- Division of Cardiovascular Medicine, School of Medicine, University of California, Davis, Sacramento, CA, United States
1. Introduction
Many patients with heart failure with reduced ejection fraction (HFrEF) are initially diagnosed at an index heart failure hospitalization. Nearly half of these patients have an ischemic etiology. In the latter group, the Surgical Treatment for Ischemic Heart Failure (STICH) trial was the first, and thus far, the only trial to show benefit of revascularization on survival in patients with left ventricular (LV) dysfunction (1). Revascularization by percutaneous coronary intervention (PCI) has also been applied to this population in anticipation of achieving similar benefit. The REVIVED-BCIS2 trial tested this hypothesis by randomizing patients with multivessel coronary artery disease (CAD) and a left ventricular ejection fraction (LVEF) < 35% to PCI and guideline directed medical therapy (GDMT) vs. GDMT alone (2). However, no benefit on survival or LV function was found with PCI compared to GDMT during a mean follow-up interval of more than 3 years. Thus, for patients with HFrEF and stable multivessel CAD, coronary artery bypass graft surgery (CABG) is the only revascularization method with documented mortality benefit. GDMT remains the foundation of care for these patients.
This finding raises an important question: For newly diagnosed HFrEF patients at high risk for CAD not presenting with acute coronary syndrome, how should revascularization be prioritized at index hospitalization?
2. Discussion
2.1. GDMT should be the focus for newly diagnosed HFrEF
The 2022 ACC/AHA/HFSA Guidelines identify 4 pillars of GDMT for HFrEF (3). Each of these agents has shown reduction in heart failure hospitalization and cardiovascular death within 30 days of initiation (4). However, due to widespread underuse of GDMT, there have been considerable and potentially avoidable losses of life and function (5). The Guidelines suggest simultaneous initiation of this regimen at diagnosis with subsequent titration at regular intervals (3). This is a documented strategy to reduce both early and long-term mortality and morbidity in HFrEF, regardless of etiology.
In fact, the STRONG-HF trial randomized patients hospitalized with heart failure, a majority with HFrEF, to a strategy of aggressive initiation and titration of GDMT vs. usual care (6). The result was a notable 8.1% absolute risk reduction in the primary endpoint of 180-day readmission for heart failure or all-cause death. N-terminal pro-brain natriuretic peptide was also reduced by 23% at 90 days in the aggressively titrated arm compared to usual care despite no difference in doses of loop diuretics. The key to this success was targeted up-titration of GDMT peri-discharge, resulting in >80% of patients on half-target dose or greater of beta-blocker, renin-angiotensin-aldosterone system inhibitor, and mineralocorticoid receptor antagonist by 2 weeks post-discharge.
While an ischemic evaluation is a crucial aspect in the evaluation of newly diagnosed HFrEF, immediate revascularization may impede GDMT initiation. Percutaneous coronary intervention-induced acute kidney injury (AKI) occurs in up to 7%–10% of cases, which may prohibit initiation or continuation of GDMT agents, which often transiently reduce glomerular filtration rate (7). Moreover, the concern for contrast induced AKI from coronary angiography can render clinicians reluctant to titrate GDMT. Additionally, GDMT use after CABG has historically been lower than with PCI, presenting another barrier to medical optimization (8).
2.2. Could there be benefit to deferred revascularization of multivessel disease?
Guidelines provide a Class I indication for revascularization with CABG for patients with high risk left main (LM) CAD and multivessel CAD associated with diabetes or LVEF < 35% (9). However, as with candidacy for implantable cardiac defibrillators for primary prevention, consideration of revascularization may shift as a patient's LVEF improves after optimization of GDMT (10). Per Guidelines, a patient with an LVEF of 30% with multivessel disease and no diabetes has a Class I indication for CABG; however, in three months if the EF improves to 35%–50% with optimal GDMT, then the recommendation for CABG drops to Class 2a; if EF improves to >50% then it becomes 2b. Moreover, marked LV dysfunction is a leading reason for rejection of surgery due to the increased risk of surgical mortality (1, 5). Historically, many of these turndowns are sent for PCI. However, results of REVIVED-BCIS2 reveal that this approach may not have been beneficial (2). But even with high rates of surgical mortality, the STICH trial showed that the clinical benefits of CABG in LV dysfunction are eventually realized (1). Prioritization of optimal GDMT before revascularization in patients whose sole indication is LVEF < 35%, could result in increased LVEF at the time of consideration for CABG, which may obviate the need for CABG, or lower operative risk if the decision is made to proceed with CABG. Therefore, deferred, i.e., postponement of this decision to the outpatient setting, may be a preferred strategy for management of these patients.
2.3. Consider a non-invasive ischemic evaluation
While invasive coronary angiography has been the gold-standard for diagnosis of ischemic cardiomyopathy, the limited role of PCI in patients with LV dysfunction and stable CAD may limit its necessity. Non-invasive imaging minimizes procedural risk while maintaining diagnostic accuracy for high risk disease. Coronary computed tomography angiography (CCTA) has upwards of 90% sensitivity and specificity for identifying obstructive CAD (11). For certain patients in which CCTA may be impractical, such as those with elevated heart rates or marginal kidney function, non-invasive stress imaging can be used to detect LM and triple vessel CAD (3, 12). Patients with high risk, inconclusive, or high likelihood of false negative findings on non-invasive testing can be considered for invasive angiography, but the possible benefits of deferred revascularization should be considered as previously noted (Figure 1). Patients whose non-invasive testing is negative or not high risk have low annual rates of ischemic events and further invasive evaluation can be performed in the outpatient setting, if indicated (13, 14). In these cases, non-ischemic causes of cardiomyopathy should also be evaluated (15).
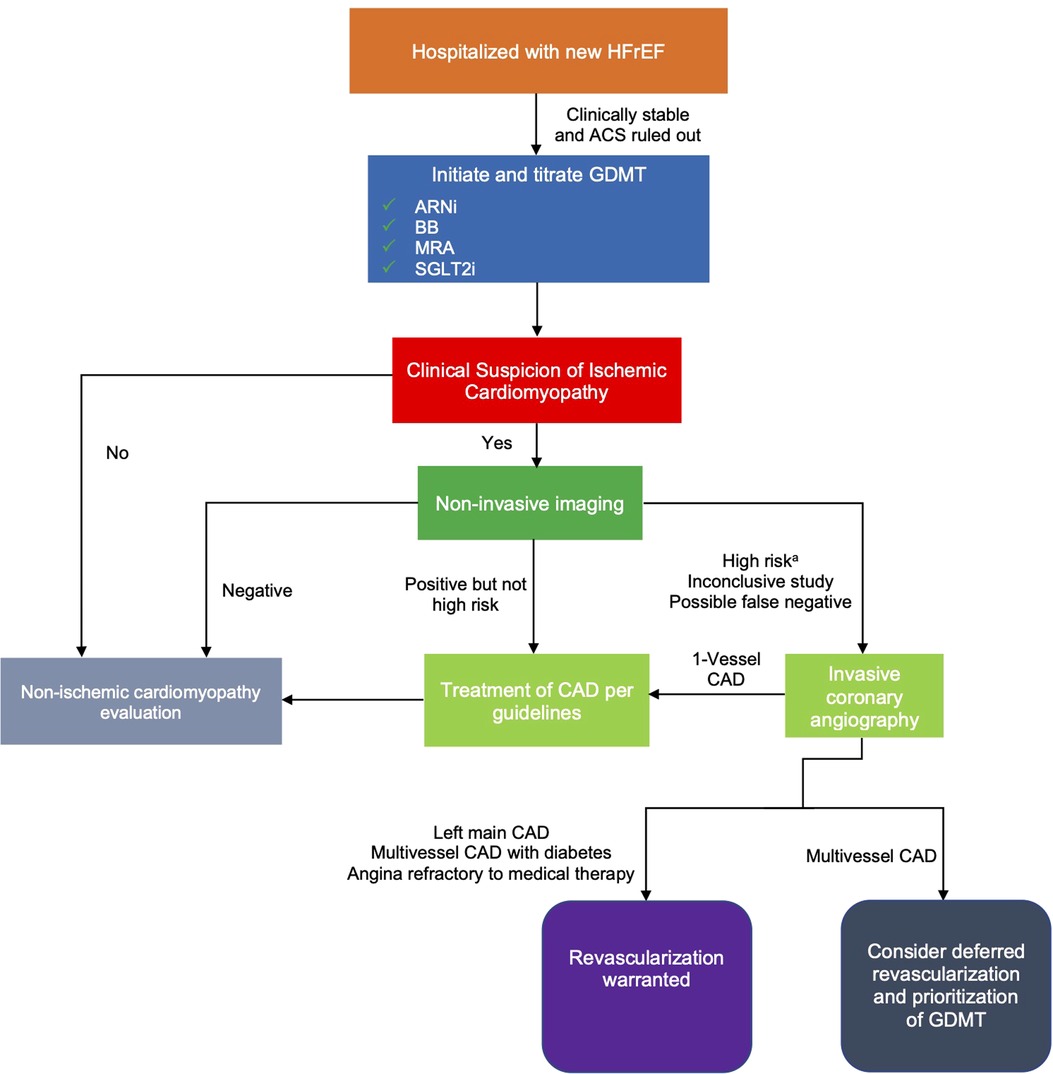
Figure 1. Proposed algorithm for evaluation of ischemic cardiomyopathy in hospitalized patients with newly diagnosed HFrEF. aSuggestive of LM or multivessel CAD. ACS, acute coronary syndrome; ARNi, angiotensin receptor-neprilysin inhibitor; BB, beta-blocker; CAD, coronary artery disease; GDMT, guideline-directed medical therapy; HFrEF, heart failure with reduced ejection fraction; MRA, mineralocorticoid receptor antagonist; SGLT2i, sodium/glucose cotransporter-2 inhibitors.
3. Conclusion
With the negative results of the REVIVED-BCIS2 trial, CABG remains the only method of revascularization in patients with ischemic cardiomyopathy to demonstrate morbidity and mortality benefit. However, deferred revascularization of multi-vessel disease in patients with new onset HFrEF should be considered to allow time for the impact of the rapid, beneficial effects of GDMT, which may lead to lower surgical risk or render CABG unnecessary. Additionally, an initial non-invasive ischemic evaluation reduces procedural risk and may better facilitate GDMT optimization than an initial invasive evaluation. Overall, for newly diagnosed HFrEF patients, a strategy prioritizing GDMT over revascularization may lead to greater long-term benefits. A randomized trial is required to provide further guidance on this approach.
Author contributions
ND: wrote the first draft of the manuscript. EA: reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Velazquez EJ, Lee KL, Jones RH, Al-Khalidi HR, Hill JA, Panza JA, et al. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med. (2016) 374(16):1511–20. doi: 10.1056/NEJMoa1602001
2. Perera D, Clayton T, O'Kane PD, Greenwood JP, Weerackody R, Ryan M, et al. Percutaneous revascularization for ischemic left ventricular dysfunction. N Engl J Med. (2022) 387(15):1351–60. doi: 10.1056/NEJMoa2206606
3. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. published correction appears in Circulation. (2022) 145(18):e1033; (2022) 146(13):e185; (2023) 147(14):e674; (2022) 145(18):e895–e1032. doi: 10.1161/CIR.0000000000001063
4. Brownell NK, Ziaeian B, Fonarow GC. The gap to fill: rationale for rapid initiation and optimal titration of comprehensive disease-modifying medical therapy for heart failure with reduced ejection fraction. Card Fail Rev. (2021) 7:e18. doi: 10.15420/cfr.2021.18
5. Greene SJ, Butler J, Albert NM, DeVore AD, Sharma PP, Duffy CI, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF registry. J Am Coll Cardiol. (2018) 72(4):351–66. doi: 10.1016/j.jacc.2018.04.070
6. Mebazaa A, Davison B, Chioncel O, Cohen-Solal A, Diaz R, Filippatos G, et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomised, trial. Lancet. (2022) 400(10367):1938–52. doi: 10.1016/S0140-6736(22)02076-1
7. Azzalini L, Candilio L, McCullough PA, Colombo A. Current risk of contrast-induced acute kidney injury after coronary angiography and intervention: a reappraisal of the literature. Can J Cardiol. (2017) 33(10):1225–8. doi: 10.1016/j.cjca.2017.07.482
8. Pinho-Gomes AC, Azevedo L, Ahn JM, Park SJ, Hamza TH, Farkouh ME, et al. Compliance with guideline-directed medical therapy in contemporary coronary revascularization trials. J Am Coll Cardiol. (2018) 71(6):591–602. doi: 10.1016/j.jacc.2017.11.068
9. Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: executive summary: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. published correction appears in Circulation. (2022) 145(11):e771; (2022) 145(3):e4–e17. doi: 10.1161/CIR.0000000000001039
10. Wilcox JE, Fang JC, Margulies KB, Mann DL. Heart failure with recovered left ventricular ejection fraction: JACC scientific expert panel. J Am Coll Cardiol. (2020) 76(6):719–34. doi: 10.1016/j.jacc.2020.05.075
11. Paech DC, Weston AR. A systematic review of the clinical effectiveness of 64-slice or higher computed tomography angiography as an alternative to invasive coronary angiography in the investigation of suspected coronary artery disease. BMC Cardiovasc Disord. (2011) 11(1):32. doi: 10.1186/1471-2261-11-32
12. Mahajan N, Polavaram L, Vankayala H, Ference B, Wang Y, Ager J, et al. Diagnostic accuracy of myocardial perfusion imaging and stress echocardiography for the diagnosis of left main and triple vessel coronary artery disease: a comparative meta-analysis. Heart. (2010) 96(12):956–66. doi: 10.1136/hrt.2009.182295
13. Reynolds HR, Shaw LJ, Min JK, Page CB, Berman DS, Chaitman BR, et al. Outcomes in the ISCHEMIA trial based on coronary artery disease and ischemia severity. Circulation. (2021) 144(13):1024–38. doi: 10.1161/CIRCULATIONAHA.120.049755
14. Hoffmann U, Ferencik M, Udelson JE, Picard MH, Truong QA, Patel MR, et al. Prognostic value of noninvasive cardiovascular testing in patients with stable chest pain: insights from the PROMISE trial (prospective multicenter imaging study for evaluation of chest pain). Circulation. (2017) 135(24):2320–32. doi: 10.1161/CIRCULATIONAHA.116.024360
Keywords: guideline-directed medical therapy, heart failure, revascularization, cardiomyopathy, coronary artery bypass surgery, percutaneous coronary intervention
Citation: Dixit NM and Amsterdam EA (2023) Should GDMT be prioritized over revascularization in new onset HFrEF? Potential lessons from the REVIVED-BCIS2 and STRONG-HF trials. Front. Cardiovasc. Med. 10:1193226. doi: 10.3389/fcvm.2023.1193226
Received: 24 March 2023; Accepted: 16 May 2023;
Published: 12 June 2023.
Edited by:
Jamshid Karimov, Cleveland Clinic, United StatesReviewed by:
Eric Rytkin, Northwestern University, United States© 2023 Dixit and Amsterdam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ezra A. Amsterdam ZWFhbXN0ZXJkYW1AdWNkYXZpcy5lZHU=
 Neal M. Dixit
Neal M. Dixit Ezra A. Amsterdam
Ezra A. Amsterdam