- Faculty of Biology and Medicine, University of Lausanne, Switzerland and Hypertension Research Foundation, St Légier, Switzerland
Gout and hyperuricaemia are two clinical situations associated with an elevated risk of developing cardiovascular (heart failure, myocardial infarction, stroke) and metabolic and renal complications. One reason is probably related to the fact that the prevalence of hyperuricaemia and gout is high in clinical situations, which themselves involve a high cardiovascular risk, such as hypertension, diabetes, chronic kidney disease or obesity. However, recent studies suggest that hyperuricaemia may promote cardiovascular complications independently of other cardiovascular risk factors, by inducing chronic inflammation, oxidative stress, and endothelial dysfunction. The questions that arise today concern primarily the treatment of asymptomatic hyperuricaemia. Should it be treated to decrease the patients' cardiovascular risk and if so, starting from which level and towards which target? There are now several pieces of evidence indicating that this might be useful, but data from large studies are not unanimous. This review will discuss this issue as well as new well-tolerated treatments, such as febuxostat or SGLT2 inhibitors, which lower uric acid levels, prevent gout and lower the risk of cardio-renal events.
Introduction
Hyperuricaemia and gout are highly prevalent in the population of developed countries particularly in men. Indeed, gout, considered for centuries to be a disease of rich, well-fed people, affects about 2%–3% of men and hyperuricaemia, defined in Europe as a uric acid blood level >360 μmol/L (408 μmol/L in the United States and 420 μmol/L in Japan (1, 2), is present in 25%–30% of men in Switzerland (3). The European and American recommendations concerning the treatment of hyperuricaemia and gout are clear today: patients with asymptomatic hyperuricaemia should not receive uric acid lowering treatments and should be treated with lifestyle corrections (2, 4, 5). By contrast, all patients who suffered an attack of gout should receive—in addition to non-pharmacological approaches—a specific treatment to lower their uric acid level below the crystallisation threshold of less than 360 μmol/l and even below 300 μmol/l in the case of severe gout. Recommendations for patients with gout are not controversial. However, the attitude towards asymptomatic hyperuricaemia and its role in kidney and cardiovascular diseases are regularly questioned (6–8). The main reason for reconsidering the therapeutic abstention in asymptomatic patients is due to the fact that hyperuricaemia is frequently found to be an independent cardiovascular risk factor in epidemiological studies. Therefore, some national recommendations propose treating asymptomatic hyperuricaemia when levels exceed a certain threshold, e.g., > 480 μmol/L or > 500 μmol/L (i.e., > 8 or 9 mg/dl) (1) and others recommend to measure plasma uric acid levels as a cardiovascular risk factor in some patients groups such as patients with hypertension (9).
Hyperuricaemia, gout, and cardiovascular risk
The first associations between the level of uric acid and the occurrence of cardiovascular events were reported in the 1950s already (10). In 1999, a first analysis of the Framingham Heart Study involving 6763 subjects with an average age of 47 years, suggested that baseline uric acid was predictive of the risk of cardiovascular mortality and coronary events in women but not in men (11). However, after correcting for other cardiovascular risk factors, this association was no longer significant, suggesting that uric acid was not the cause of the occurrence of cardiovascular events but rather a marker. In contrast, the first analysis of the National Health and Nutrition Examination Survey (NHANES I) in the United States, using data collected between 1971 and 1975 with a follow-up of 16 years, concluded that uric acid was an independent risk factor for cardiovascular events in men as well as in women, even after correcting for other known cardiovascular risk factors (12). Several more recent analyses from the NHANES program subsequently confirmed the link between uric acid and hypertension and the risk of global and cardiovascular mortality in diabetic patients (13, 14). Moreover, numerous analyses have been conducted in various populations to analyse the association between uric acid and the cardiovascular risk in greater details (15–20). In these studies, hyperuricaemia is described as a predictive factor, not only for hypertension and diabetes but also for heart disease, atrial fibrillation, myocardial infarction, heart failure, cerebrovascular events and chronic kidney disease. A systematic review and dose-response meta-analysis of over one million subjects has found a significant positive association between uric acid levels and the risk of cardiovascular mortality with a stronger association in women than men (21). Mendelian randomization studies have produced conflicting results regarding the causal implication of uric acid in the development of cardiovascular complications. Thus, Keenan et al. did not found any evidence supporting a causal role of circulating serum urate levels in type-2 diabetes, coronary heart disease, ischemic stroke, or heart failure (22). In contrast, Yang et al. reported some evidence for a causal effect of genetically determined serum urate level on heart failure (23). A similar causal effect was found for cardiovascular death and sudden cardiac death in 3,315 patients of the Ludwigshafen Risk and Cardiovascular Health Study (24). Similar results were found in the UK biobank but Mendelian Randomization analyses suggested a causal effect of hyperuricemia, but not gout, on cardiovascular diseases (25). In hypertension, the Mendelian randomization and clinical trial data from the UK Biobank tended to support an effect of higher serum urate on increasing blood pressure (26). Yet, as reviewed by Sanchez-Losada et al, though several studies were positive, the causal effect of elevated serum urate on blood pressure remains uncertain (27). Therefore, the causal character of the association between uric acid and cardiovascular events always remains a topic of debate because hyperuricaemia is very often associated with other risk factors such as dyslipidaemia, hypertension, obesity or diabetes. Hence, one cannot exclude an inverse causality or the effect of residual confounding factors, such as alcohol or excessive consumption of certain sugars. In this respect, in the Swiss investigation, conducted among a large group of general practitioners, the patients who presented with hyperuricaemia and/or gout were hypertensive in nearly 70% of cases and diabetic in ∼25% of cases (3). A quarter of these patients also had chronic kidney disease. Of note, in hyperuricemic adolescents with newly diagnosed hypertension, lowering uric acid has been reported to lower BP suggesting a pathogenic role of uric acid in the development of hypertension in some subjects (28). However, similar results were not always obtained in adults (27, 29). Most recent studies investigated the impact on cardiovascular risk of the trajectory of the uric acid level over several decades in younger populations. In this context, the CARDIA study showed that subjects (men and women) whose uric acid level increases the most over an average period of 10 years have a 2.89 fold increase in the risk of developing heart disease, heart failure, or a cerebrovascular events when compared with those whose uric acid level remains stable over time (30). As expected, hypertension, diabetes and obesity were much more frequent in subjects whose uric acid level increased over time.
Why is hyperuricaemia related to an elevated cardiovascular risk?
A priori, one could simply conclude that hyperuricaemia and gout are associated with a high cardiovascular risk because they develop in patients whose intrinsic cardiovascular risk is elevated, such as patients with hypertension, diabetes, renal failure, and ischaemic cardiomyopathy. However, looking at renal damages caused by hyperuricaemia, histological lesions are characterised by uric acid crystalline and also microcrystalline deposits associated with interstitial inflammation and renal fibrosis indicating that an excess of uric acid in itself causes tissue lesions. Today, numerous experimental studies have enable to better define the negative impact of hyperuricaemia and gout on the cardiovascular system (18, 19). As indicated in Figure 1, several mechanisms are involved, one of which is an accumulation of uric acid crystals in tissues (including in the heart and certain vessels (31, 32), the development of systemic inflammation with activation of neutrophils and macrophages and stimulation of pro-inflammatory cytokines associated with an inflammasome activation, endothelial dysfunction, and increase oxidative stress (33). These factors, some of which are responsible for joint disorders, are nowadays recognized as being implicated in the development of atherosclerosis and cardiovascular complications (34, 35). Another mechanism of toxicity of uric acid is the intracellular deposition of uric acid leading to elevated concentrations of uric acid within cells (36, 37). This may occur in the kidneys and in the liver. In this latter organ, intracellular accumulation of uric acid is thought to mediate oxidative stress to the mitochondria and has been associated with metabolic effects such as insulin resistance and hepatic fat accumulation and the development of atherosclerosis (37). As intracellular uric acid levels cannot be measured, the determination of plasma xanthine oxidase activity may be another diagnostic approach to identify patients in whom intracellular accumulation of uric acid may occur (38).
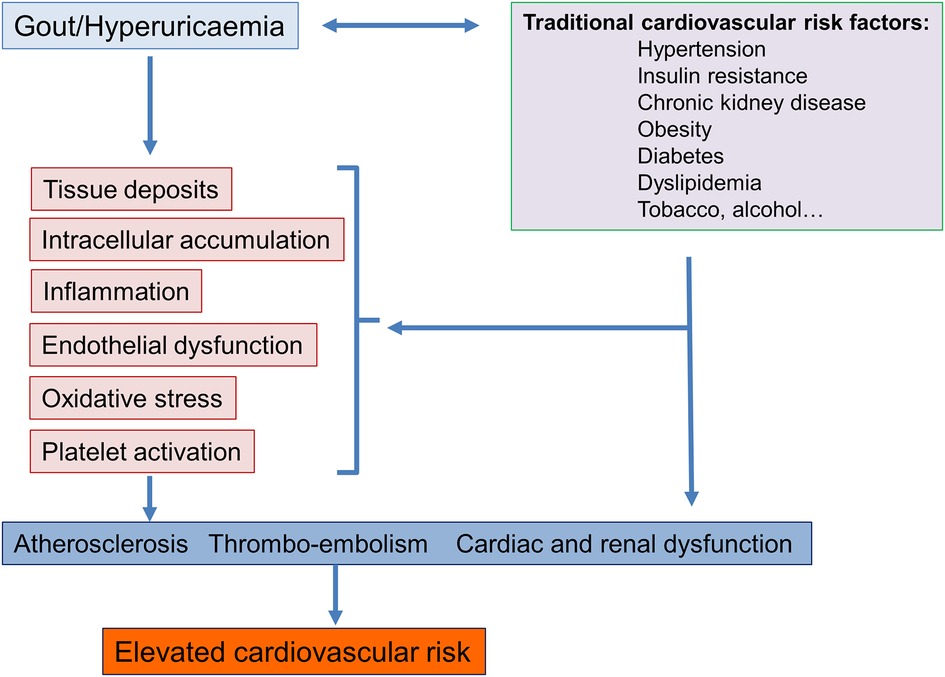
Figure 1. Potential mechanisms involved in the association between gout and hyperuricaemia and an elevated cardiovascular risk.
Prevention of cardiovascular complications in gout and hyperuricaemia: which target?
As discussed above, the main therapeutic objective of the treatment of gout and hyperuricaemia is to decrease the plasma uric acid levels to below 360 μmol/L in order to prevent tissue deposits and gout attacks (39). Is this target adequate to prevent cardiovascular complications associated with hyperuricaemia? This is the question asked by a working group of the Italian Hypertension Society in its project entitled URRAH for “Uric acid Right for heArt Health project” (40). In this program, several threshold analyses were performed using data from the general population of Italy that involved 23,475 subjects with an average age of 57 ± 15 years, 49% of whom were women and whose average blood pressure was 143/85 ± 24/13 mmHg. The subjects were followed for nearly 20 years. As indicated in Table 1, the thresholds of the plasma uric acid levels associated with the highest risk of cardiovascular outcomes are all lower than the threshold actually recommended for the prevention of gout (40–43). These observations thus pose the question of future recommendations but these thresholds need to be validated in prospective randomised controlled studies. One possible explanation may be that some patients with an apparently “normal” serum uric acid develop cardiovascular complications due to the intracellular accumulation of uric acid. More recently, the same Italian authors proposed using the ratio of urinary uric acid/creatinine as a predictive factor of cardiovascular risk, a ratio greater than 5.35 having good predictive value of the cardiovascular risk associated with hyperuricaemia (44).
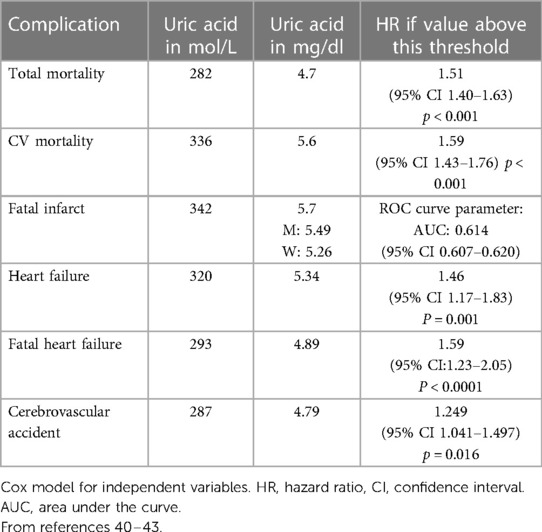
Table 1. Thresholds of plasma levels of uric acid associated with better prevention of events according to the URRAH (uric acid right for heart health) project of the Italian hypertension society.
Impact of uric acid lowering drugs on cardiovascular and renal complications
If hyperuricaemia and gout should play a significant role in the development of cardiovascular and renal outcomes, a decrease in uric acid levels brought about by xanthine oxidase inhibitors (allopurinol or febuxostat) or an inhibition of the inflammation caused by deposits of uric acid should be accompanied by a significant reduction of mortality and cardiovascular and renal events. Today the level of evidence of such protection from a reduction of uricemia remains low due to the heterogeneity of the results. Several cohort studies have suggested that treatment with allopurinol is associated with a reduction in cardiac events, such as myocardial infarction or heart failure (45–48). Patients on a high dose of allopurinol appear to have a lower incidence of cardiovascular complications than those on low doses (45). However, these findings have not always been confirmed (49). In a meta-analysis of 10 clinical studies with 738 participants systolic BP decreased by 3.3 mm Hg (95% confidence interval [CI], 1.4–5.3 mm Hg; P = .001) and diastolic BP decreased by 1.3 mm Hg (95% CI, 0.1–2.5 mm Hg; P = .03) in patients treated with allopurinol when compared with the control group (50).
A decrease in the incidence of cardiovascular outcomes has been reported with treatments that inhibit the inflammatory reaction in gout, such as colchicine and interleukin 1 inhibitors, but these treatments are not frequently used chronically (19). With regard to xanthine oxidase inhibitors (allopurinol, febuxostat), the ALL-HEARTstudy, a multicentre, prospective, randomised, open-label, blinded-endpoint trial, 5,937 patients with ischemic heart disease but no history of gout were randomly assigned to receive allopurinol or usual care (51). After a mean follow-up time of 4.8 years, there was no difference in the incidence of cardiovascular endpoints such as non-fatal myocardial infarction, non-fatal stroke, or cardiovascular death or in all-cause mortality. Based on these results, authors concluded that allopurinol should not be used for the secondary prevention of cardiovascular events in patients with ischaemic heart disease. More recent studies compared the cardiovascular effects of allopurinol and febuxostat in patients with a high cardiovascular risk. The first studies comparing two xanthine oxidase inhibitors [CARES (52), FAST (53)] did not show superiority of one compound over the other. In fact, a slight increase in the cardiovascular risk was observed on febuxostat, which was also seen in a large national monitoring study in Austria (54). However, this difference was not seen in the analysis of another large registry in Korea (55). Moreover, major flaws in the conduct of the CARES trial have been identified, which may relativize the difference in cardiovascular risk observed between allopurinol and febuxostat (56). In the FREED (Febuxostat for Cerebral and CaRdiorenovascular Events PrEvEntion StuDy) study, which included elderly patients with hyperuricaemia and an elevated risk of cerebral, cardiovascular or renal complications, patients were randomised to receive either febuxostat for 3 years or a conventional treatment (57). The use of febuxostat was associated with a significant decrease in the primary objective, which comprised 3 elements (cerebral, cardiac and renal events) (23.3% vs. 28.7%, p = 0.02). However, the statistical significance was essentially due to a decrease in the risk of albuminuria (58). More recently, the analysis of the largest cohort allowing allopurinol to be compared to febuxostat did not demonstrate any difference between the two treatments in terms of cardiovascular events. Nonetheless, the risk of total mortality was 16% lower in the febuxostat group (59).
Among the new drugs that have an effect on uric acid, sodium-glucose cotransporter 2 (SGLT2) inhibitors should be mentioned since they have a well-documented uricosuric effect. In a meta-analysis of 62 studies, SGLT2 inhibitors reduced circulating uric acid by 0.63 mg/dl (95% CI 0.59–0.61; 38 μmol/L, 95% CI 41–35) (60). In another epidemiological study using the Danish nationwide health registries, the three year risk of gout was assessed in 11,047 pairs of matched SGLT2 inhibitors and glucagon-like peptide-1 receptor agonists (GLP1-RA) users (61). The incidence rate of gout was significantly lower in patients initiated with a SGLT2 inhibitors with a hazard ratio of 0.58 (0.44 to 0.75).The current hypothesis on the mechanisms whereby SGLT2 inhibitors lower serum uric acid is that glucose promotes urinary uric acid excretion through the high urine flow rate induced by the glycosuria. SGLT2-inhibitors-induced uricosuria is mediated by cellular mechanisms involving the transporters URAT1 and the suppression of the activity of GLUT9 transporting both glucose and uric acid (62). The SIRTUIN pathway may also be involved by reducing xanthine oxidase activity and hence serum uric acid levels. In addition, several studies have recently demonstrated that the SGLT2 inhibitors decrease the risk of gout in patients with type 2 diabetes (63–66, 67). The contribution of the reduction in uric acid levels to the observed cardiovascular and renal protection induced by SGLT2 inhibitors is now being evaluated (68).
With regard to renal protection by xanthine oxidase inhibitors, several reviews have concluded that there is insufficient evidence to support the renoprotective effects of urate-lowering agents in CKD patients with hyperuricemia, but some specific patients groups may benefit from lowering their uric acid levels (6, 7, 69–71). Three prospective, randomised, placebo-controlled studies (FEATHER (72), PERL and CKD-FIX (73) were conducted to demonstrate the ability of these drugs to slow the worsening of renal function in patients with stage 3 chronic renal failure, one of which involved patients with type 1 diabetes (74). These three studies did not demonstrate a significant effect of the inhibition of xanthine oxidase on worsening of renal function. However, it is possible that they included patients with renal stages that were too advanced to be slowed down. To support this hypothesis, a post-hoc analysis of the FEATHER trial has shown that febuxostat retarded the decline in kidney function among stage 3 CKD patients with asymptomatic hyperuricemia without proteinuria (75). Earlier treatment in CKD patients should therefore be studied in more detail.
Conclusions
Asymptomatic hyperuricaemia and gout are two clinical conditions associated with a high risk of cardiovascular events and mortality. One of the main reasons is that hyperuricaemia develops mainly in patients at high cardiovascular risk. Nonetheless, there is growing experimental evidence suggesting that hyperuricaemia contributes to the progression of cardiovascular pathologies through tissue deposits and intracellular uric acid accumulation leading to a chronic inflammation, particularly in patients with gout. Today, it is not recommended to treat asymptomatic hyperuricaemia, unless the uric acid level is very elevated. Recently, the safety of reaching lower uric acid levels (independently of the drug class used) has been questioned in line with the U-shaped association of urate with mortality in some observational studies (76). Nonetheless, measurement of the uric acid level is now called for in several recommendations of international hypertension societies to optimise management of cardiovascular risk factors (9, 77). Current data suggest that gout and hyperuricaemia are modifiable cardiovascular risk factors, but to prevent the development of cardiovascular complications, it is possible that one must reach uric acid thresholds that are lower than those currently recommended as suggested by Italian researchers, but this remains to be demonstrated in randomised prospective studies. To reach these targets, xanthine oxidase inhibitors remain the first-line treatment, but treatment persistence is often low (78). In the long-term management of hyperuricaemia and the prevention of gout attacks, febuxostat is as effective as allopurinol but with better tolerance and persistence of the treatment (78) which allows a larger number of patients to be correctly treated. The place of new therapeutic approaches such as SGLT2 inhibition remains to be precised. Today, there is a consensus to further investigate the role of uric acid in the development of renal and cardio-metabolic complications through newly designed clinical trials using all new technologies (38, 79) to test the real benefits of lowering uric acid in hyperuricemic subjects.
Author contributions
The author confirms being the sole contributor of this work and has approved it for publication
Acknowledgment
This publication was supported by a grant from Menarini GmbH, Switzerland to MB.
Conflict of interest
The author has received speakers fees from Menarini, Servier, Sanofi, Bayer, Boehringer Ingelheim.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hisatome I, Li P, Miake J, Taufiq F, Mahati E, Maharani N, et al. Uric acid as a risk factor for chronic kidney disease and cardiovascular disease- Japanese guideline on the management of asymptomatic hyperuricemia. Circ J. (2021) 85:130–8. doi: 10.1253/circj.CJ-20-0406
2. FitzGerald JD, Dalbeth N, Mikuls T, Brignardello-Petersen R, Guyatt G, Abeles AM, et al. 2020 American college of rheumatology guideline for the management of gout. Arthritis Care Res (Hoboken). (2020) 72(6):744–60. doi: 10.1002/acr.24180. Erratum in: Arthritis Care Res (Hoboken). 2020 Aug;72(8):1187. Erratum in: Arthritis Care Res (Hoboken). (2021) 73(3):458.32391934
3. Meier R, di Gangi S, Valeri F, Rosemann T, Zechmann S. Gout management in Swiss primary care—a retrospective observational study. Swiss Med Wkly. (2020) 150:w20209. doi: 10.4414/smw.2020.20209
4. Russell MD, Rutherford AI, Ellis B, Norton S, Douiri A, Gulliford MC, et al. Management of gout following 2016/2017 European (EULAR) and British (BSR) guidelines: an interrupted time-series analysis in the United Kingdom. The Lancet Regional Health—Europe. (2022) 18:100416. doi: 10.1016/j.lanepe.2022.100416
5. Richette P, Doherty M, Pascual E, Barskova V, Becce F, Castañeda-Sanabria J, et al. 2016 Updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis. (2017) 76(1):29–42. doi: 10.1136/annrheumdis-2016-209707
6. Goldberg A, Garcia-Arroyo F, Sasai F, Rodriguez-Iturbe B, Sanchez-Lozada LG, Lanaspa MA, et al. Mini review: reappraisal of uric acid in chronic kidney disease. Am J Nephrol. (2021) 52:837–44. doi: 10.1159/000519491
7. Johnson RJ, Sanchez Lozada LG, Lanaspa MA, Piani F, Borghi C. Uric acid and chronic kidney disease: still more to do. Kidney Int Rep. (2023) 8:229–39. doi: 10.1016/j.ekir.2022.11.016
8. Johnson RJ, Sanchez-Lozada LG, Mazzali M, Feig DI, Kanbay M, Sautin YY. What are the key arguments against uric acid as a true risk factor for hypertension? Hypertension. (2013) 61:948–51. doi: 10.1161/HYPERTENSIONAHA.111.00650
9. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. Authors/Task Force M. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European society of cardiology and the European society of hypertension: the task force for the management of arterial hypertension of the European society of cardiology and the European society of hypertension. J Hypertens. (2018) 36(10):1953–2041. doi: 10.1097/HJH.0000000000001940
10. Gertler MM, Garn SM, Levine SA. Serum uric acid in relation to age and physique in health and in coronary heart disease. Ann Intern Med. (1951) 34:1421–31. doi: 10.7326/0003-4819-34-6-1421
11. Culleton BF, Larson MG, Kannel WB, Levy D. Serum uric acid and risk for cardiovascular disease and death: the framingham heart study. Ann Intern Med. (1999) 131:7–13. doi: 10.7326/0003-4819-131-1-199907060-00003
12. Fang J, Alderman MH. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971-1992. National health and nutrition examination survey. Jama. (2000) 283:2404–10. doi: 10.1001/jama.283.18.2404
13. Ding N, He L, Li C, Su Y. Uric acid and blood pressure in NHANES dated from 2009 to 2018: a cross-sectional research. Nutr Metab Cardiovasc Dis. (2022) 32:2568–78. doi: 10.1016/j.numecd.2022.08.017
14. Li B, Chen L, Hu X, Tan T, Yang J, Bao W, et al. Association of Serum uric acid with all-cause and cardiovascular mortality in diabetes. Diabetes Care. (2023) 46(2):425–33.36490263
15. Saito Y, Tanaka A, Node K, Kobayashi Y. Uric acid and cardiovascular disease: a clinical review. J Cardiol. (2021) 78:51–7. doi: 10.1016/j.jjcc.2020.12.013
16. Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. (2008) 359:1811–21. doi: 10.1056/NEJMra0800885
17. Borghi C, Agabiti-Rosei E, Johnson RJ, Kielstein JT, Lurbe E, Mancia G, et al. Hyperuricaemia and gout in cardiovascular, metabolic and kidney disease. Eur J Intern Med. (2020) 80:1–11. doi: 10.1016/j.ejim.2020.07.006
18. Hansildaar R, Vedder D, Baniaamam M, Tausche AK, Gerritsen M, Nurmohamed MT. Cardiovascular risk in inflammatory arthritis: rheumatoid arthritis and gout. Lancet Rheumatol. (2021) 3:e58–70. doi: 10.1016/S2665-9913(20)30221-6
19. Choi HK, McCormick N, Yokose C. Excess comorbidities in gout: the causal paradigm and pleiotropic approaches to care. Nat Rev Rheumatol. (2022) 18:97–111. doi: 10.1038/s41584-021-00725-9
20. Li M, Hu X, Fan Y, Li K, Zhang X, Hou W, et al. Hyperuricemia and the risk for coronary heart disease morbidity and mortality a systematic review and dose-response meta-analysis. Sci Rep. (2016) 6:19520. doi: 10.1038/srep19520
21. Rahimi-Sakak F, Maroofi M, Rahmani J, Bellissimo N, Hekmatdoost A. Serum uric acid and risk of cardiovascular mortality: a systematic review and dose-response meta-analysis of cohort studies of over a million participants. BMC Cardiovasc Disord. (2019) 19:218. doi: 10.1186/s12872-019-1215-z
22. Keenan T, Zhao W, Rasheed A, Ho WK, Malik R, Felix JF, et al. Causal assessment of Serum urate levels in cardiometabolic diseases through a Mendelian randomization study. J Am Coll Cardiol. (2016) 67:407–16. doi: 10.1016/j.jacc.2015.10.086
23. Yang F, Hu T, Cui H. Serum urate and heart failure: a bidirectional Mendelian randomization study. Eur J Prev Cardiol. (2022) 29:1570–8. doi: 10.1093/eurjpc/zwac100
24. Kleber ME, Delgado G, Grammer TB, Silbernagel G, Huang J, Kramer BK, et al. Uric acid and cardiovascular events: a Mendelian randomization study. J Am Soc Nephrol. (2015) 26:2831–8. doi: 10.1681/ASN.2014070660
25. Zhu J, Zeng Y, Zhang H, Qu Y, Ying Z, Sun Y, et al. The association of hyperuricemia and gout with the risk of cardiovascular diseases: a cohort and Mendelian randomization study in UK biobank. Front Med (Lausanne). (2021) 8:817150. doi: 10.3389/fmed.2021.817150
26. Gill D, Cameron AC, Burgess S, Li X, Doherty DJ, Karhunen V, et al. Urate, blood pressure, and cardiovascular disease: evidence from Mendelian randomization and meta-analysis of clinical trials. Hypertension. (2021) 77:383–92. doi: 10.1161/HYPERTENSIONAHA.120.16547
27. Sanchez-Lozada LG, Rodriguez-Iturbe B, Kelley EE, Nakagawa T, Madero M, Feig DI, et al. Uric acid and hypertension: an update with recommendations. Am J Hypertens. (2020) 33:583–94. doi: 10.1093/ajh/hpaa044
28. Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. (2008) 300:924–32. doi: 10.1001/jama.300.8.924
29. Gunawardhana L, McLean L, Punzi HA, Hunt B, Palmer RN, Whelton A, et al. Effect of febuxostat on ambulatory blood pressure in subjects with hyperuricemia and hypertension: a phase 2 randomized placebo-controlled study. J Am Heart Assoc. (2017) 6(11):e006683. doi: 10.1161/JAHA.117.006683
30. Morikawa N, Bancks MP, Yano Y, Kuwabara M, Gaffo AL, Duprez DA, et al. Serum urate trajectory in young adulthood and incident cardiovascular disease events by middle age: cARDIA study. Hypertension. (2021) 78:1211–8. doi: 10.1161/HYPERTENSIONAHA.121.17555
31. Klauser AS, Halpern EJ, Strobl S, Gruber J, Feuchtner G, Bellmann-Weiler R, et al. Dual-Energy computed tomography detection of cardiovascular monosodium urate deposits in patients with gout. JAMA Cardiol. (2019) 4:1019–28. doi: 10.1001/jamacardio.2019.3201
32. Hammer HB, Rollefstad S, Semb AG, Jensen G, Karoliussen LF, Terslev L, et al. Urate crystal deposition is associated with inflammatory markers and carotid artery pathology in patients with intercritical gout: results from the NOR-gout study. RMD Open. (2022) 8(2):e002348. doi: 10.1136/rmdopen-2022-002348
33. Diaz-Torne C, Ortiz MA, Garcia-Guillen A, Jeria-Navarro S, Sainz L, Fernandez-Sanchez S, et al. The inflammatory role of silent urate crystal deposition in intercritical gout. Rheumatology (Oxford). (2021) 60:5463–72. doi: 10.1093/rheumatology/keab335
34. Ruparelia N, Chai JT, Fisher EA, Choudhury RP. Inflammatory processes in cardiovascular disease: a route to targeted therapies. Nat Rev Cardiol. (2017) 14:133–44. doi: 10.1038/nrcardio.2016.185
35. Strandberg TE, Kovanen PT. Coronary artery disease: “gout” in the artery? Eur Heart J. (2021) 42:2761–4. doi: 10.1093/eurheartj/ehab276
36. El Ridi R, Tallima H. Physiological functions and pathogenic potential of uric acid: a review. J Adv Res. (2017) 8:487–93. doi: 10.1016/j.jare.2017.03.003
37. Kimura Y, Tsukui D, Kono H. Uric acid in inflammation and the pathogenesis of atherosclerosis. Int J Mol Sci. (2021) 22(22):12394. doi: 10.3390/ijms222212394
38. Gondouin B, Jourde-Chiche N, Sallee M, Dou L, Cerini C, Loundou A, et al. Plasma Xanthine oxidase activity is predictive of cardiovascular disease in patients with chronic kidney disease, independently of uric acid levels. Nephron. (2015) 131:167–74. doi: 10.1159/000441091
39. O'Dell JR, Brophy MT, Pillinger MH, Neogi T, Palevsky PM, Wu H, et al. Comparative effectiveness of allopurinol and febuxostat in gout management. NEJM Evid. (2022) 1(3):10.1056/evidoa2100028. doi: 10.1056/evidoa2100028
40. Maloberti A, Giannattasio C, Bombelli M, Desideri G, Cicero AFG, Muiesan ML, et al. Hyperuricemia and risk of cardiovascular outcomes: the experience of the URRAH (uric acid right for heart health) project. High Blood Press Cardiovasc Prev. (2020) 27:121–8. doi: 10.1007/s40292-020-00368-z
41. Muiesan ML, Salvetti M, Virdis A, Masi S, Casiglia E, Tikhonoff V, et al. Serum uric acid, predicts heart failure in a large Italian cohort: search for a cut-off value the URic acid right for heArt health study. J Hypertens. (2021) 39:62–9. doi: 10.1097/HJH.0000000000002589
42. Tikhonoff V, Casiglia E, Spinella P, Barbagallo CM, Bombelli M, Cicero AFG, et al. Identification of a plausible serum uric acid cut-off value as prognostic marker of stroke: the uric acid right for heart health (URRAH) study. J Hum Hypertens. (2022) 36:976–82. doi: 10.1038/s41371-021-00613-5
43. Virdis A, Masi S, Casiglia E, Tikhonoff V, Cicero AFG, Ungar A, et al. From the working group on uric A and cardiovascular risk of the Italian society of H. Identification of the uric acid thresholds predicting an increased total and cardiovascular mortality over 20 years. Hypertension. (2020) 75:302–8. doi: 10.1161/HYPERTENSIONAHA.119.13643
44. Casiglia E, Tikhonoff V, Virdis A, Grassi G, Angeli F, Barbagallo CM, et al. Serum uric acid/serum creatinine ratio as a predictor of cardiovascular events. Detection of prognostic cardiovascular cut-off values. J Hypertens. (2023) 41:180–6. doi: 10.1097/HJH.0000000000003319
45. Wei L, Mackenzie IS, Chen Y, Struthers AD, MacDonald TM. Impact of allopurinol use on urate concentration and cardiovascular outcome. Br J Clin Pharmacol. (2011) 71:600–7. doi: 10.1111/j.1365-2125.2010.03887.x
46. Grimaldi-Bensouda L, Alpérovitch A, Aubrun E, Danchin N, Rossignol M, Abenhaim L, et al. Impact of allopurinol on risk of myocardial infarction. Ann Rheum Dis. (2015) 74:836–42. doi: 10.1136/annrheumdis-2012-202972
47. Thanassoulis G, Brophy JM, Richard H, Pilote L. Gout, allopurinol use, and heart failure outcomes. Arch Intern Med. (2010) 170:1358–64. doi: 10.1001/archinternmed.2010.198
48. Yu W, Cheng JD. Uric acid and cardiovascular disease: an update from molecular mechanism to clinical perspective. Front Pharmacol. (2020) 11:582680. doi: 10.3389/fphar.2020.582680
49. White WB. Gout, Xanthine oxidase inhibition, and cardiovascular outcomes. Circulation. (2018) 138:1127–9. doi: 10.1161/CIRCULATIONAHA.118.036148
50. Agarwal V, Hans N, Messerli FH. Effect of allopurinol on blood pressure: a systematic review and meta-analysis. J Clin Hypertens (Greenwich). (2013) 15:435–42. doi: 10.1111/j.1751-7176.2012.00701.x
51. Mackenzie IS, Hawkey CJ, Ford I, Greenlaw N, Pigazzani F, Rogers A, et al. Allopurinol versus usual care in UK patients with ischaemic heart disease (ALL-HEART): a multicentre, prospective, randomised, open-label, blinded-endpoint trial. Lancet. (2022) 400:1195–205. doi: 10.1016/S0140-6736(22)01657-9
52. White WB, Saag KG, Becker MA, Borer JS, Gorelick PB, Whelton A, et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med. (2018) 378:1200–10. doi: 10.1056/NEJMoa1710895
53. Mackenzie IS, Ford I, Nuki G, Hallas J, Hawkey CJ, Webster J, et al. Long-term cardiovascular safety of febuxostat compared with allopurinol in patients with gout (FAST): a multicentre, prospective, randomised, open-label, non-inferiority trial. Lancet. (2020) 396:1745–57. doi: 10.1016/S0140-6736(20)32234-0
54. Weisshaar S, Litschauer B, Reichardt B, Gruber F, Leitner S, Sibinovic S, et al. Cardiovascular risk and mortality in patients with hyperuricemia treated with febuxostat or allopurinol: a retrospective nation-wide cohort study in Austria 2014-2017. Rheumatol Int. (2022) 42:1597–603. doi: 10.1007/s00296-022-05139-8
55. Zhang M, Solomon DH, Desai RJ, Kang EH, Liu J, Neogi T, et al. Assessment of cardiovascular risk in older patients with gout initiating febuxostat versus allopurinol: population-based cohort study. Circulation. (2018) 138:1116–26. doi: 10.1161/CIRCULATIONAHA.118.033992
56. Jansen T, Janssen M. Gout lessons from 2018: cARES, a direct comparison of febuxostat vs allopurinol, and CANTOS, IL1 blocker for cardiovascular risk minimisation. Clin Rheumatol. (2019) 38:263–5. doi: 10.1007/s10067-018-4396-4
57. Kojima S, Matsui K, Hiramitsu S, Hisatome I, Waki M, Uchiyama K, et al. Febuxostat for cerebral and CaRdiorenovascular events PrEvEntion StuDy. Eur Heart J. (2019) 40:1778–86. doi: 10.1093/eurheartj/ehz119
58. Messerli FH, Burnier M. Cardiovascular disease and uric acid: is the not-so-innocent bystander becoming a true culprit and does the US black box warning for febuxostat indicate that not all uric acid lowering is beneficial? Eur Heart J. (2019) 40:1787–9. doi: 10.1093/eurheartj/ehz199
59. Shin A, Choi SR, Han M, Ha YJ, Lee YJ, Lee EB, et al. Cardiovascular safety associated with febuxostat versus allopurinol among patients with gout: update with accumulated use of febuxostat. Semin Arthritis Rheum. (2022) 56:152080. doi: 10.1016/j.semarthrit.2022.152080
60. Zhao Y, Xu L, Tian D, Xia P, Zheng H, Wang L, et al. Effects of sodium-glucose co-transporter 2 (SGLT2) inhibitors on serum uric acid level: a meta-analysis of randomized controlled trials. Diabetes Obes Metab. (2018) 20:458–62. doi: 10.1111/dom.13101
61. Lund LC, Hojlund M, Henriksen DP, Hallas J, Kristensen KB. Sodium-glucose cotransporter-2 inhibitors and the risk of gout: a danish population based cohort study and symmetry analysis. Pharmacoepidemiol Drug Saf. (2021) 30:1391–5. doi: 10.1002/pds.5252
62. Novikov A, Fu Y, Huang W, Freeman B, Patel R, van Ginkel C, et al. SGLT2 Inhibition and renal urate excretion: role of luminal glucose, GLUT9, and URAT1. Am J Physiol Renal Physiol. (2019) 316:F173–f185. doi: 10.1152/ajprenal.00462.2018
63. Chung M-C, Hung P-H, Hsiao P-J, Wu L-Y, Chang C-H, Wu M-J, et al. Association of sodium-glucose transport protein 2 inhibitor use for type 2 diabetes and incidence of gout in Taiwan. JAMA Network Open. (2021) 4:e2135353–e2135353. doi: 10.1001/jamanetworkopen.2021.35353
64. Somagutta MKR, Luvsannyam E, Jain M, Cuddapah GV, Pelluru S, Mustafa N, et al. Sodium glucose co-transport 2 inhibitors for gout treatment. Discoveries (Craiova). (2022) 10:e152. doi: 10.15190/d.2022.11
65. Suijk DLS, van Baar MJB, van Bommel EJM, Iqbal Z, Krebber MM, Vallon V, et al. SGLT2 Inhibition and uric acid excretion in patients with type 2 diabetes and normal kidney function. Clin J Am Soc Nephrol. (2022) 17:663–71. doi: 10.2215/CJN.11480821
66. Sheu WH. Lowering the risk of gout: another benefits from the use of sodium-glucose cotransporter 2 inhibitors. J Diabetes Investig. (2020) 11:1115–6. doi: 10.1111/jdi.13254
67. Fralick M, Chen SK, Patorno E, Kim SC. Assessing the risk for gout with sodium-glucose cotransporter-2 inhibitors in patients with type 2 diabetes: a population-based cohort study. Ann Intern Med. (2020) 172:186–94. doi: 10.7326/M19-2610
68. Bailey CJ. Uric acid and the cardio-renal effects of SGLT2 inhibitors. Diabetes Obes Metab. (2019) 21:1291–8. doi: 10.1111/dom.13670
69. Liu X, Qiu Y, Li D, Tan J, Liang X, Qin W. Effectiveness of drug treatments for lowering uric acid on renal function in patients with chronic kidney disease and hyperuricemia: a network meta-analysis of randomized controlled trials. Front Pharmacol. (2021) 12:690557. doi: 10.3389/fphar.2021.690557
70. Chen Q, Wang Z, Zhou J, Chen Z, Li Y, Li S, et al. Effect of urate-lowering therapy on cardiovascular and kidney outcomes: a systematic review and meta-analysis. Clin J Am Soc Nephrol. (2020) 15:1576–86. doi: 10.2215/CJN.05190420
71. Kaul S, Gupta M, Bandyopadhyay D, Hajra A, Deedwania P, Roddy E, et al. Gout pharmacotherapy in cardiovascular diseases: a review of utility and outcomes. Am J Cardiovasc Drugs. (2021) 21:499–512. doi: 10.1007/s40256-020-00459-1
72. Kimura K, Hosoya T, Uchida S, Inaba M, Makino H, Maruyama S, et al. Febuxostat therapy for patients with stage 3 CKD and asymptomatic hyperuricemia: a randomized trial. Am J Kidney Dis. (2018) 72:798–810. doi: 10.1053/j.ajkd.2018.06.028
73. Gonzalez-Martin G, Cano J, Carriazo S, Kanbay M, Perez-Gomez MV, Fernandez-Prado R, et al. The dirty little secret of urate-lowering therapy: useless to stop chronic kidney disease progression and may increase mortality. Clin Kidney J. (2020) 13:936–47. doi: 10.1093/ckj/sfaa236
74. Leoncini G, Barnini C, Manco L, Nobili G, Dotta D, Penso M, et al. Uric acid lowering for slowing CKD progression after the CKD-FIX trial: a solved question or still a dilemma? Clin Kidney J. (2022) 15:1666–74. doi: 10.1093/ckj/sfac075
75. Kataoka H, Mochizuki T, Ohara M, Tsuruta Y, Iwasa N, Yoshida R, et al. Urate-lowering therapy for CKD patients with asymptomatic hyperuricemia without proteinuria elucidated by attribute-based research in the FEATHER study. Sci Rep. (2022) 12:3784. doi: 10.1038/s41598-022-07737-9
76. Perez-Gomez MV, Bartsch LA, Castillo-Rodriguez E, Fernandez-Prado R, Kanbay M, Ortiz A. Potential dangers of Serum urate-lowering therapy. Am J Med. (2019) 132:457–67. doi: 10.1016/j.amjmed.2018.12.010
77. Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International society of hypertension global hypertension practice guidelines. Hypertension. (2020) 75:1334–57. doi: 10.1161/HYPERTENSIONAHA.120.15026
78. Kim A, Kim Y, Kim GT, Ahn E, So MW, Lee SG. Comparison of persistence rates between allopurinol and febuxostat as first-line urate-lowering therapy in patients with gout: an 8-year retrospective cohort study. Clin Rheumatol. (2020) 39:3769–76. doi: 10.1007/s10067-020-05161-w
Keywords: uric acid, hypertension, gout, cardiovascular mortality, tissue deposits, uric acid lowering treatments
Citation: Burnier M (2023) Gout and hyperuricaemia: modifiable cardiovascular risk factors?. Front. Cardiovasc. Med. 10:1190069. doi: 10.3389/fcvm.2023.1190069
Received: 20 March 2023; Accepted: 9 May 2023;
Published: 25 May 2023.
Edited by:
Mateusz Siedlinski, Jagiellonian University Medical College, PolandReviewed by:
Federica Piani, University of Bologna, Italy© 2023 Burnier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michel Burnier bWljaGVsLmJ1cm5pZXJAY2h1di5jaA==
 Michel Burnier
Michel Burnier