- 1Department of Cardiology, Department of Internal Medicine, Chonnam National University Hospital, Gwangju, Republic of Korea
- 2Department of Nuclear Medicine, Chonnam National University Hospital, Gwangju, Republic of Korea
- 3Department of Cardiology, Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Republic of Korea
Introduction: Although most cases of myocardial bridge (MB) are clinically benign, sometimes it can be one of potential threats of myocardial infarction (MI) and life-threatening arrhythmia. In the present study, we present a case of ST-segment elevation MI caused by MB and concomitant vasospasm.
Case Presentation: A 52-year-old woman was brought to our tertiary hospital due to resuscitated cardiac arrest. Because the 12-lead electrocardiogram indicated ST-segment elevation MI, coronary angiogram was promptly commenced, which showed near-total occlusion at the middle portion of left anterior descending coronary artery (LAD). After intracoronary nitroglycerin administration, this occlusion was dramatically relieved, however, systolic compression at this site remained, indicative of myocardial bridge (MB). Intravascular ultrasound also showed eccentric compression with a “half-moon” sign, which is consistent with MB. Coronary computed tomography also showed a bridged coronary segment surrounded by myocardium at the middle portion of LAD. To assess the severity and extent of myocardial damages and ischemia, myocardial single photon emission computed tomography (SPECT) was additionally conducted, showing a moderate fixed perfusion defect around the cardiac apex, suggesting MI. After receiving optimal medical therapy, the patient's clinical symptoms and signs were improved then the patient was discharged from the hospital successfully and uneventfully.
Conclusion: We demonstrated a case of MB-induced ST-segment elevation MI which was confirmed with its perfusion defects via myocardial perfusion SPECT. There have been proposed a number of diagnostic modalities to examine its anatomic and physiologic significance. Among them, myocardial perfusion SPECT can be available as one of useful modalities to evaluate the severity and extent of myocardial ischemia in patients with MB.
1. Introduction
Myocardial bridge (MB) is a congenital coronary anomaly manifested by coronary artery segment tunnelling through the myocardial bands (1), and this term was first mentioned in 1961 in a case report about angiographic narrowing during the systole (2). Although many clinicians tend to consider it to be a benign condition, sometimes it can be clinically fatal (3, 4). In other words, MB may contribute to the development of acute coronary syndrome such as myocardial infarction (MI) or cardiac arrest (5–7). Especially, coronary vasospasm (CVS) may act as a trigger factor for these situations in patients with MB (7). We present a case of ST-segment elevation MI triggered by CVS within the site of MB.
2. Case presentation
A 52-year-old Korean woman with essential hypertension was admitted to our cardiovascular center because of resuscitated cardiac arrest as the chief complaint. Several hours before the presentation, the patient experienced squeezing chest pain and was suddenly collapsed then received bystander cardiopulmonary resuscitation. During the transportation via ambulance, the patient had received electrical defibrillation for documented ventricular fibrillation (Supplementary Figure S1). There was no documentation of previous cardiovascular events. On the physical examination, temperature was 36.5°C, heart rate was 90 beats per minute, respiratory rate was 20 beats per minute, and blood pressure was 120/90 mmHg. A 12-lead electrocardiogram showed abnormal ST-segment elevation with pathologic Q-waves in precordial leads (Figure 1). In the laboratory test, high-sensitivity troponin-I was elevated to 1.332 ng/ml (reference: 0–0.050 ng/ml). Since we initially concluded a diagnosis of ST-segment elevation MI, the catheterization laboratory was activated then emergent coronary angiogram (CAG) was promptly planned for percutaneous coronary intervention.
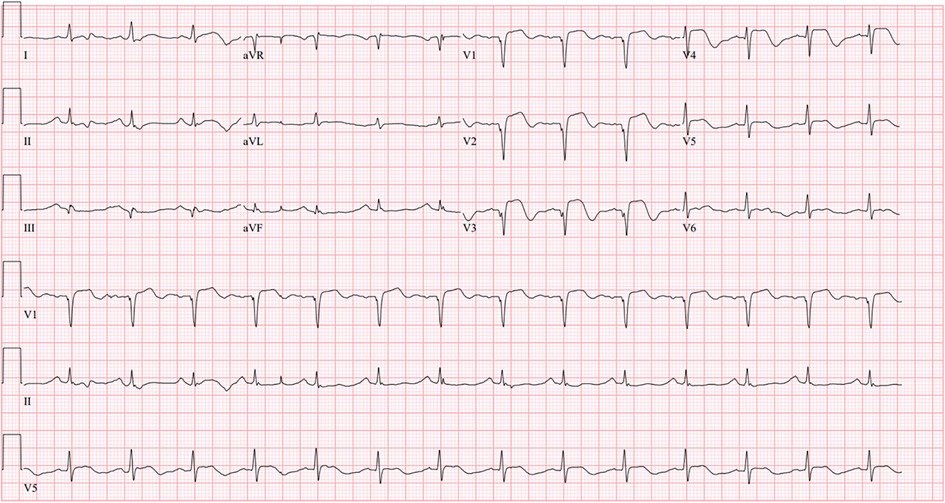
Figure 1. Initial 12-lead electrocardiogram: A 12-lead electrocardiogram showed abnormal ST-segment elevation with pathologic Q-waves in precordial leads.
Initial CAG was performed via the right femoral artery at the catheterization laboratory. It revealed near-total occlusion at the middle portion of left anterior descending coronary artery (LAD) with reduced antegrade coronary flow (Figure 2A). After intracoronary nitroglycerin (I-NTG) administration, however, this stenosis was dramatically relieved (Figure 2B), but there was found systolic compression at the same site, suggestive of MB (Figures 2C,D, Supplementary Video S1). In quantitative coronary analysis, the systolic diameter was 0.84 mm and the diastolic diameter was 1.17 mm, which meant that the diameter change from diastole to systole was about 28.2% (Supplementary Figure S2). For the further evaluation, intravascular ultrasound (IVUS) study was examined with a guidance system (Eagle Eye® Platinum RX Digital IVUS Catheter, Volcano Corporation, Rancho Cordova, CA, USA). In IVUS, eccentric compression was seen in the MB segment with a half-moon-like echo-lucent space between the MB segment and epicardial tissue (Figures 2E,F, Supplementary Video S2). There was no definite evidence of atherosclerotic plaque formation within the MB segment. The cross-sectional area at this segment was 4.99 mm2 during the diastole, and 3.42 mm2 during the systole (Supplementary Figure S3). In multiple-slice coronary computed tomography angiography (CCTA), there was seen a coronary segment surrounded by myocardium at the middle portion of LAD (Figure 2G). We concluded that the final diagnosis of this patient was MI with non-obstructive coronary arteries (MINOCA). The patient was transferred to the intensive care unit for the hemodynamic monitoring.
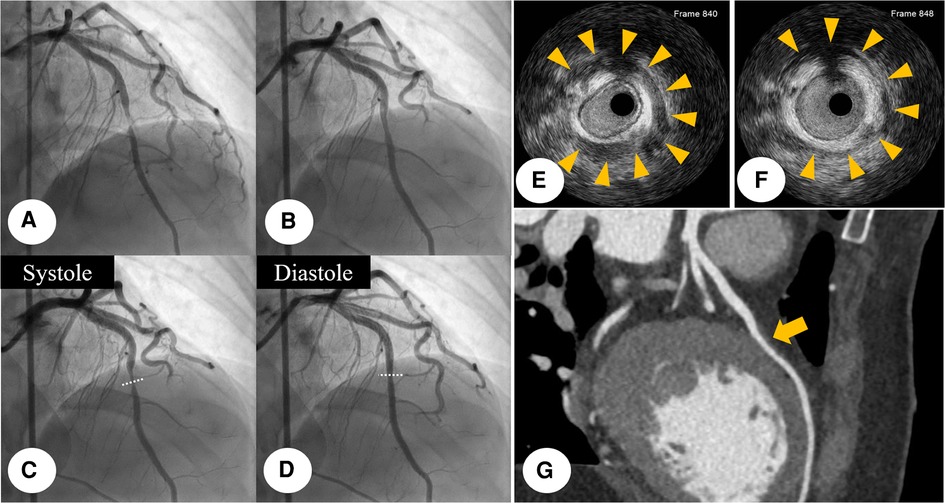
Figure 2. Initial CAG finding: initial CAG demonstrated near-total occlusion (yellowish arrow) at the middle portion of LAD with reduced antegrade coronary flow. (A) This stenosis was dramatically relieved (yellowish arrow), after I-NTG administration (B) After I-NTG administration, systolic compression was seen at the middle portion of LAD, which means MB. (C,D) IVUS was further examined with a guidance system (Eagle Eye® Platinum RX Digital IVUS Catheter, Volcano Corporation, Rancho Cordova, CA, USA), demonstrating eccentric compression with a half-moon-like echo-lucent space between the MB segment and epicardial tissue. (E,F) Multiple-slice CCTA imaging showed a bridged coronary segment surrounded by myocardium at the middle portion of LAD (yellowish arrow). (G) CAG, coronary angiogram; CCTA, coronary computed tomography angiography; I-NTG, intracoronary nitroglycerin; LAD, left anterior descending coronary artery; MB, myocardial bridge.
To determine its functional significance, Tc-99 m methylisobutyl isonitrile single photon emission computed tomography (SPECT) was additionally conducted. It demonstrated a medium-sized, moderate fixed perfusion defect around the apex, downstream to the bridging segment of the LAD, suggesting MI (Figure 3). The cardiac SPECT/CCTA hybrid imaging also confirmed excellent correlation between the extent of perfusion defects and the anatomical location of MB (Figure 4A), which was also correlated with CAG finding (Figure 4B).
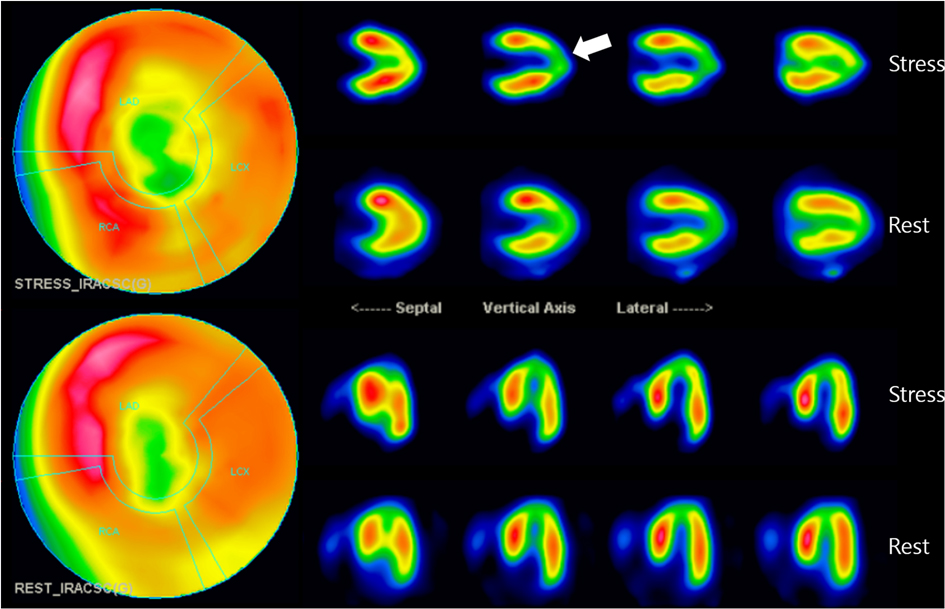
Figure 3. Myocardial perfusion SPECT imaging: SPECT scan showed medium-sized, non-transmural infarction around the apex (whitish arrow). This defect correlates to the downstream myocardium to the bridging segment of the mid-LAD, as marked by arrows. LAD, left anterior descending coronary artery; SPECT, single photon emission computed tomography.

Figure 4. The cardiac SPECT/CCTA hybrid imaging also confirmed excellent correlation between the extent of perfusion defects and the anatomical location of MB, which was well correlated with CAG finding (whitish arrow and yellowish arrow) (A,B). CCTA, coronary computed tomography angiography; MB, myocardial bridge; SPECT, single photon emission computed tomography.
As the patient received optimal antiplatelet agents, high-intensity statins, and non-dihydropyridine calcium channel blockers (CCBs), the patient's clinical symptoms and signs were improved. The patient was discharged from the hospital then has remained symptom-free on follow-up outpatient visits.
3. Discussion and conclusion
MB is one of normal variants of coronary artery which is manifested by intramuscular course of a coronary artery. Although the true prevalence of MB is not fully understood, its angiographic detection seems not uncommon with the detection rates varying from 0.5–12% in the resting CAG to about 40% in I-NTG administration or coronary reactivity testing (8). Since most patients with MBs have no clinical symptoms, many clinicians are reluctant to manage it with intensive medical treatments then just tend to recommend simple observation with not much clinical significance. Although most cases with MBs are clinically benign (7), as suggested in the present case, sometimes it can be one of potential threats of MI and life-threatening arrhythmia (9, 10). According to an observational retrospective study, MB seems to be a potential cause of MI, and its prevalence was significantly higher in patients with MINOCA than in their counterparts (10). Moreover, since MB itself is one of independent risk factors for ischemia-induced myocardial fibrosis and MINOCA (11), it also act as a crucial factor contributing to the onset of fatal arrhythmia (12).
There have been proposed a number of diagnostic modalities to examine its anatomic and physiologic significance (13, 14). Invasive modalities include CAG, and intracoronary imaging/physiology studies. CAG may demonstrate the diameter change between systole and diastole within the segment of MB. “Milking effect” is one of characteristic findings with a significant (≥70%) reduction in minimum lumen diameter (MLA) during the systole and persistent ≥35% decrease in MLA (15). After I-NTG administration, systolic compression can be accentuated by vasodilation of non-bridged coronary segments (16). As shown in the present case, IVUS can show the characteristic “half-moon” sign which is an echo-lucent space between the MB segment and epicardial tissue. Atherosclerotic plaque can also be seen proximal to MB in IVUS. Coronary physiologic studies such as fractional flow reserve can be useful, but its role of MB has remained challenging (14).
Non-invasive modalities include CCTA, stress gated SPECT, and stress echocardiogram. CCTA is able to identify bridging segment surrounded by myocardium (17), whereas stress gated SPECT can visualize myocardial perfusion defects then quantify the degree of myocardial ischemia (18). In the present case, we utilized the myocardial perfusion SPECT. Although most cases of MB can be found in anatomical imaging modalities, functional imaging like myocardial perfusion SPECT can also be feasible and useful to evaluate its functional significance in the form of perfusion defects (19). Lee and his colleagues demonstrated that high-grade MB could induce perfusion defects on territories of LAD and its branches in dipyridamole TI-201 SPECT findings (20). Such findings may reflect the degree of luminal narrowing by systolic contraction (18). A retrospective study by Huang et al. showed abnormal myocardial perfusion in patients with MB at the middle portion of LAD (21).
The treatment options can be subdivided into two categories: (A) pharmacological therapy; (B) interventional therapy. In the pharmacological therapy, beta-blocker is the mainstay treatment which can relieve the hemodynamic disturbance caused by MB through its negative chronotropic effect (15). CCBs are also available with similar pharmacologic mechanisms of beta-blockers. Like in this case, they may be more beneficial in patients with MB and concomitant CVS (13). In the literature review, there have been no available comparative studies of beta-blocker vs. CCB in patients with MB. In contrast, pure vasodilators such as long-acting nitrates should be avoided or applied with caution because they can worsen symptoms by accentuating systolic compression of the MB segment (22). If refractory to pharmacological therapy, interventional therapy should be considered. Interventional therapy includes percutaneous coronary intervention with stenting for the bridged coronary segment, supra-arterial myotomy, or coronary artery bypass grafting.
Meanwhile, we should know that this case shows MINOCA with concomitant presence of MB and CVS. MB seems to be closely related to CVS (11, 23, 24). That is, patients having MB tended to have a higher proportion of CVS compared to those not having MB (11, 25). Although not fully accountable, it is plausible that sustained shear stress (i.e., contraction-relaxation effect) of the site of MB may induce the alteration in endothelial function (11, 23), resulting in coronary vasomotor disorders. Since patients with both of them tend to have a higher risk of adverse cardiac events such as readmission for recurrent angina pectoris (11, 25), therefore, coronary reactivity testing may be a useful diagnostic tool to elicit CVS then verify these high-risk patients (26).
In the present case, we detected the fatal case of MB-induced MINOCA through multimodality imaging tools. While there are a number of useful diagnostic modalities, myocardial perfusion SPECT can be available as one of useful modalities to evaluate the severity and extent of myocardial ischemia in patients with MB.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Chonnam National University Hospital. Written informed consent was obtained from the patient for the publication of any potentially identifiable images or data included in this article.
Author contributions
SO and DH: drafted the manuscript. SO, DH: designed the study methodology. SO, DH, SC: collected the data. YH, JK, YA, and MJ: reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We sincerely thank SO, DH, and MJ for providing and interpreting CAG images. We also thank SC for providing and interpreting myocardial perfusion SPECT images.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1188095/full#supplementary-material
Abbreviations
CAG, coronary angiogram; CCB, calcium channel blocker; CCTA, coronary computed tomography; CVS, coronary vasospasm; I-NTG, intracoronary nitroglycerin; IVUS, intravascular ultrasound; LAD, left anterior descending coronary artery; MB, myocardial bridge; MI, myocardial infarction; MINOCA, with non-obstructive coronary arteries; MLA, minimum lumen diameter; SPECT, single photon emission computed tomography.
References
1. Alegria JR, Herrmann J, Holmes DR Jr., Lerman A, Rihal CS. Myocardial bridging. Eur Heart J. (2005) 26(12):1159–68. doi: 10.1093/eurheartj/ehi203
2. Angelini P, Trivellato M, Donis J, Leachman D R. Myocardial bridges: a review. Prog Cardiovasc Dis. (1983) 26(1):75–88. doi: 10.1016/0033-0620(83)90019-1
3. Ripa C, Melatini MC, Olivieri F, Antonicelli R. Myocardial bridging: a ‘forgotten’ cause of acute coronary syndrome—a case report. Int J Angiol. (2007) 16(3):115–8. doi: 10.1055/s-0031-1278262
4. Thej MJ, Kalyani R, Kiran J. Atherosclerosis and myocardial bridging: not a benign combination. An autopsy case report. J Cardiovasc Dis Res. (2012) 3(2):176–8. doi: 10.4103/0975-3583.95380
5. Abe T, Jujo K, Matsukage T. Myocardial bridging may shed light on one aspect of unexplained myocardial infarction with non-obstructive coronary arteries. Eur Heart J Acute Cardiovasc Care. (2022) 11(6):508–9. doi: 10.1093/ehjacc/zuac074
6. Husmann L, Nkoulou R, Wolfrum M, Kaufmann PA. Myocardial bridging causing infarction and ischaemia. Eur Heart J. (2011) 32(7):790. doi: 10.1093/eurheartj/ehq423
7. Ki YJ. Myocardial bridging presenting as myocardial ischaemia induced cardiac arrest: a case report. BMC Cardiovasc Disord. (2021) 21(1):178. doi: 10.1186/s12872-021-01975-x
8. Tarantini G, Migliore F, Cademartiri F, Fraccaro C, Iliceto S. Left anterior descending artery myocardial bridging: a clinical approach. J Am Coll Cardiol. (2016) 68(25):2887–99. doi: 10.1016/j.jacc.2016.09.973
9. Mizuno A, Nishi Y, Niwa K. Deferring coronary stenting for myocardial bridging by fractional flow reserve and optical coherence tomography. J Invasive Cardiol. (2012) 24(2):E27–9.22294543
10. Matta A, Nader V, Canitrot R, Delmas C, Bouisset F, Lhermusier T, et al. Myocardial bridging is significantly associated to myocardial infarction with non-obstructive coronary arteries. Eur Heart J Acute Cardiovasc Care. (2022) 11(6):501–7. doi: 10.1093/ehjacc/zuac047
11. Montone RA, Gurgoglione FL, Del Buono MG, Rinaldi R, Meucci MC, Iannaccone G, et al. Interplay between myocardial bridging and coronary spasm in patients with myocardial ischemia and non-obstructive coronary arteries: pathogenic and prognostic implications. J Am Heart Assoc. (2021) 10(14):e020535. doi: 10.1161/jaha.120.020535
12. Kazbanov IV, ten Tusscher KH, Panfilov AV. Effects of heterogeneous diffuse fibrosis on arrhythmia dynamics and mechanism. Sci Rep. (2016) 6:20835. doi: 10.1038/srep20835
13. Corban MT, Hung OY, Eshtehardi P, Rasoul-Arzrumly E, McDaniel M, Mekonnen G, et al. Myocardial bridging: contemporary understanding of pathophysiology with implications for diagnostic and therapeutic strategies. J Am Coll Cardiol. (2014) 63(22):2346–55. doi: 10.1016/j.jacc.2014.01.049
14. Aleksandric SB, Djordjevic-Dikic AD, Dobric MR, Giga VL, Soldatovic IA, Vukcevic V, et al. Functional assessment of myocardial bridging with conventional and diastolic fractional flow reserve: vasodilator versus inotropic provocation. J Am Heart Assoc. (2021) 10(13):e020597. doi: 10.1161/JAHA.120.020597
15. Bourassa MG, Butnaru A, Lesperance J, Tardif JC. Symptomatic myocardial bridges: overview of ischemic mechanisms and current diagnostic and treatment strategies. J Am Coll Cardiol. (2003) 41(3):351–9. doi: 10.1016/s0735-1097(02)02768-7
16. Ferreira AG Jr., Trotter SE, Konig B Jr., Decourt LV, Fox K, Olsen EG. Myocardial bridges: morphological and functional aspects. Br Heart J. (1991) 66(5):364–7. doi: 10.1136/hrt.66.5.364
17. Kim PJ, Hur G, Kim SY, Namgung J, Hong SW, Kim YH, et al. Frequency of myocardial bridges and dynamic compression of epicardial coronary arteries: a comparison between computed tomography and invasive coronary angiography. Circulation. (2009) 119(10):1408–16. doi: 10.1161/CIRCULATIONAHA.108.788901
18. Gawor R, Kusmierek J, Plachcinska A, Bienkiewicz M, Drozdz J, Piotrowski G, et al. Myocardial perfusion GSPECT imaging in patients with myocardial bridging. J Nucl Cardiol. (2011) 18(6):1059–65. doi: 10.1007/s12350-011-9406-8
19. Singh D, Vadi SK, Parmar M, Sood A, Mehrotra S, Mittal BR. Role of (99 m)Tc-sestamibi gated SPECT/CT myocardial perfusion imaging in the management of patients with myocardial bridging and its correlation with coronary angiography. J Cardiol Cases. (2017) 15(4):136–40. doi: 10.1016/j.jccase.2016.12.006
20. Lee YS, Moon DH, Shin JW, Park SW, Park SJ, Lee HK. Dipyridamole TI-201 SPECT imaging in patients with myocardial bridging. Clin Nucl Med. (1999) 24(10):759–64. doi: 10.1097/00003072-199910000-00006
21. Huang WS, Chang HD, Yang SP, Tsao TP, Cheng CY, Cherng SC. Abnormal 201Tl myocardial single photon emission computed tomography in energetic male patients with myocardial bridge. Nucl Med Commun. (2002) 23(11):1123–8. doi: 10.1097/00006231-200211000-00013
22. Hongo Y, Tada H, Ito K, Yasumura Y, Miyatake K, Yamagishi M. Augmentation of vessel squeezing at coronary-myocardial bridge by nitroglycerin: study by quantitative coronary angiography and intravascular ultrasound. Am Heart J. (1999) 138(2 Pt 1):345–50. doi: 10.1016/s0002-8703(99)70123-7
23. Herrmann J, Higano ST, Lenon RJ, Rihal CS, Lerman A. Myocardial bridging is associated with alteration in coronary vasoreactivity. Eur Heart J. (2004) 25(23):2134–42. doi: 10.1016/j.ehj.2004.08.015
24. Kim JW, Seo HS, Na JO, Suh SY, Choi CU, Kim EJ, et al. Myocardial bridging is related to endothelial dysfunction but not to plaque as assessed by intracoronary ultrasound. Heart. (2008) 94(6):765–9. doi: 10.1136/hrt.2007.115980
25. Nam P, Choi BG, Choi SY, Byun JK, Mashaly A, Park Y, et al. The impact of myocardial bridge on coronary artery spasm and long-term clinical outcomes in patients without significant atherosclerotic stenosis. Atherosclerosis. (2018) 270:8–12. doi: 10.1016/j.atherosclerosis.2018.01.026
26. Montone RA, Rinaldi R, Del Buono MG, Gurgoglione F, La Vecchia G, Russo M, et al. Safety and prognostic relevance of acetylcholine testing in patients with stable myocardial ischaemia or myocardial infarction and non-obstructive coronary arteries. EuroIntervention. (2022) 18(8):e666–76. doi: 10.4244/eij-d-21-00971
Keywords: cardiac imaging techniques, myocardial bridging, myocardial infarction, coronary angiography, SPECT CT
Citation: Oh S, Hyun DY, Cho S-G, Hong YJ, Kim JH, Ahn Y and Jeong MH (2023) Case report: A fatal case of myocardial infarction due to myocardial bridge and concomitant vasospasm: the role of stress gated SPECT. Front. Cardiovasc. Med. 10:1188095. doi: 10.3389/fcvm.2023.1188095
Received: 16 March 2023; Accepted: 10 May 2023;
Published: 30 May 2023.
Edited by:
Antonios Karanasos, Hippokration General Hospital, GreeceReviewed by:
Hiroki Teragawa, JR Hiroshima Hospital, JapanRocco Antonio Montone, Agostino Gemelli University Polyclinic (IRCCS), Italy
© 2023 Oh, Hyun, Cho, Hong, Kim, Ahn and Jeong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Myung Ho Jeong bXl1bmdob0BjaG9sbGlhbi5uZXQ=
†These authors have contributed equally to this work
 Seok Oh
Seok Oh Dae Young Hyun1,†
Dae Young Hyun1,† Sang-Geon Cho
Sang-Geon Cho Youngkeun Ahn
Youngkeun Ahn