
Meanwhile, chi-square tests and I-squared (I2) were used to quantify and assess statistical heterogeneity among studies. If the I2 value was more than 50% and/or P < 0.05 for the chi-squared test, we considered the between-study heterogeneity to be significant, and we would adopt a random-effect model. Otherwise, we would adopt fixed-effect model. Sensitivity analysis was performed by sequentially omitting one study at a time to assess the effect of a single study on the overall risk, and potential publication bias was also evaluated via Egger's test.
In addition, subgroup analysis was conducted to screen potential determinants of LAAO outcomes between ICE and TEE groups. According to the characteristics of eligible studies, a total of eight subgroup factors were identified, including study design, age cutoff, ICE group sample size, AF type, male proportion, hypertension proportion, device types, and duration of follow up. If the study design included more than one center, it was defined as a multicenter subgroup; otherwise, it was defined as a single-center subgroup. According to age cutoff values of 75, two subgroups were divided, including ≥75 years subgroup and <75 years subgroups. If over 50% of patients had paroxysmal AF (PAF), they were classified as ≥50% PAF subgroup, otherwise they were classified as <50% PAF subgroup. According to the proportion of the male, they were divided into ≥70% subgroups and <70% subgroups. Similarly, the proportion of hypertension with ≥90% subgroup and <90 subgroup, respectively, also was defined. According to the sealing position, the existing sealers could be roughly divided into plug type and disc type. Plug type sealers, also known as single sealers, included Watchman, Plaato, and Lefort. Disc sealer was also called dual sealer, including ACP, Lambre, Lacbes, and Leftear. If the LAAO devices included only dual-seal mechanism devices, it was assigned to dual-seal mechanism subgroup, and if the LAAO devices included only single-seal mechanism devices, it was assigned to the single-seal mechanism subgroup. In addition, studies using both dual-seal mechanism devices and single-seal mechanism devices were divided into muti-seal mechanism subgroup. Follow-up time was divided into two subgroups (≥12 months and <12 months).
3. Results
3.1. Study selection and quality assessment
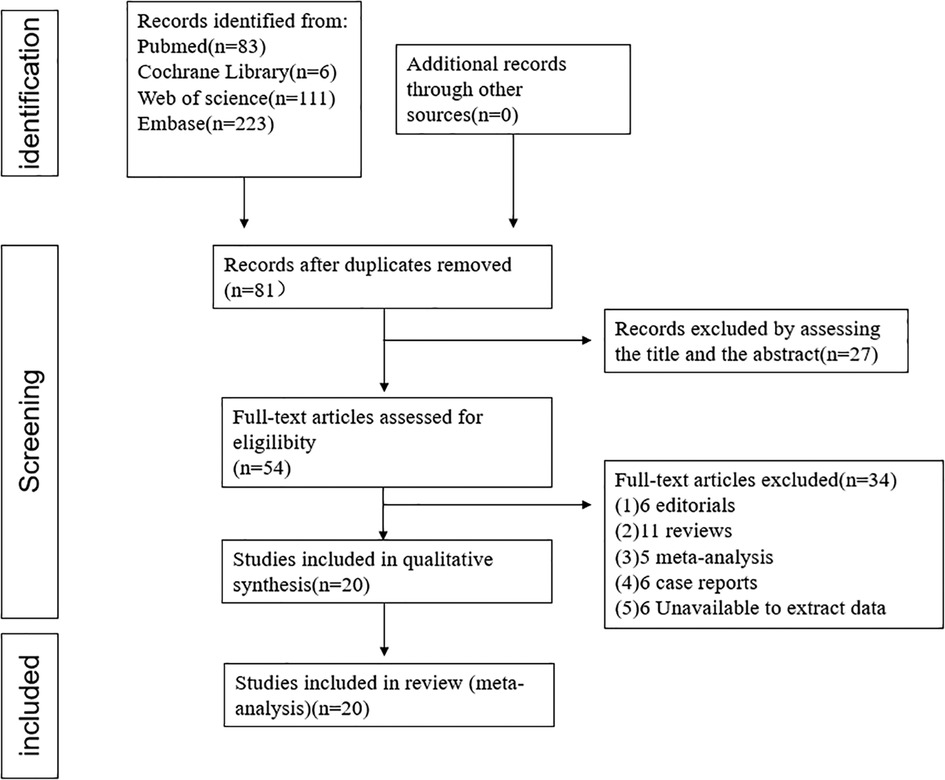
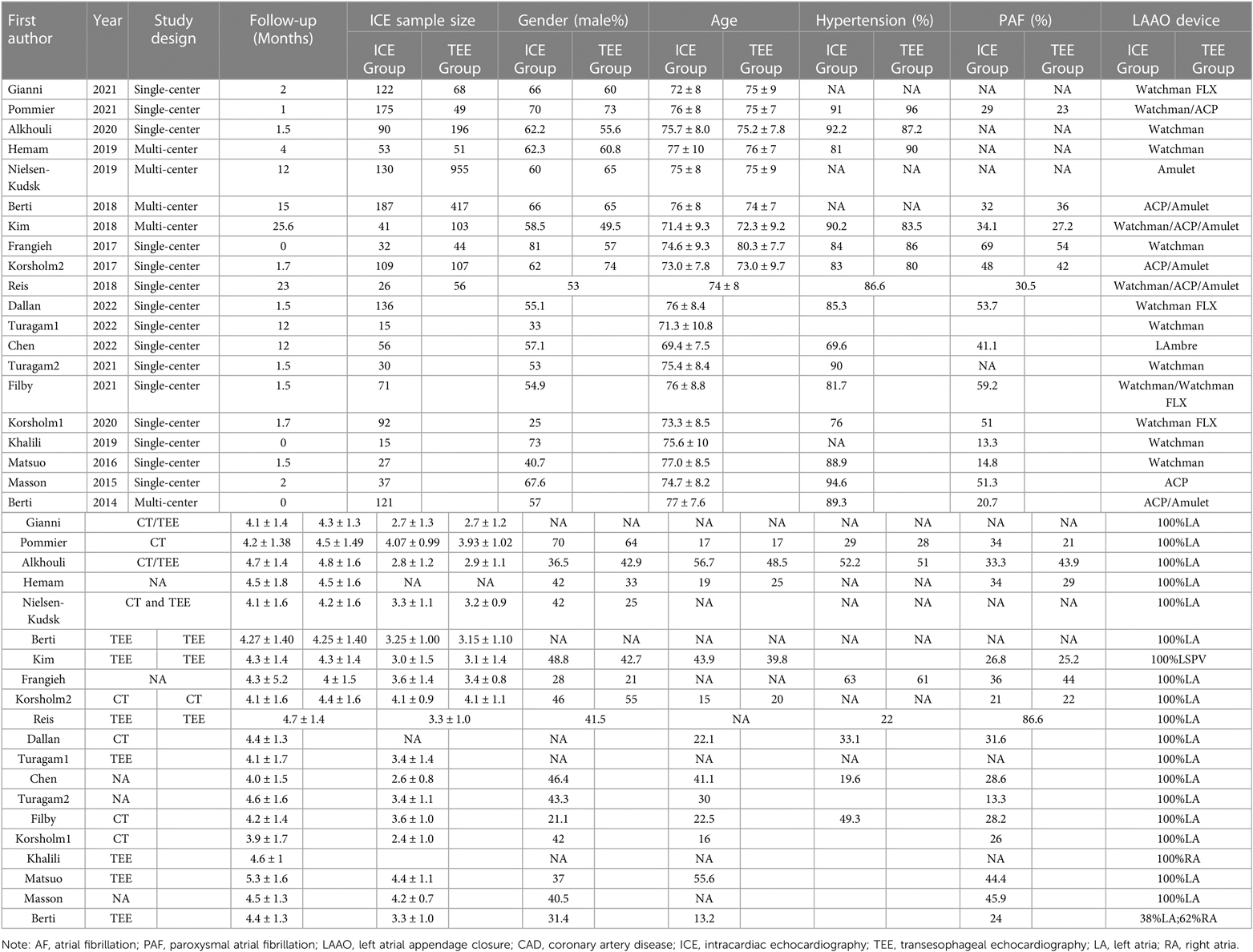
This meta-analysis included 20 studies with a total of 3,610 AF patients (1,564 patients for ICE and 2,046 patients for TEE) consisting of 10 observational two-arm studies (965 ICE patients and 2,046 TEE patients) (6–15) and 10 single-arm studies (599 ICE patients) (16–25). The selection flowchart was displayed in Figure 1. The average age of the patients included in the studies ranged from 71.3 to 80.3 years. Among the included clinical studies, the mean CHA2DS2-VASc score and HAS-BLED score ranged from 3.9 to 5.3 and 2.4 to 4.4. Eleven studies included Watchman or Watchman FLX (6, 8, 9, 13, 16, 17, 19–23), six included the ACP or Amulet device (10, 11, 14, 18, 24, 25) and three studies included both (7, 12, 14). The baseline characteristics and procedure-related indexes of the eligible studies were presented in Table 1. In this meta-analysis, all two-arm studies had a moderate-to-high quality (Supplementary Table S1). Ten single-arm studies all had a score higher than fifteen (Supplementary Table S1).
3.2. Primary outcome
3.2.1. Procedural success rate
All eligible two-arms studies reported the acute procedural success data and there was no significant difference in procedural success rate (RR = 1.01; 95% CI: 1.00, 1.02; P = 0.171; I2 = 0.00%) between two groups (Figure 2) (6–15). Our result was consistent with those of several other meta-analyses (26–28). Subgroup analysis was performed with a total of eight subgroup factors for the acute procedural success of LAAO, and the results are displayed in Figure 3. There was no significant difference between TEE group and ICE group in the study design subgroup, follow-up subgroup, ICE sample size subgroup, male proportion subgroup, age cutoff subgroup, hypertension proportion subgroup, PAF proportion subgroup, and device types subgroup, suggesting that all subgroup results were consistent with the pooled result.
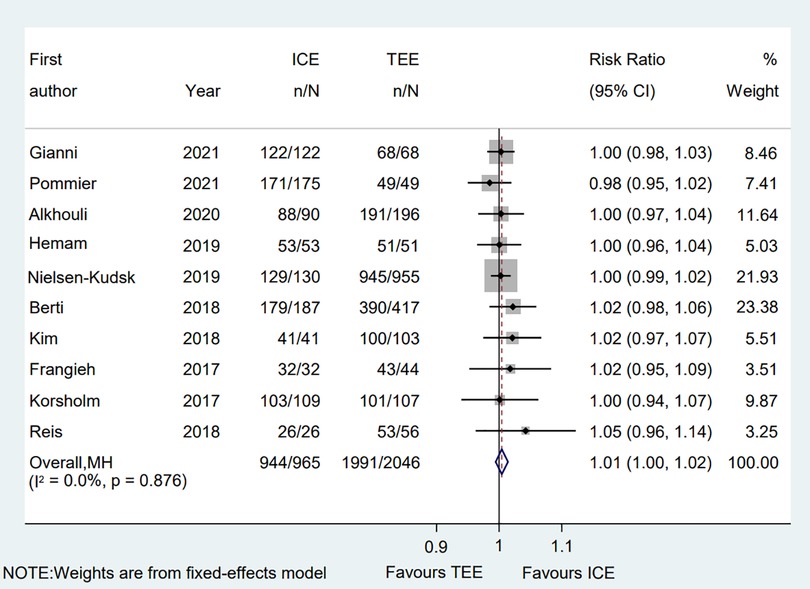
Figure 2. Forest plot of the procedural success between ICE and TEE groups. Comparison of the rates of the procedural success between ICE and TEE groups. ICE, intracardiac echocardiography; TEE, transesophageal echocardiography; RR, risk ratio; CI, confidence interval.
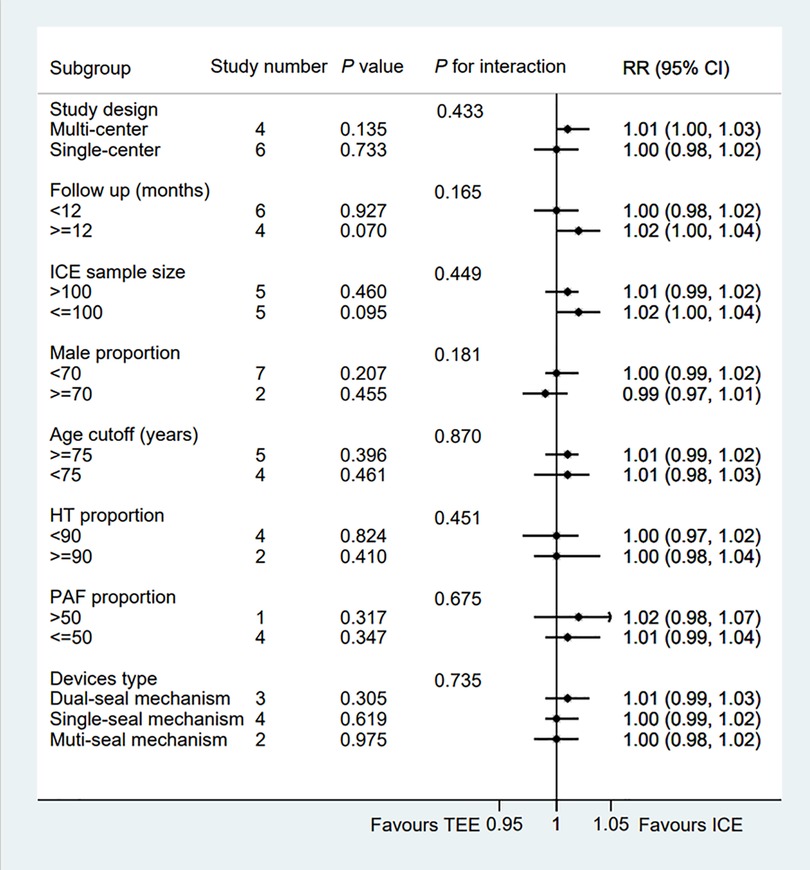
Figure 3. Forest plot of subgroup analysis of the procedural success between ICE and TEE groups. Subgroup analysis of the rates of the procedural success between ICE and TEE groups. ICE, intracardiac echocardiography; TEE, transesophageal echocardiography; RR, risk ratio; CI, confidence interval.
We also performed a sensitivity analysis and the results showed no significant change, ranging from 1.00 (95% CI: 0.99, 1.02) to 1.01 (95% CI: 1.00, 1.03), in the overall combined proportion, suggesting that there was no single study in the domination of the combined proportion and heterogeneity. Moreover, no publication bias was presented in Egger's test (P = 0.208).
3.2.2. Pooled rate of procedural success in ICE group
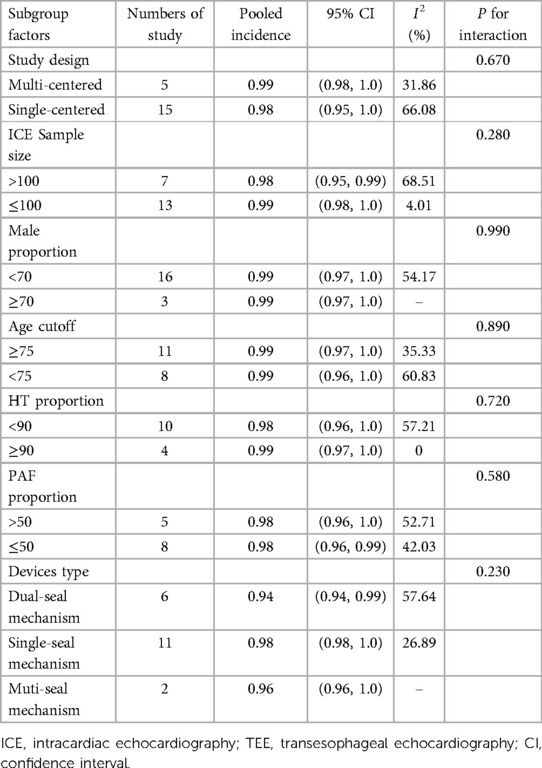
A total of 20 eligible studies (1,564 patients undergoing LAAO with ICE procedural guidance) reported the rate of procedural success in ICE Group (6–15). The pooled rate of procedural success was 0.99 (95% CI: 0.98, 1.00; P = 0.02; I2 = 43.69%) with the random-effect model (Figure 4). Meanwhile we performed a subgroup analysis with eight subgroup factors for procedural success in ICE group, and the results are shown in Table 2. Overall, the pooled rate of procedural success in ICE Group does not differ significantly between subgroups.
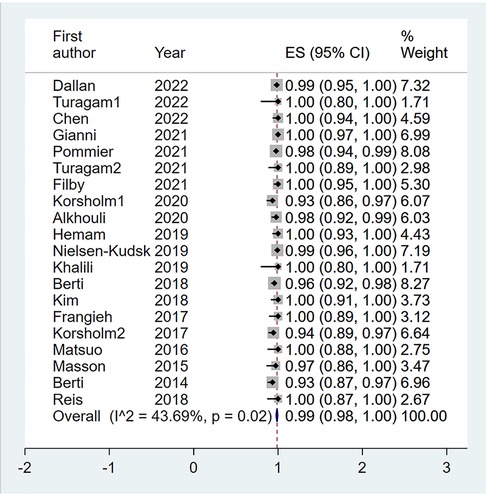
Figure 4. Forest plot of the pooled rate of the procedural success in ICE groups. The line of equity refers to the pooled result of eligible studies in the forest plots. ICE, intracardiac echocardiography; TEE, transesophageal echocardiography; ES, effect size; CI, confidence interval.
Also, sensitivity analysis showed that no significant change was detected in the overall combined proportion, ranging from 0.98 (95% CI: 0.97, 0.99) to 0.99 (95% CI: 0.98, 1.00), indicating that no single study dominated the combined proportion and heterogeneity. Moreover, Egger's test was performed and result showed no publication bias (P = 0.068), which indicated that the results were robust.
3.3. Secondary outcome
3.3.1. Total procedure time
A total of ten clinical studies provided the total procedural time, and the data on the total procedural time was similar between groups (WMD = −5.58; 95% CI: −15.97, 4.81; P = 0.29) (6–15). Significant heterogeneity was observed (I2 = 96.4%) (Figure 5). Subgroup analysis was performed with a total of seven subgroup factors for total procedure time, and the results are displayed in Supplementary Table S2. Interestingly, in the PAF proportion ≥50% subgroup, the procedural time in the TEE group was shorter than in the ICE group (WMD = 14.20; 95% CI: 7.6, 20.8; P = 0.000). Meanwhile, compared with the TEE group, the ICE group was associated with shorter procedural time in the muti-seal mechanism devices subgroup (WMD = −31.56; 95% CI: −55.57, −7.5; P = 0.010; I2 = 95.8%). Sensitivity analysis showed that no significant change, ranging from −7.80 (95% CI: −18.72, 3.11) to −1.35 (95% CI: −10.13, 7.44), was detected in the overall combined proportion. Moreover, no publication bias was shown in Egger's test (P = 0.535).
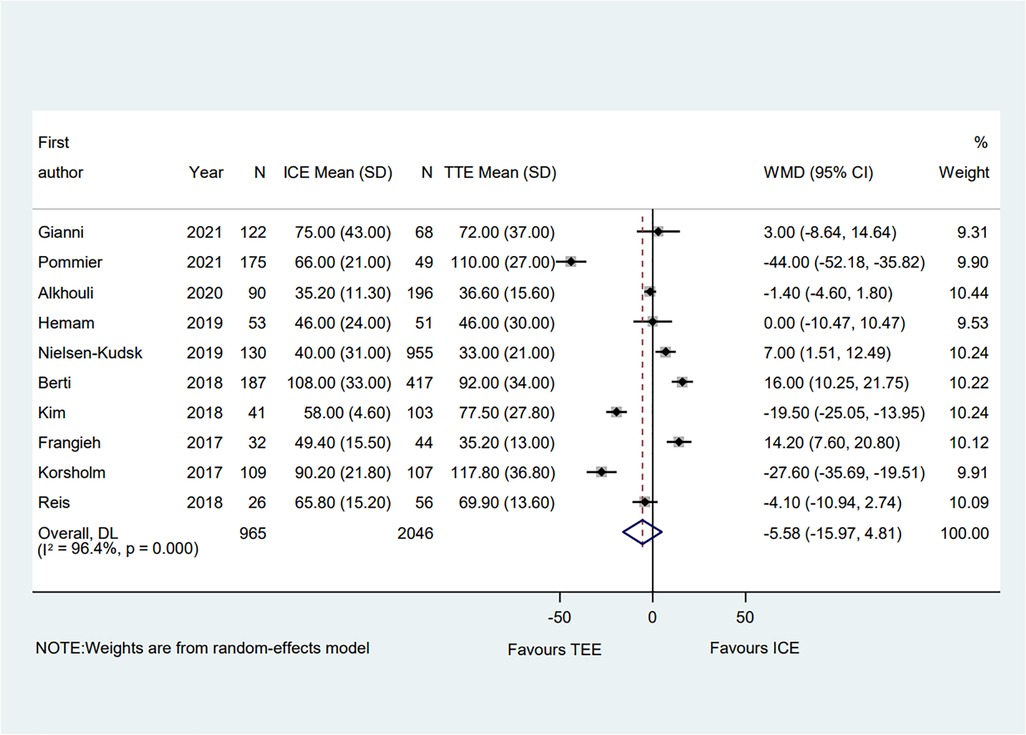
Figure 5. Forest plot of the total procedural time between ICE and TEE groups. Comparison of the rates of the total procedural time between ICE and TEE roups. ICE, intracardiac echocardiography; TEE, transesophageal echocardiography; WMD, weighted mean difference; CI, confidence interval.
3.3.2. Contrast volume
A total of six eligible studies reported the contrast volume (6–8, 10, 13, 14). The pooled results indicated that compared with the TEE procedure, the ICE procedure showed no significant difference (WMD = −2.61; 95% CI: −12.25, 7.02; P = 0.595; I2 = 84.80%) (Figure 6). The subgroup analysis showed that in the PAF proportion <50% subgroup, the ICE group's contrast volume was significantly decreased compared with the TEE group (WMD = −15.02; 95% CI: −27.08, −2.97; P = 0.015; I2 = 78.60%). Moreover, in the hypertension proportion <90% subgroup, the contrast volume in the ICE group was much lower than that in the TEE group (WMD = −12.95; 95% CI: −22.83, −3.07; P = 0.010; I2 = 62.90%). Meanwhile, the ICE group was associated with less contrast volume than the TEE group in the muti-seal mechanism devices subgroup (WMD = −22.00; 95% CI: −32.01, −11.99; P = 0.000). Interestingly, in the muti-centered subgroup, ICE-guided LAAO required a greater amount of contrast volume than TEE-guided LAAO (WMD = 47.00; 95% CI: 19.59, 74.42; P = 0.001) (Supplementary Table S3).
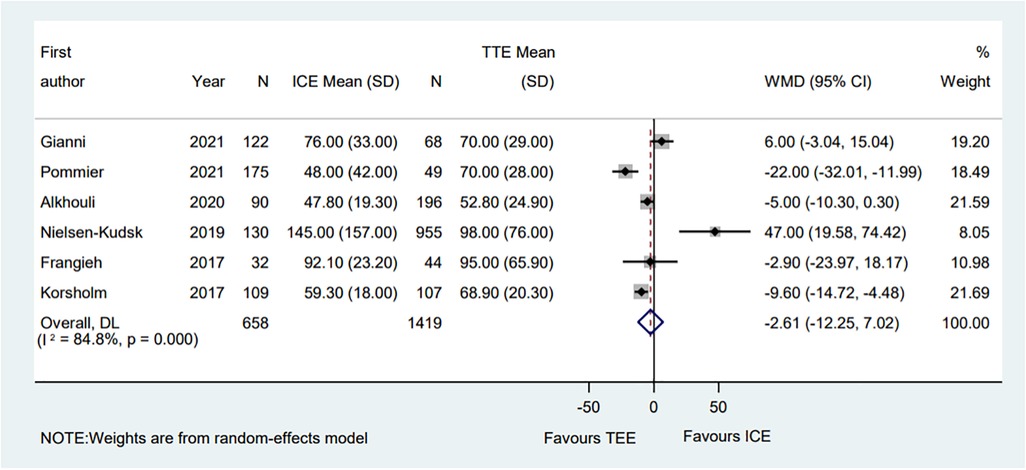
Figure 6. Forest plot of the contrast volume between ICE and TEE groups. Comparison of the rates of the contrast volume between ICE and TEE groups. ICE, intracardiac echocardiography; TEE, transesophageal echocardiography; WMD, weighted mean difference; CI, confidence interval.
Meanwhile, sensitivity analysis showed that no single study dominated the combined proportion and heterogeneity, ranging from −4.81 (95% CI: −15.29, 5.66) to 1.26 (95% CI: −8.55, 11.06). Moreover, Egger's test was performed and result showed no publication bias (P = 0.371), which suggested that the results were robust.
3.3.3. Fluoroscopic time
A total of ten eligible studies reported the fluoroscopic time and the pooled result showed that the fluoroscopic time guided by ICE was significantly equivalent to that guided by TEE (WMD = −0.34; 95% CI: −2.09, 1.41; P = 0.705; I2 = 82.80%) (Figure 7) (6–15). Subgroup analysis was performed with a total of seven subgroup factors for the fluoroscopic time, and the results were displayed in Supplementary Table S4. Compared with the TEE group, the fluoroscopic time in the ICE group was much shorter in the hypertension proportion <90% subgroup (WMD = −1.49; 95% CI: −2.87, −0.10; P = 0.035; I2 = 33.50%) as well as the muti-seal mechanism devices subgroup (WMD = −3.49; 95% CI: −5.53, −1.45; P = 0.001; I2 = 0.00%). No significant change was detected in the overall combined proportion by sensitivity analysis, ranging from −0.72 (95% CI: −2.48, 1.03) to 0.07 (95% CI: −1.74, 1.87). Moreover, no publication bias was shown in Egger's test (P = 0.941).
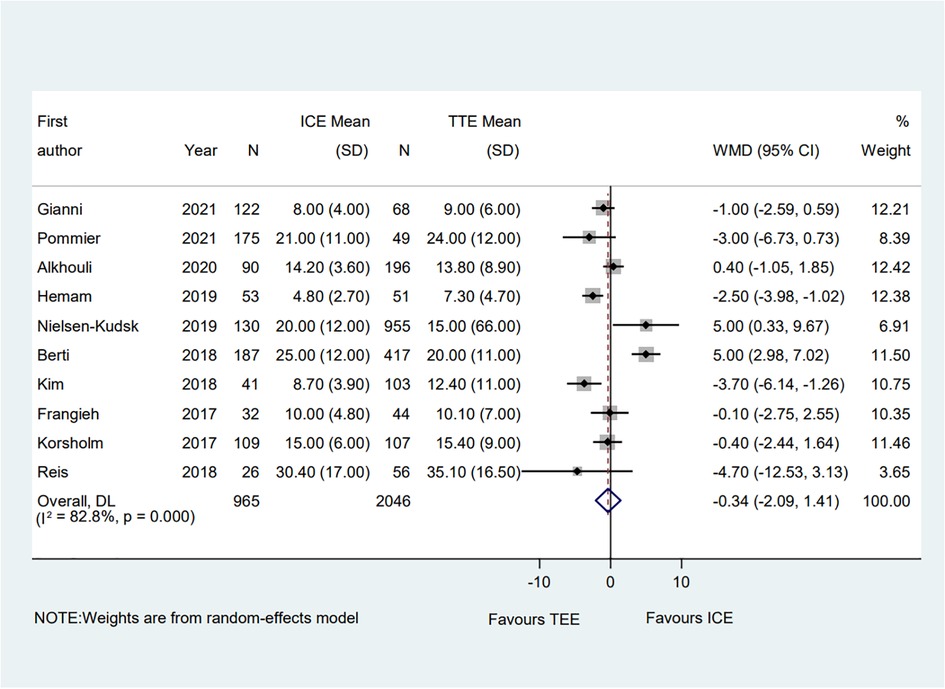
Figure 7. Forest plot of the fluoroscopic time between ICE and TEE groups. Comparison of the rates of the fluoroscopic time between ICE and TEE groups. ICE, intracardiac echocardiography; TEE, transesophageal echocardiography; WMD, weighted mean difference; CI, confidence interval.
3.3.4. Pooled safety outcomes
Common perioperative complications include cardiac effusion, cardiac tamponade, device migration, device thrombus, stroke/TIA, bleeding, hematoma, renal complications, cardiac arrest, and death. The data on procedural complications was available in nine clinical studies (6–8, 10–15). Complications from each eligible study were listed independently in Supplementary Tables S5, S6. The rate of procedural complications in ICE group was similar with that of TEE group (RR = 0.82; 95% CI: 0.58, 1.16; P = 0.261; I2 = 23.50%) (Figure 8). Sensitivity analysis was performed and the results showed no significant change in the overall combined proportion, ranging from 0.70 (95% CI: 0.45, 1.10) to 0.87 (95% CI: 0.61, 1.25). Egger's test also showed no publication bias (P = 0.696). Meanwhile, seven clinical studies were followed up and reported long-term adverse events (6–11, 14). In terms of long-term adverse events, the ICE group showed a similar result to TEE group (RR = 0.86; 95% CI: 0.64, 1.16; P = 0.329; I2 = 41.10%) (Figure 9).
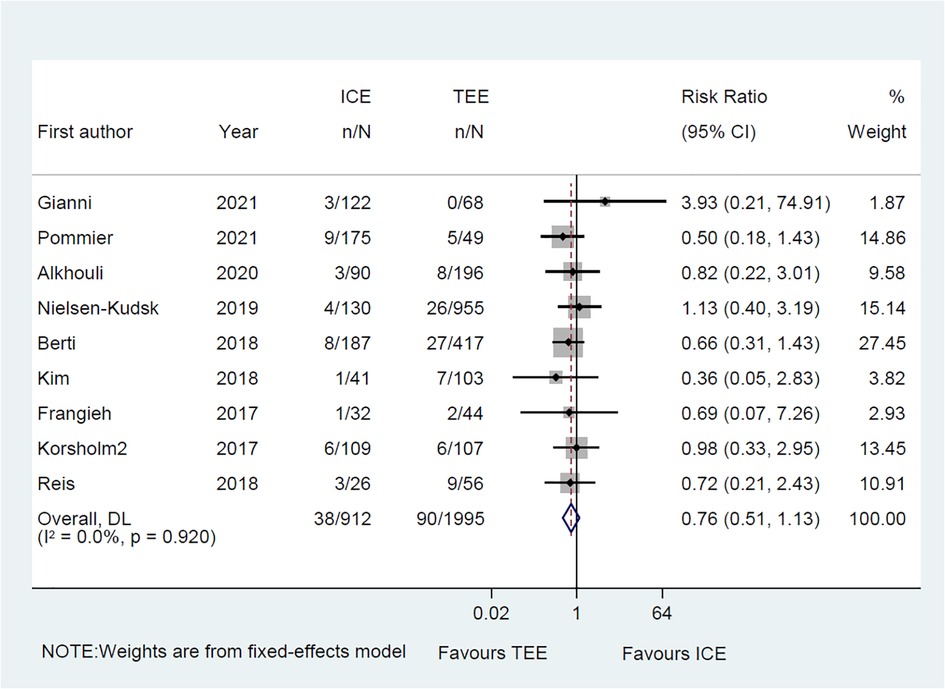
Figure 8. Forest plot of the preprocedural complications between ICE and TEE groups. Comparison of the rates of the preprocedural complications between ICE and TEE groups. ICE, intracardiac echocardiography; TEE, transesophageal echocardiography; RR, risk ratio; CI, confidence interval.
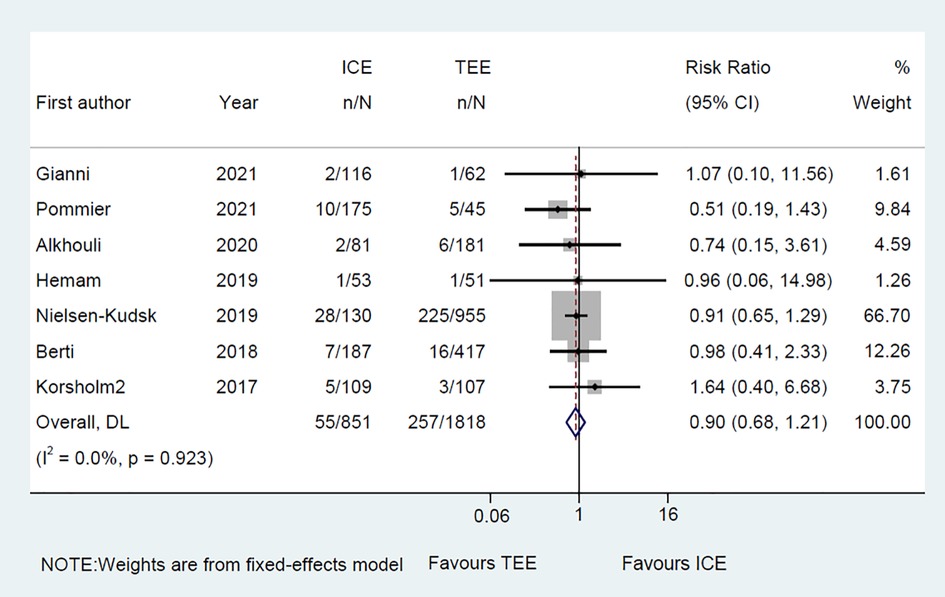
Figure 9. Forest plot of the long-term adverse events between ICE and TEE groups. Comparison of the rates of the long-term adverse events between ICE and TEE groups. ICE, intracardiac echocardiography; TEE, transesophageal echocardiography; RR, risk ratio; CI, confidence interval.
4. Discussion
Among twenty enrolled published original articles, a total of 3,610 patients (including 1,564 patients for ICE and 2,046 patients for TEE) were evaluated. Compared with previous meta-analysis, we included recent publications and single-arm studies. Meanwhile we performed subgroup analysis for each endpoint event. Our main findings were as follows. Compared with TEE group, (1) ICE group showed comparable efficacy and safety outcomes for LAAO, including the acute procedural success rate, total procedure time, contrast volume, the fluoroscopic time, and safety outcomes; (2) ICE group might reduce the use of contrast agent and fluoroscopic time in the hypertension proportion <90 subgroup; (3) ICE group might be associated with lower total procedure time, contrast volume, and the fluoroscopic time in device type subgroup with multi-seal mechanism; (4) The total procedure time might be longer in PAF proportion >50 subgroup while the contrast use might be less in PAF proportion ≤50 subgroup for ICE group; (5) ICE group might be related in an increased use of contrast in multi-center subgroup.
AF is an important pathogenesis of ischemic stroke, with approximately 5% of stroke patients being associated with AF each year, ultimately resulting in high rates of mortality and morbidity (3). LAAO has been demonstrated to be an alternative to prevent stroke in AF patients, particularly for individuals who are intolerant to oral anticoagulants. Intraoperative imaging is a crucial factor for LAAO. While TEE is currently the mainstream method, ICE is increasingly being used as an alternative to TEE.
In this meta-analysis, we compared the acute procedural success between the TEE and ICE groups. Similar with previous studies (26–28), we found no significant difference between the two groups. We then conducted a subgroup analysis to further compare the advantages and disadvantages of the two groups. The result showed that, regardless of the subgroup, there was no significant difference in acute procedural success rate. TEE is the gold standard imaging method for LAAO, providing clear images of the right atrium, left atrium, atrial septum, and left atrial appendage anatomy for LAAO. However, TEE-guided LAAO has some disadvantages, such as increased pain with local or conscious anesthesia, prolonged procedure time and hospitalization burden with general anesthesia, aggravated risk of possible esophageal injury under “one-stop” ablation, and high dependence on a dedicated echocardiography operator. To explore the safety of ICE and TEE, we recorded both the preprocedural complications and the long-term complications. For the short-term adverse events, the results showed that ICE was not inferior to TEE in guiding LAA occlusion procedures in terms of peri-procedural complications. Additionally, the long-term adverse events were comparable between groups, indicating that ICE had a reliable performance on safety.
Hypertension is one of the common comorbidities and modifiable risk factors in cardiovascular diseases, which could lead to the enlargement of left atria diameter, promotion of atrial fibrosis, and impairment of the endothelial function, ultimately causing the initiation and progression of AF and related stroke (29). However, few studies reported the role of hypertension on the procedure of LAAO for AF. Our subgroup results showed that ICE group might reduce the use of contrast agent and fluoroscopic time in the hypertension proportion <90 subgroup in comparison with the TEE group, suggesting that the lower proportion of hypertension may be associated with the more benefit for AF patients with LAAO procedure. This result might provide a basis for a randomized control trial to further evaluate the role of hypertension on the use of contrast agent and the fluoroscopic time between ICE-guided and TEE-guided LAAO.
At present, multiple types of devices for LAAO were applied in clinical procedure, mainly including single-seal mechanism device, dual-seal mechanism devices, and both mechanism device (30). Accumulated studies had revealed that selective application of the device type for LAAO might showed a similarly clinical outcomes based on the specific morphologies of LAA (31). Interestingly, ICE-guided LAAO might be associated with lower total procedure time, contrast volume, and the fluoroscopic time in device type subgroup with multi-seal mechanism. We could make a reasonable speculation that the application of multi-seal mechanism devices is associated with the mastery of the ICE-guided LAAO procedure by operators. Whereas, more studies should be performed to demonstrate this result.
Studies on the impact of AF type during LAAO procedure are emerging. A recent lesson from the prospective Left Atrial Appendage Occluder Registry Germany (LAARGE) had suggested that the procedure time and fluoroscopy time were longer for LAAO procedure in PAF patients than non-PAF patients, which might be significantly related in the challenge of LAA movement due to the higher rate of sinus rhythm in PAF patients during LAAO procedure (32). Similarly, our subgroup also indicated that the total procedure time in ICE-guided LAAO might be longer in PAF proportion >50 subgroup. In addition, the contrast use might be less in PAF proportion ≤50 subgroup for ICE-guided LAAO group, potentially suggesting that ICE-guided LAAO might reduce the contrast use for non-PAF patients.
Additionally, our subgroup results suggested that ICE-guided LAAO might be associated with an increased use of contrast in multi-center subgroup, which indicated that ICE-guided LAAO showed unsatisfied performance on the contrast use in multi-center subgroup in comparison with single-center subgroup. This might be explained by the multiple possibilities, including center heterogeneity, team quality heterogeneity, and relatively rigid procedure protocol rarely with decision-making strategy in multi-center study. Moreover, only one multi-center study (10) reported the contrast use for subgroup analysis, which might cause potential bias due to the limited sample size. Therefore, more prospective studies are needed to further demonstrate our results.
Also, a total of two studies compared the cost of hospitalization between ICE group and TEE group (8, 9), which showed that the global charges were similar between the ICE-guided LAAO and TEE-guided LAAO in American centers. Whereas, in other medical centers, the hospital charges of ICE-guided LAAO might be higher in comparison with TEE-guided LAAO due to the higher cost of ICE catheter (33). Also, local medical team experience and environment would play an important role on the determination of the appropriate imaging modality to be implemented. Therefore, more prospective, randomized studies will probably clarify the comparison of ICE and TEE for guiding the LAAO procedure, especially in terms of efficacy, safety, and hospital charges.
5. Limitations
Our study has several limitations. First, the studies included in this meta-analysis were nonrandomized and observational in design, which might lead to potential selection bias. Second, the sample size included in the study is small which may affect the stability of the result indicators, reduce the efficiency of the test, and introduce potential research bias. Third, different studies were followed with different tests, which may have affected the follow-up results. In addition, clinical studies lacked a uniform definition of procedural success and procedure-related complications. Therefore, a prospective, randomized study is needed to clarify the clinical outcomes of LAAO with the comparison of ICE vs. TEE monitoring.
6. Conclusions
Our results demonstrate that ICE may have comparable efficacy and safety compared to TEE for LAAO.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author contributions
R-XW developed the concept of the study; Z-YZ, FL and JZ designed this study and carried out the data analysis; FL, and Z-YZ conducted meta-analysis registration in the PROSPERO platform with help from R-XW; Z-YZ wrote the manuscript with help from FL, JZ, LZ, H-HL, NZ, FY, QK; Y-TZ, L-LQ and R-XW provided critical reviews of the paper. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1194771/full#supplementary-material
References
1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. (2021) 42(5):373–498. doi: 10.1093/eurheartj/ehaa612
2. Jame S, Barnes G. Stroke and thromboembolism prevention in atrial fibrillation. Heart. (2020) 106(1):10–7. doi: 10.1136/heartjnl-2019-314898
3. Berti S, Pastormerlo L E, Korsholm K, Saw J, Alkhouli M, Costa MP, et al. Intracardiac echocardiography for guidance of transcatheter left atrial appendage occlusion: an expert consensus document. Catheter Cardiovasc Interv. (2021) 98(4):815–25. doi: 10.1002/ccd.29791
4. Furlan A D, Pennick V, Bombardier C, van Tulder M. 2009 Updated method guidelines for systematic reviews in the cochrane back review group. Spine. (2009) 34(18):1929–41. doi: 10.1097/BRS.0b013e3181b1c99f
5. Guo B, Moga C, Harstall C, Schopflocher D. A principal component analysis is conducted for a case series quality appraisal checklist. J Clin Epidemiol. (2016) 69:199–207.e2. doi: 10.1016/j.jclinepi.2015.07.010
6. Gianni C, Horton RP, Della Rocca DG, Mohanty S, Al-Ahmad A, Bassiouny MA, et al. Intracardiac echocardiography- versus transesophageal echocardiography-guided left atrial appendage occlusion with watchman FLX. J Cardiovasc Electrophysiol. (2021) 32(10):2781–4. doi: 10.1111/jce.15220
7. Pommier T, Guenancia C, Richard C, Sagnard A, Fichot M, Salignon-Vernay C, et al. Safety and efficacy of left atrial appendage occlusion with the ACP or watchman device guided by intracardiac echocardiography from the left atrium. Clin Cardiol. (2021) 44(10):1402–8. doi: 10.1002/clc.23696
8. Alkhouli M, Chaker Z, Alqahtani F, Raslan S, Raybuck B. Outcomes of rroutine intracardiac echocardiography to guide left atrial appendage occlusion. JACC Clin Electrophysiol. (2020) 6(4):393–400. doi: 10.1016/j.jacep.2019.11.014
9. Hemam ME, Kuroki K, Schurmann PA, Dave AS, Rodríguez DA, Sáenz LC, et al. Left atrial appendage closure with the watchman device using intracardiac vs transesophageal echocardiography: procedural and cost considerations. Heart Rhythm. (2019) 16(3):334–42. doi: 10.1016/j.hrthm.2018.12.013
10. Nielsen-kudsk JE, Berti S, De backer O, Aguirre D, Fassini G, Cruz-Gonzalez I, et al. Use of intracardiac compared with transesophageal echocardiography for left atrial appendage occlusion in the amulet observational study. JACC Cardiovasc Interv. (2019) 12(11):1030–9. doi: 10.1016/j.jcin.2019.04.035
11. Berti S, Pastormerlo LE, Santoro G, Brscic E, Montorfano M, Vignali L, et al. Intracardiac versus ttransesophageal echocardiographic guidance for left atrial appendage occlusion: the LAAO Italian multicenter registry. JACC Cardiovasc Interv. (2018) 11(11):1086–92. doi: 10.1016/j.jcin.2018.05.008
12. Kim DY, Shin SY, Kim J-S, Kim SH, Kim YH, Lim HE. Feasibility of intracardiac echocardiography imaging from the left superior pulmonary vein for left atrial appendage occlusion. Int J Cardiovasc Imaging. (2018) 34(10):1571–9. doi: 10.1007/s10554-018-1374-5
13. Frangieh AH, Alibegovic J, Templin C, Gaemperli O, Obeid S, Manka R, et al. Intracardiac versus transesophageal echocardiography for left atrial appendage occlusion with watchman. Catheter Cardiovasc Interv. (2017) 90(2):331–8. doi: 10.1002/ccd.26805
14. Korsholm K, Jensen JM, Nielsen-kudsk JE. Intracardiac echocardiography from the left aatrium for procedural guidance of transcatheter left atrial appendage occlusion. JACC Cardiovasc Interv. (2017) 10(21):2198–206. doi: 10.1016/j.jcin.2017.06.057
15. Reis L, Paiva L, Costa M, Silva J, Teixeira R, Botelho A, et al. Registry of left atrial appendage closure and initial experience with intracardiac echocardiography. Rev Port Cardiol (Engl Ed). (2018) 37(9):763–72. doi: 10.1016/j.repc.2018.03.009
16. Dallan LAP, Arruda M, Yoon S-H, Rana MA, Mogalapalli A, Carneiro HA, et al. Novel computed tomography angiography-based sizing methodology for WATCHMAN FLX device in left atrial appendage closure. J Cardiovasc Electrophysiol. (2022) 33(8):1781–7. doi: 10.1111/jce.15548
17. Turagam MK, Neuzil P, Hala P, Hala P, Mraz T, Dukkipati SR, Reddy VY. Intracardiac echocardiography-guided left atrial appendage closure with a novel foam-based conformable device: safety and 1-year outcomes. JACC Clin Electrophysiol. (2022) 8(2):197–207. doi: 10.1016/j.jacep.2021.10.001
18. Chen Y-H, Wang L-G, Zhou X-D, Fang Y, Su L, Wu SJ, et al. Outcome and safety of intracardiac echocardiography guided left atrial appendage closure within zero-fluoroscopy atrial fibrillation ablation procedures. J Cardiovasc Electrophysiol. (2022) 33(4):667–76. doi: 10.1111/jce.15370
19. Turagam MK, Neuzil P, Petru J, Hala P, Mraz T, Baroch J, et al. Intracardiac echocardiography-guided implantation of the watchman FLX left atrial appendage closure device. J Cardiovasc Electrophysiol. (2021) 32(3):717–25. doi: 10.1111/jce.14927
20. Filby SJ, Dallan LAP, Cochet A, Kobayashi A, Attizzani GF, Rashid I, et al. Left aatrial appendage occlusion using cardiac CT angiography and intracardiac echocardiography: a prospective, ssingle-center study. J Invasive Cardiol. (2021) 33(11):E851–E6.34619655
21. Korsholm K, Samaras A, Andersen A, Jensen JM, Nielsen-Kudsk JE. The watchman FLX device: first European experience and feasibility of intracardiac echocardiography to guide implantation. JACC Clin Electrophysiol. (2020) 6(13):1633–42. doi: 10.1016/j.jacep.2020.06.028
22. Khalili H, Patton M, Taii HA, Bansal P, Brady M, Taylor J, et al. 4D Volume intracardiac echocardiography for intraprocedural guidance of ttranscatheter left aatrial appendage closure. J Atr Fibrillation. (2019) 12(4):2200. doi: 10.4022/jafib.2200
23. Matsuo Y, Neuzil P, Petru J, Chovanec M, Janotka M, Choudry S, et al. Left atrial appendage closure under intracardiac echocardiographic guidance: feasibility and ccomparison with transesophageal echocardiography. J Am Heart Assoc. (2016) 5(10):e003695. doi: 10.1161/JAHA.116.003695
24. Masson J-B, Kouz R, Riahi M, Nguyen Thanh HK, Potvin J, Naim C, et al. Transcatheter left atrial appendage closure using intracardiac echocardiographic gguidance from the left atrium. Can J Cardiol. (2015) 31(12):1497.e7–1497.e14. doi: 10.1016/j.cjca.2015.04.031
25. Berti S, Paradossi U, Meucci F, Trianni G, Tzikas A, Rezzaghi M, et al. Periprocedural intracardiac echocardiography for left atrial appendage closure: a dual-center experience. JACC Cardiovasc Interv. (2014) 7(9):1036–44. doi: 10.1016/j.jcin.2014.04.014
26. Ribeiro JM, Teixeira R, Puga L, Costa M, Gonçalves L. Comparison of intracardiac and transoesophageal echocardiography for guidance of percutaneous left atrial appendage occlusion: a meta-analysis. Echocardiography. (2019) 36(7):1330–7. doi: 10.1111/echo.14415
27. Liang G, Xu B, Wang S, Li C, Zhong G. Imaging with intracardiac echocardiography compared to transesophageal echocardiography during left atrial appendage occlusion. Rev Cardiovasc Med. (2020) 21(1):93–101. doi: 10.31083/j.rcm.2020.01.569
28. Akella K, Murtaza G, Turagam M, Sharma S, Madoukh B, Amin A, et al. Evaluating the role of transesophageal echocardiography (TEE) or intracardiac echocardiography (ICE) in left atrial appendage occlusion: a meta-analysis. J Interv Card Electrophysiol. (2021) 60(1):41–8. doi: 10.1007/s10840-019-00677-x
29. Brundel B, Ai X, Hills MT, Kuipers MF, Lip GYH, de Groot NMS. Atrial fibrillation. Nat Rev Dis Prim. (2022) 8(1):21. doi: 10.1038/s41572-022-00347-9
30. Saw J, Holmes DR, Cavalcante JL, Freeman JV, Goldsweig AM, Kavinsky CJ, et al. SCAI/HRS expert consensus statement on transcatheter left atrial appendage closure. Heart Rhythm. (2023) 20(5):e1–e16. doi: 10.1016/j.hrthm.2023.01.007
31. Holmes DR Jr, Korsholm K, Rodés-Cabau J, Saw J, Berti S, Alkhouli MA. Left atrial appendage occlusion. EuroIntervention. (2023) 18(13):e1038–65. doi: 10.4244/EIJ-D-22-00627
32. Kany S, Brachmann J, Lewalter T, Akin I, Sievert H, Zeymer U, et al. Impact of atrial fibrillation pattern on outcomes after left atrial appendage closure: lessons from the prospective LAARGE registry. Clin Res Cardiol. (2022) 111(5):511–21. doi: 10.1007/s00392-021-01874-3
Keywords: atrial fibrillation, intracardiac echocardiography, transesophageal echocardiography, left atrial appendage closure, implantable devices, cardiac mapping
Citation: Zhang Z-Y, Li F, Zhang J, Zhang L, Liu H-H, Zhao N, Yang F, Kong Q, Zhou Y-T, Qian L-L and Wang R-X (2023) A comparable efficacy and safety between intracardiac echocardiography and transesophageal echocardiography for percutaneous left atrial appendage occlusion. Front. Cardiovasc. Med. 10:1194771. doi: 10.3389/fcvm.2023.1194771
Received: 27 March 2023; Accepted: 9 May 2023;
Published: 24 May 2023.
Edited by:
Francesca Innocenti, Careggi University Hospital, ItalyReviewed by:
Eustaquio Maria Onorato, U.O. Cardiologia Universitaria, Ospedale Galeazzi—Sant’Ambrogio, Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), ItalyTobias Zeus, University Hospital of Düsseldorf, Germany
© 2023 Zhang, Li, Zhang, Zhang, Liu, Zhao, Yang, Kong, Zhou, Qian and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ru-Xing Wang cnV4aW5nd0BhbGl5dW4uY29t
†These authors have contributed equally to this work
 Zhi-Yuan Zhang†
Zhi-Yuan Zhang† Feng Li
Feng Li Ning Zhao
Ning Zhao Ru-Xing Wang
Ru-Xing Wang