- 1Research Group Cardiovascular Diseases, University of Antwerp, Antwerp, Belgium
- 2Department of Cardiology, Heart Centre Hasselt, Jessa Hospital, Hasselt, Belgium
- 3Faculty of Medicine and Life Sciences, Hasselt University, Hasselt, Belgium
- 4Department of Cardiology, Antwerp University Hospital, Edegem, Belgium
- 5Department of Cardiology, University Hospital Gasthuisberg, Leuven, Belgium
Background: As the prevalence of atrial fibrillation (AF) increases worldwide and AF management becomes ever more diversified and personalised, insights into (regional) AF patient demographics and contemporary AF management are needed. This paper reports the current AF management and baseline demographics of a Belgian AF population recruited for a large multicenter integrated AF study (AF-EduCare/AF-EduApp study).
Methods: We analyzed data from 1,979 AF patients, assessed between 2018 and 2021 for the AF-EduCare/AF-EduApp study. The trial randomised consecutive patients with AF (irrespective of AF history duration) into three educational intervention groups (in person-, online-, and application-based), compared with standard care. Baseline demographics of both the included and excluded/refused patients are reported.
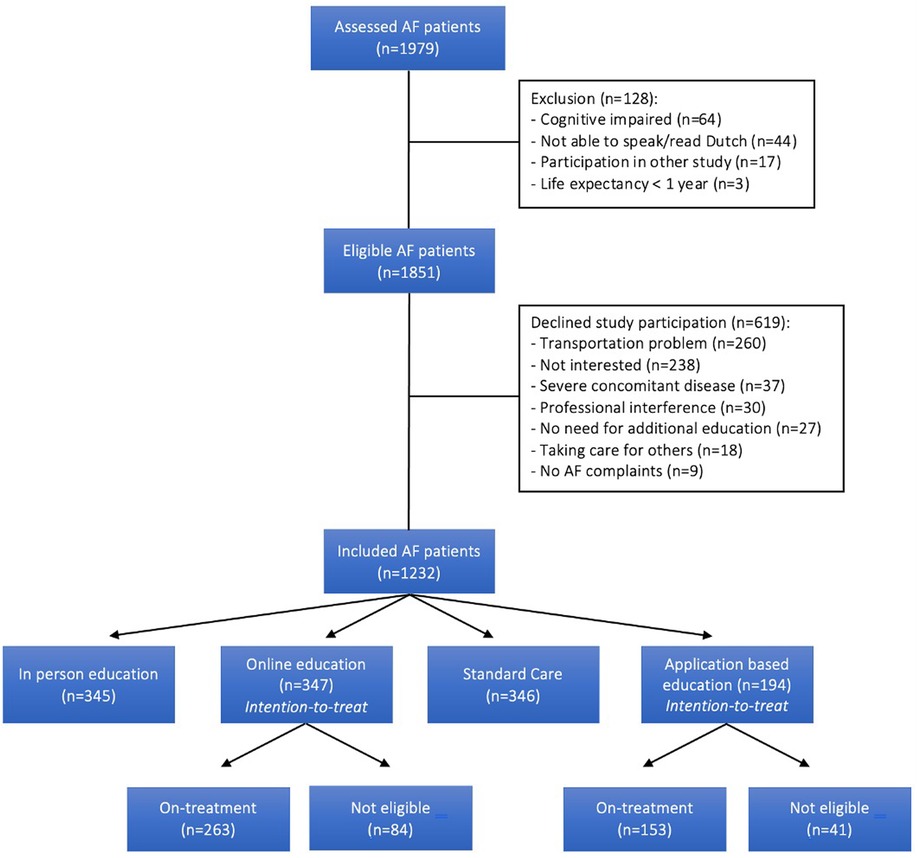
Results: The mean age of the trial population was 71.2 ± 9.1 years, with a mean CHA2DS2-VASc score of 3.4 ± 1.8. Of all screened patients, 42.4% were asymptomatic at presentation. Being overweight was the most common comorbidty, present in 68.9%, while 65.0% were diagnosed with hypertension. Anticoagulation therapy was prescribed in 90.9% of the total population and in 94.0% of the patients with an indication for thromboembolic prophylaxis. Of the 1,979 assessed AF patients, 1,232 (62.3%) were enrolled in the AF-EduCare/AF-EduApp study, with transportation problems (33.4%) as the main reason for refusal/non-inclusion. About half of the included patients were recruited at the cardiology ward (53.8%). AF was first diagnosed, paroxysmal, persistent and permanent in 13.9%, 47.4%, 22.8% and 11.3%, respectively. Patients who refused or were excluded were older (73.3 ± 9.2 vs. 69.8 ± 8.9 years, p < 0.001) and had more comorbidities (CHA2DS2-VASc 3.8 ± 1.8 vs. 3.1 ± 1.7, p < 0.001). The four AF-EduCare/AF-EduApp study groups were comparable across the vast majority of parameters.
Conclusions: The population showed high use of anticoagulation therapy, in line with current guidelines. In contrast to other AF trials about integrated care, the AF-EduCare/AF-EduApp study managed to incorporate all types of AF patients, both out-patient and hospitalised, with very comparable patient demographics across all subgroups. The trial will analyze whether different approaches to patient education and integrated AF care have an impact on clinical outcomes.
Clinical Trial Registration: https://clinicaltrials.gov/ct2/show/NCT03707873?term=af-educare&draw=2&rank=1, identifier: NCT03707873; https://clinicaltrials.gov/ct2/show/NCT03788044?term=af-eduapp&draw=2&rank=1, identifier: NCT03788044.
1. Introduction
Atrial Fibrillation (AF) is an emerging epidemic in Western countries and creates a high burden on patients, healthcare providers and healthcare systems (1). Currently, the lifetime risk for developing AF in adults above 55 years old is 37% and it is estimated that in 2060, AF will affect 17.9 million European citizens (i.e., 3.5% of the total population) (2, 3). This is due to ageing of the population and the increasing prevalence of modifiable AF risk factors such as hypertension, diabetes mellitus, obesity, heart failure and obstructive sleep apnea (OSA), which all contribute to the development and the progression of AF (4).
AF is related with several clinical outcomes like an overall 3.5-fold mortality risk, responsible for 20%–30% of all ischemic strokes, a 10%–40% annual hospitalisation rate, left ventricular dysfunction in 20%–30%, and impaired quality of life in more than 60% of AF patients (4).
AF care is multidimensional. This complexity requires great efforts of all health care providers and big investments from healthcare systems. Ideally, it requires a patient-centered, multidisciplinary, integrated and structured approach as proposed by the 2016 and 2020 European Society of Cardiology (ESC) guidelines. Moreover, the management of AF needs to be tailored to regional AF patient characteristics and health care realities (4, 5). The optimal determining components and global approach of such integrated care is not fully established and requires further study.
Our research group had shown before that short tailored education sessions based on patients' knowledge gaps assessed with the Jessa Atrial fibrillation Knowledge Questionnaire (JAKQ) significantly improved their knowledge both via in-person and online education (6, 7). Therefore, the innovative integrated care approach of the AF-EduCare/AF-EduApp studies (NCT03707873 & NCT03788044) is based on this type of (I) education combined with (II) systematic assessment of AF risk factors, (iii) patient involvement to improve self-care capabilities, improvement of adherence to oral anticoagulation (OAC) therapy and (iv) low-threshold accessibility for study patients to the care team in case of AF-related questions or problems. The main goal is to improve several clinical outcomes (8).
This paper describes the contemporary Belgian AF population of unselected consecutive AF patients, recruited for the trial from both outpatient clinics as hospitalisation wards. Moreover, we explore the uniformity of the baseline demographic data in the different AF-EduCare/AF-EduApp study groups.
2. Methods
The AF-EduCare study (ClinicalTrials.gov—NCT03707873) is an open, prospective, randomised clinical trial (RCT) conducted in three Belgian tertiary centers (Antwerp University Hospital, the Jessa Hospital in Hasselt and the University Hospitals Leuven) (8). A total of 1,038 AF patients were randomised to three study groups (in-person education—online education—standard care).
The AF-EduApp study (ClinicalTrials.gov—NCT03788044) evaluates an integrated care application (operating on a tablet or smartphone) for AF patients with the primary aim to improve adherence to non-vitamin K-antagonist oral anticoagulants (NOAC). This extra study arm was integrated in the AF-Educare study (and in its randomisation process) for which an additional 153 AF patients (on-treatment) were recruited at the Antwerp University Hospital and the Jessa Hospital in Hasselt.
The Ethics Committees of the participating centers approved both trials and its amendments Belgian study number B300201836720—Ethics committee approval n° 18/12/171). These studies are being conducted in compliance with the Declaration of Helsinki.
2.1. Study population and procedure
Patients with AF (diagnosed with a single-lead ECG recording of ≥30 s or a 12-lead ECG), hospitalised at the department of cardiology or who presented for an outpatient visit, were assessed for the AF-EduCare/AF-EduApp study. All types of AF patients were eligible (1) with a minimum age of 18 years, (2) AF or atrial flutter diagnosed with an electrocardiogram, and (3) capable of signing the informed consent. Exclusion criteria were (1) not able to speak and read Dutch, (2) cognitively impaired (e.g., severe dementia), (3) life expectancy estimated <1 year, (4) participation in another randomised clinical trial and (5) pregnant women. After enrolment and providing written consent, their clinical data and profile were registered in the electronic Case Report Form (eCRF) before the start of any intervention. AF patients who were not eligible or not willing to participate were also logged in the eCRF to avoid readdressing these candidates twice during the inclusion period. Only baseline demographic data of these patients were retrieved from the patients' hospital files.
2.2. Data
All collected data were stored in an encoded eCRF. Each participating center kept a separate list linking the eCRF study number with the study patient identification. This list was only accessible by the local investigators so that this information was maximally secured. Baseline data of all patients was defined as the data on the date of study inclusion or on the date of the study enrolment proposal for the excluded/refused patients.
3. Statistics
Data were analysed using IBM SPSS version 28.0. Variables were described as numbers and percentages or as mean ± standard deviation, as appropriate. Normal distribution was assumed as all study subgroups were large enough. For continuous variables, differences between two or more groups were compared using the independent T-test or one-way ANOVA analysis. The chi-squared test and Fisher's exact test were used for categorical variables, when appropriate. P-values < 0.05 were considered statistically significant. Of note, non-objectified parameters in the medical file of the excluded/refused patients were considered to be unknown and were left out in the analysis and comparisons.
4. Results
4.1. Enrolment
Enrolment of patients began in September 2018 and ended in March 2021. Due to the development and validation of the AF-EduApp, inclusions for this study started in October 2019. The anticipated 18 month inclusion period was expanded due to the COVID-19 outbreak in 2020, which abruptly interrupted patient recruitment in all centers.
A total of 1,979 AF patients were assessed for study participation (Figure 1). Of these, 128 (6.5%) were excluded, mainly due to cognitive impairment (50.0%). Of the resulting 1,851 eligible AF patients, 619 declined participation (33.4%) primarily because of transportation problems (e.g., distance to the hospital; depending on others; living abroad) and insufficient interest in the study (e.g., no time; only preferring follow-up by their treating physician) in 42.0% and 38.4% of patients, respectively. A total of 1,232 AF patients were eventually included of which 1,038 (84.3%) and 194 (15.7%) patients were enrolled in the AF-EduCare- and AF-EduApp trial, respectively. Of the patients randomised to the online- (n = 347) and application-based (n = 194) education groups, 75.8% (n = 263) and 78.8% (n = 153) respectively were eligible of using the online platform or using the in-house developed AF application (=on-treatment subgroups). Future outcome analysis of these two study arms will be performed as intention-to-treat.
4.2. AF patient demographics
Of the 1,979 AF patients, slightly more than half were approached at the cardiology ward (59.2%), of which 79.6% were hospitalised primarily related to their AF while 23.7% were cardiovascular unplanned admissions.
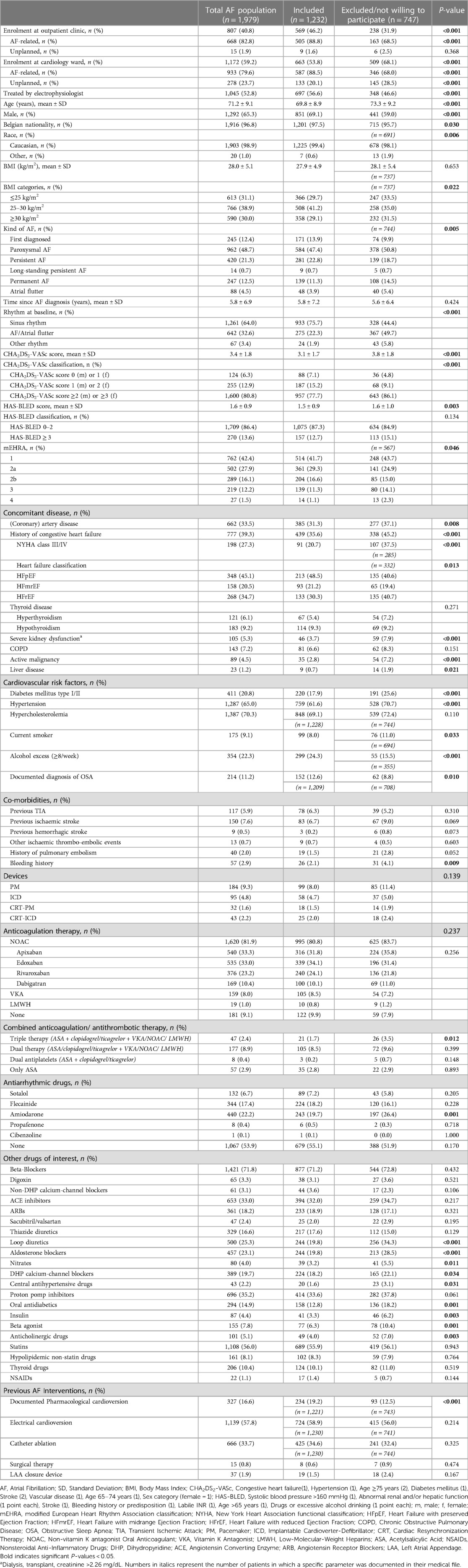
Table 1 presents the baseline characteristics of the entire patient cohort, i.e., the included, excluded and declined patients. Mean age was 71.2 ± 9.1 years, 65.3% were male and 98.9% were Caucasian. Almost half of the patients had paroxysmal AF (48.7%) and mean duration since AF diagnosis was 5.8 ± 6.9 years.
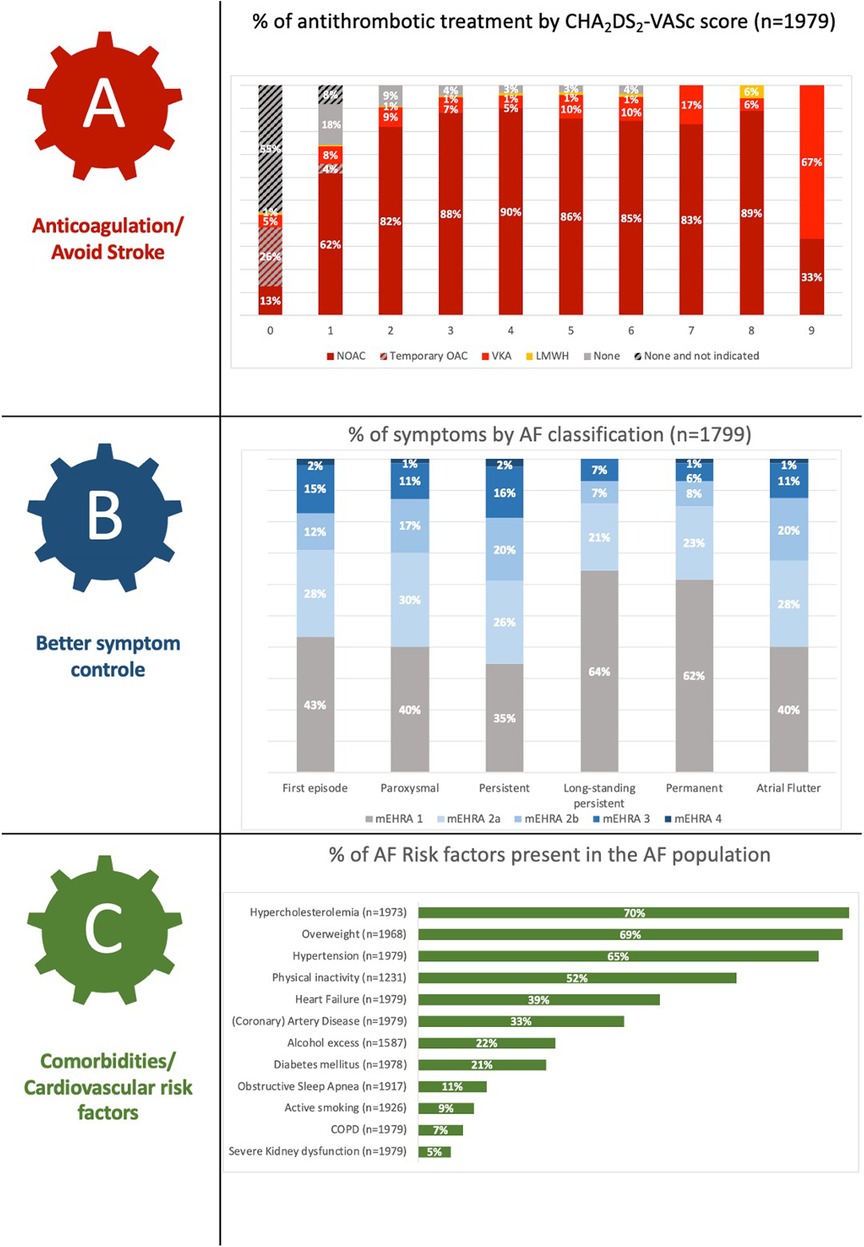
Figure 2 depicts the global characterisation of the Flemish AF population according to the “ABC pathway” focusing on anticoagulant treatment by CHA2DS2-VASc score (panel A), symptom severity by AF classification (panel B) and cardiovascular risk factors (panel C) (9). The mean CHA2DS2-VASc score was 3.4 ± 1.8 and anticoagulation therapy was prescribed in 90.9% of AF patients with the majority receiving NOACs (90.1%). A total of 112 (6.0%) out of 1,855 AF patients in whom thromboembolic prophylaxis was indicated did not receive any kind of anticoagulation therapy.
At the time of assessment for study inclusion, 42.4% of patients had no AF symptoms [scored as “1” by the modified European Heart Rhythm Association (mEHRA) symptom scale] (10). Antiarrhythmic drugs were used in 46.1% of patients and rate control was primarily obtained using beta-blockers (71.8%). Figure 3 shows the percentage of antiarrhythmics (panel A) and previous AF interventions by AF classification (panel B).
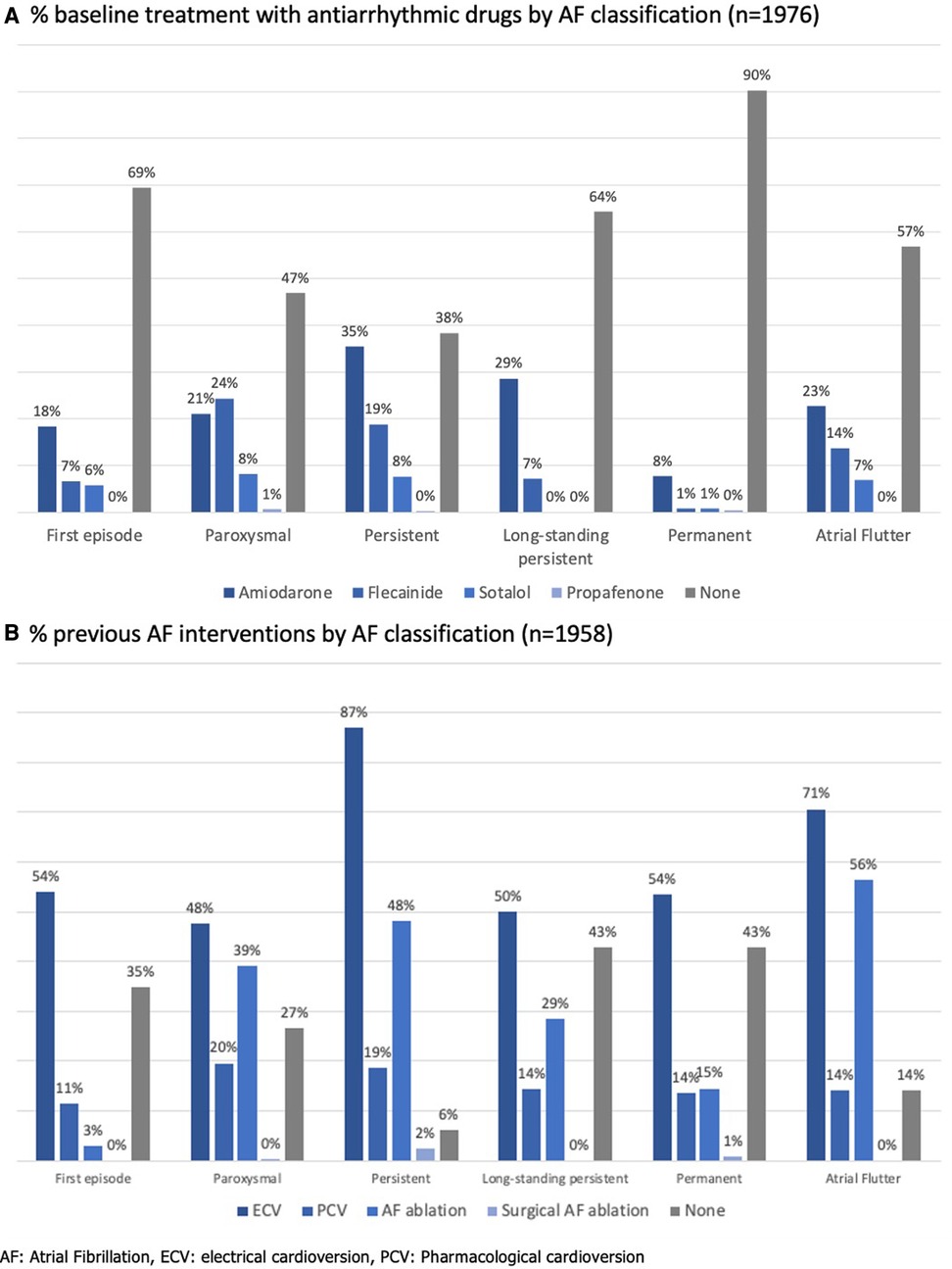
Figure 3. Antiarrhythmic therapy in the Flemish AF cohort. (A) % baseline treatment with antiarrhythmic drugs by AF classification (n = 1,976). (B) % previous AF interventions by AF classification (n = 1,958).
Regarding AF risk factors, overweight was present in 68.9% of the patients, 9.1% still actively smoked, 22.3% (excessively) consumed alcohol (≥8 units/week) and in only 11.2% OSA was diagnosed. Furthermore, 65.0% and 39.3% of patients were known with hypertension or a history of congestive heart failure (CHF), respectively.
4.3. Comparison of the study participants vs. non-participants
As shown in Table 1, there were several significant differences between the included patients and the excluded/declined patients. The majority of the non-participants were assessed while on the cardiology ward (68.1%). These patients were older (73.3 ± 9.2 years), more frequently women (41.0%), and had more comorbidities such as coronary artery disease (CAD; 37.1%), CHF (45.2%), diabetes mellitus (25.6%) and hypertension (70.7%). Consequently, these factors led to a significantly higher mean CHA2DS2-VASc score (3.8 ± 1.8) and more frequent use of diuretics, diabetic therapy and antihypertensive drugs.
4.4. Demographics of the AF-EduCare and AF-EduApp population
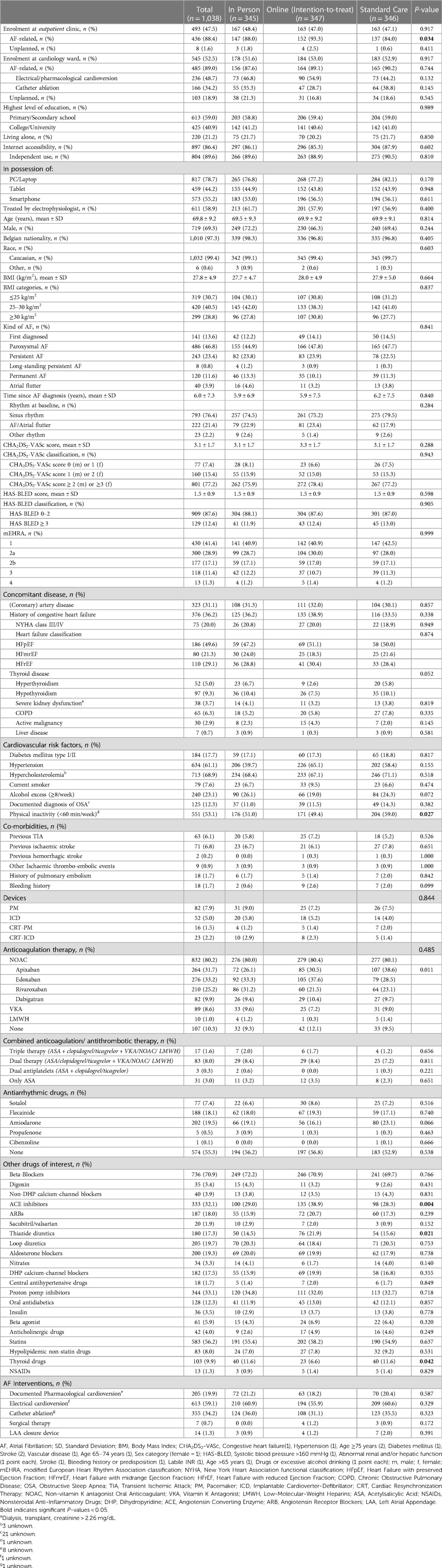
The three AF-EduCare study groups were very well balanced across the vast majority of parameters (Table 2). The AF-EduCare intervention groups were more physically active at baseline than the standard care group (p = 0.027). Furthermore, angiotensin-converting enzyme inhibitors (ACE-I) and thiazide diuretics were taken more often in the online education group (38.9% and 21.9%, respectively) compared with the in-person education (29.0% and 14.5%, respectively) and standard care groups (28.3% and 15.6% respectively) with p-values 0.004 and 0.021, respectively. Also, less use of thyroid drugs was seen in the online group (6.6%) compared to the in-person and standard care groups (both 11.6%, p = 0.042). Supplementary Table S1 shows the demographic characteristics of the on-treatment vs. not-eligible online education subgroups. On-treatment AF patients were higher educated, younger with fewer comorbidities such as CAD, CHF, and hypertension, resulting in lower CHA2DS2-VASc and HAS-BLED scores. This is also reflected on the therapy usage in this group with lesser use of OAC therapy (84.4% vs. 98.8%, p < 0.001) and diuretics and a higher prescription rate of flecainide and prior catheter ablations.
Both the AF-EduCare and AF-EduApp study populations were also comparable across the demographic parameters (Table 3). Remarkably, 23.1% and 30.4% of the AF-EduCare and AF-EduApp study patients respectively, consumed ≥8 alcoholic beverages/week (p = 0.030). The AF-EduApp group was physically more active than the AF-EduCare group (p = 0.017). Supplementary Table S2 shows the demographic characteristics of the on-treatment vs. not-eligible application subgroups. Several significant differences were seen including younger age, lower CHA2DS2-VASc score, more first diagnosed and less permanent AF patients, less diabetes mellitus, less hypercholesterolemia (with less use of statins), less treatment with antiarrhythmic drugs in the on-treatment group, in line with expectations for a group of patients that accept intervention with a mobile Health (mHealth) app.
Regarding internet accessibility and multimedia, a high proportion of the included patients had internet access (87.5%) and 90.9% of these patients could independently work with it. When comparing the AF-EduCare group with the AF-EduApp group, study patients of the latter group had significantly more internet accessibility and more possession of a tablet (54.1% vs. 44.2%, p = 0.011) or a smartphone (80.4% vs. 55.2%, p < 0.001).
5. Discussion
Our results provide data from the largest and most recent contemporary AF population in Flanders (the Dutch-speaking north of Belgium) regarding comorbidities and current management. As AF prevalence is anticipated to rise further in the coming decades, it is important to have up-to-date insights into AF population characteristics and current AF management. Compared with international registries, there is a higher use of anticoagulation (mainly with NOACs), conform the most recent guidelines. Also rhythm control therapies were more frequently applied, both pharmacologically and especially ablation.
The education and integrated care intervention of the AF-EduCare/AF-EduApp study was targeted at an unselected AF population, in contrast to previous integrated care trials which included selected AF patient populations (11–18). Nevertheless, almost 35% of the patients had to be excluded or declined participation, illustrating the difficulties in reaching all AF patients with integrated care approaches. The clinical outcome events in this large AF study cohort will provide extra information on the effectiveness and best strategies to implement integrated AF care.
5.1. Current flemish AF population compared to other cohorts
When comparing our cohort with other European/Western prospective studies and international registries, similarities and important evolutions can be noticed (Supplementary Table S3).
Concerning the general demographic characteristics, our total cohorts' mean age of 71.2 years was similar to other registries ranging between 68.8–73.5 years old (19–25). Our population showed a slightly higher proportion of men (65.3%) compared with other studies (range between 55%–60%) (19–25). In our study, 59.2% of AF patients were approached at the cardiology ward, whereas the EURObservational Research Programme on Atrial Fibrillation (EORP-AF) Pilot and Long-Term General Registries included 62.8% and 52.2% hospitalised AF patients, respectively (19, 20).
The management of AF shows several evolutions. In line with the “ABC” pathway as proposed in the 2020 ESC guidelines, an important aspect in the treatment of AF is stroke prevention (“A—Anticoagulation/Avoid stroke”) in patients with high thromboembolic risk, for which NOACs are now the preferred therapy over vitamin K antagonists (VKA) (4). Therapy with NOACs and VKAs were prescribed in 81.9% and 8.0% of our cohort, respectively. AF patients with increasing thromboembolic risk (i.e., CHA2DS2-VASc score) were more increasingly treated with OAC, which reflects a good adherence to the Guidelines (Figure 2) (4). The use of OACs in AF patients with CHA2DS2-VASc score 0 (male) or 1 (female) could partly be explained by the enrolment of patients with a mechanical heart valve or who recently underwent direct current cardioversion or AF ablation for which temporary OAC use was indicated (26). Of the 255 patients with a CHA2DS2-VASc score of 1 (male) or 2 (female), 45 (17.6%) were not receiving OAC, although this therapy should be considered in these patients (Class IIa, level B recommendation) (4). The AF stroke risk factors that were most prevalent In this patient group were age (65–75 years old; 45.5%) and hypertension (35.3%), which are both clearly recognised as risk markers which on their own justify anticoagulation. Prior (Western-)European registries reported OAC use in 73.0%–85.0% of AF patients, and lower use of NOACs, although there is a clear temporal trend for increase in the more recent studies (19–24).
For “B—Better symptom control”, beta-blockers were primarily used as rate control therapy conform other registries. Digoxin was only prescribed in 3.3% of our cohort compared to 14.7% in the latest EORP-AF long-term registry (20).
Remarkably, in our cohort, 53% and 62% of the paroxysmal AF and persistent AF patients respectively took antiarrhythmic drugs. The majority of both patient groups had also undergone a previous AF intervention. Only 40% and 35% respectively were asymptomatic at the time of inclusion in our cohorts. In the EORP-AF long-term general registry, less antiarrhythmics were prescribed for paroxysmal (44.0%) and persistent AF (42.5%) patients, although the asymptomatic proportion in these patients were similar to our cohort (42.7% and 35.2%, respectively) (20). Overall, rhythm control was pursued more in this Flemish cohort than in the EORP-AF population, since cardioversions and ablations were performed in 65.5% vs. 42.2%, and 34.5% vs. 5.8% of patients. Moreover, 40.3% of these patients were enrolled during an admission for AF ablation or cardioversion.
The third pillar of the ABC pathway, namely “C—Comorbidities/Cardiovascular risk factors”, showed interesting findings in the Flemish population. Being overweight forms a major problem (mean BMI 28.0 kg/m2), comparable with international registries (BMI range 27.7–31.2 kg/m2) (19, 23, 24). We noted a slightly lower prevalence of hypertension (65.0% vs. >70% in the majority of registries) (19–25). Our findings are in line with the hypertension prevalence noted in the regional subanalysis of the ETNA-AF Europe study (61.6%), GARFIELD-AF (68.3%), and EORP-AF long-term general registry (49.8%). It indicates that other AF risk factors play a more important role in Belgian patients. Our study recorded a higher presence of hypercholesterolemia in our AF patients (70.3%) than the two EORP-AF registries (41%–48%) (19, 20). Furthermore, when systematically asking about AF patients' lifestyle, a rather high prevalence of regular alcohol consumption (≥8 units/week) was noted in almost a quarter of our included AF patients. This risk factor may not be underestimated as moderate to heavy alcohol consumption is related to AF progression and recurrence (27, 28). Lastly, the likely underrecognition of OSA (11.2%) is noteworthy, as the prevalence of even moderate OSA in AF patients has been estimated to be between 42.1%–56.1% (29). This calls for better screening of OSA in Flemish (and other) AF patient populations.
5.2. Patients' willingness to participate in integrated AF care programs
Thirty-three percent of the eligible AF patients could not, or refused to, participate in the AF-EduCare/AF-EduApp study. This high rate can partly be explained by the design of the trial: candidates could not choose their preferred treatment group, and the possibility for extra in-hospital study visits (i.e., in-person education group) was perceived as difficult, leading to patient refusal. This is reflected in the primary reason for non-participation, i.e., transportation problems. Hence, future integrated AF programs should offer at least the possibility for remote care through teleconsultations and mHealth technology (e.g., telemonitoring of blood pressure, heart rate and rhythm,…). This technology opportunity has also been the reason to implement the additional AF-EduApp study arm to gain experience and to evaluate intermediate patient-related outcomes when using mHealth technology. The COVID-19 pandemic certainly has accelerated the development, validation and use of remote AF technology which could support integrated AF care (30). Many challenges still await concerning remote health technology, as extensively discussed in the international collaborative statement paper by Varma et al. (31).
On the other hand, when looking at the clinical profile of the non-participating patients, who were older, were more often hospitalised (i.e., sicker), and often had limited access to or affinity with mHealth, such AF patients may derive most benefit from integrated care. This group is more vulnerable to complications due to its higher prevalence of comorbidities and AF risk factors. These patients, therefore, should not be forgotten and care pathways that motivate them for integrated AF care should be explored.
5.3. Comparison of the AF-EduCare/AF-EduApp cohort with other integrated AF care trials
Over the last decade, several integrated AF care trials were conducted with varying success on clinical outcomes (Supplementary Table S4) (11–18). These studies show important differences in the characteristics of the included AF patients.
The RCT by Hendriks et al., the study by Carter et al. and the RACE 4 trial included AF patients with a new AF diagnosis and/or patients seen at the outpatient clinic (11, 13, 14). Consequently, these patients were younger and had fewer comorbidities, resulting in a lower overall thromboembolic risk (range CHA2DS2-VASc score 2.2–2.4). OACs were taken in 57.0 to 67.6% of patients.
In contrast to these three studies, the SAFETY trial included hospitalised patients with chronic AF but without chronic heart failure (12). This cohort had a mean age of 72 years and had more comorbidities. Only VKAs were available at that time and were prescribed in just 55.5% of patients despite a mean CHA2DS2-VASc score of 3.6.
Two recent integrated AF trials conducted in primary care included older patients but with variable prevalence of comorbidities compared with our results, i.e., CHF, diabetes mellitus and TIA/stroke in 16.8%−25.7%, 25.5%–28.9% and 14.4%–18.4% of patients, respectively (15, 16). OAC was used in the 71.3%–90.7% of patients.
Lastly, the mAFA-II trial included in- and outpatient candidates but with a CHA2DS2-VASc score ≥2 (18). This population was slightly younger (mean 68.5 years old) compared to the AF-EduCare/AF-EduApp population. Comorbidities were generally less common except for CAD which was more prevalent in the mAFA population (40.9%) compared with our included study population. Median CHA2DS2-VASc score was 3 and OACs were used in 57.1% of patients.
It will be important to consider the different populations when the medical outcomes in our intervention groups will be compared with those of the mentioned prior trials.
6. Limitations
Our sample has an underrepresentation of non-Caucasian AF patients compared to the composition of the current Flemish population. This is related to the “need to understand Dutch language” exclusion criterion for the AF-EduCare/AF-EduApp studies for which AF patients with an already documented other native language than Dutch (i.e., majority of non-Caucasian candidates), were not addressed and thus not registered in the eCRF. Secondly, all AF patients were recruited at tertiary/university centers in which all AF treatment possibilities were available. This may have led to a selection of AF patients with more complex medical (AF) histories and to a higher prevalence of rhythm control interventions than in the general population. Fourthly, a possible overestimation of paroxysmal AF and underestimation of persistent AF could be present. A correct AF classification often proved difficult due to a lack of documented temporal relationship in the medical files on the duration of the AF before an AF intervention. Finally, as data for the excluded patients were retrospectively collected, some parameters could not be retrieved or were uncertain.
7. Conclusions
The studied Belgian (Flemish) AF cohort is largely comparable with previous international registries regarding demographic and clinical cardiovascular profile. It shows, however, a higher use of anticoagulation and antiarrhythmic therapy (both drugs and interventions). Some AF risk factors likely are underrecognised (mainly OSA) and alcohol usage is reported to be higher (maybe due to more thorough questioning the patients about their lifestyle). In contrast to prior integrated AF trials, the AF-EduCare/AF-EduApp study incorporated all types of AF patients. Nevertheless, about a third of patients were not motivated enough to be included, pointing to the attention needed to attract all AF patients for active participation to integrated care. Follow-up and analysis of clinical outcomes of the study patients will provide further insights into the effectiveness and optimal modalities of integrated AF care.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics committee University of Antwerp/Antwerp University Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
MD, LK, LD, HH contributed to conception and design of the study. All authors contributed to the execution of the study in the three participating hospitals. MD organised the database. MD performed the statistical analysis. MD wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The AF-Educare study is a project supported by the Fund for Scientific Research, Flanders (T002917N) and is part of Limburg Clinical Research Center, supported by the foundation Limburg Sterk Merk, province of Limburg, Flemish government, Hasselt University, Ziekenhuis Oost-Limburg and Jessa Hospital. The AF-Eduapp study was supported by an BMS/Pfizer European Thrombosis Investigator Initiated Research Program (ERISTA) grant (CV185-696). We would also like to thank the study nurses and AF nurse specialists of the involved hospitals for recruiting and following up the patients in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1186453/full#supplementary-material.
References
1. Johnsen SP, Dalby LW, Tackstrom T, Olsen J, Fraschke A. Cost of illness of atrial fibrillation: a nationwide study of societal impact. BMC Health Serv Res. (2017) 17:714. doi: 10.1186/s12913-017-2652-y
2. Staerk L, Wang B, Preis SR, Larson MG, Lubitz SA, Ellinor PT, et al. Lifetime risk of atrial fibrillation according to optimal, borderline, or elevated levels of risk factors: cohort study based on longitudinal data from the framingham heart study. Br Med J. (2018) 361:k1453. doi: 10.1136/bmj.k1453
3. Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman A, et al. Projections on the number of individuals with atrial fibrillation in the European union, from 2000 to 2060. Eur Heart J. (2013) 34:2746–51. doi: 10.1093/eurheartj/eht280
4. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. (2021) 42:373–498. doi: 10.1093/eurheartj/ehaa612.32860505
5. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. (2016) 37:2893–962. doi: 10.1093/eurheartj/ehw210.27567408
6. Desteghe L, Engelhard L, Vijgen J, Koopman P, Dilling-Boer D, Schurmans J, et al. Effect of reinforced, targeted in-person education using the Jessa atrial fibrillation knowledge questionnaire in patients with atrial fibrillation: a randomized controlled trial. Eur J Cardiovasc Nurs. (2019) 18:194–203. doi: 10.1177/1474515118804353
7. Desteghe L, Germeys J, Vijgen J, Koopman P, Dilling-Boer D, Schurmans J, et al. Effectiveness and usability of an online tailored education platform for atrial fibrillation patients undergoing a direct current cardioversion or pulmonary vein isolation. Int J Cardiol. (2018) 272:123–9. doi: 10.1016/j.ijcard.2018.07.065
8. Delesie M, Knaepen L, Dendale P, Vijgen J, Ector J, Verbeeck J, et al. Effect of targeted education for atrial fibrillation patients: design of the EduCare-AF study. Eur J Clin Invest. (2021) 51:e13442. doi: 10.1111/eci.13442
9. Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol. (2017) 14:627–8. doi: 10.1038/nrcardio.2017.153
10. Wynn GJ, Todd DM, Webber M, Bonnett L, McShane J, Kirchhof P, et al. The European heart rhythm association symptom classification for atrial fibrillation: validation and improvement through a simple modification. Europace. (2014) 16:965–72. doi: 10.1093/europace/eut395
11. Hendriks JM, de Wit R, Crijns HJ, Vrijhoef HJ, Prins MH, Pisters R, et al. Nurse-led care vs. Usual care for patients with atrial fibrillation: results of a randomized trial of integrated chronic care vs. Routine clinical care in ambulatory patients with atrial fibrillation. Eur Heart J. (2012) 33:2692–9. doi: 10.1093/eurheartj/ehs071
12. Stewart S, Ball J, Horowitz JD, Marwick TH, Mahadevan G, Wong C, et al. Standard versus atrial fibrillation-specific management strategy (SAFETY) to reduce recurrent admission and prolong survival: pragmatic, multicentre, randomised controlled trial. Lancet. (2015) 385:775–84. doi: 10.1016/S0140-6736(14)61992-9
13. Carter L, Gardner M, Magee K, Fearon A, Morgulis I, Doucette S, et al. An integrated management approach to atrial fibrillation. J Am Heart Assoc. (2016) 5. doi: 10.1161/JAHA.115.002950
14. Wijtvliet E, Tieleman RG, van Gelder IC, Pluymaekers N, Rienstra M, Folkeringa RJ, et al. Nurse-led vs. Usual-care for atrial fibrillation. Eur Heart J. (2020) 41:634–41. doi: 10.1093/eurheartj/ehz666
15. van den Dries CJ, van Doorn S, Rutten FH, Oudega R, van de Leur S, Elvan A, et al. Integrated management of atrial fibrillation in primary care: results of the ALL-IN cluster randomized trial. Eur Heart J. (2020) 41:2836–44. doi: 10.1093/eurheartj/ehaa055
16. Cox JL, Parkash R, Foster GA, Xie F, MacKillop JH, Ciaccia A, et al. Integrated management program advancing community treatment of atrial fibrillation (IMPACT-AF): a cluster randomized trial of a computerized clinical decision support tool. Am Heart J. (2020) 224:35–46. doi: 10.1016/j.ahj.2020.02.019
17. Guo Y, Lane DA, Wang L, Zhang H, Wang H, Zhang W, et al. Mobile health technology to improve care for patients with atrial fibrillation. J Am Coll Cardiol. (2020) 75:1523–34. doi: 10.1016/j.jacc.2020.01.052
18. Guo Y, Guo J, Shi X, Yao Y, Sun Y, Xia Y, et al. Mobile health technology-supported atrial fibrillation screening and integrated care: a report from the mAFA-II trial long-term extension cohort. Eur J Intern Med. (2020) 82:105–11. doi: 10.1016/j.ejim.2020.09.024
19. Lip GY, Laroche C, Dan GA, Santini M, Kalarus Z, Rasmussen LH, et al. A prospective survey in European society of cardiology member countries of atrial fibrillation management: baseline results of EURObservational research programme atrial fibrillation (EORP-AF) pilot general registry. Europace. (2014) 16:308–19. doi: 10.1093/europace/eut373
20. Boriani G, Proietti M, Laroche C, Fauchier L, Marin F, Nabauer M, et al. Contemporary stroke prevention strategies in 11 096 European patients with atrial fibrillation: a report from the EURObservational research programme on atrial fibrillation (EORP-AF) long-term general registry. Europace. (2018) 20:747–57. doi: 10.1093/europace/eux301
21. Fosbol EL, Holmes DN, Piccini JP, Thomas L, Reiffel JA, Mills RM, et al. Provider specialty and atrial fibrillation treatment strategies in United States community practice: findings from the ORBIT-AF registry. J Am Heart Assoc. (2013) 2:e000110. doi: 10.1161/JAHA.113.000110
22. Kirchhof P, Ammentorp B, Darius H, De Caterina R, Le Heuzey JY, Schilling RJ, et al. Management of atrial fibrillation in seven European countries after the publication of the 2010 ESC guidelines on atrial fibrillation: primary results of the PREvention oF thromboemolic events–European registry in atrial fibrillation (PREFER in AF). Europace. (2014) 16:6–14. doi: 10.1093/europace/eut263
23. Potpara TS, Dan GA, Trendafilova E, Goda A, Kusljugic Z, Manola S, et al. Stroke prevention in atrial fibrillation and “real world” adherence to guidelines in the Balkan region: the BALKAN-AF survey. Sci Rep. (2016) 6:20432. doi: 10.1038/srep20432
24. Steinberg BA, Gao H, Shrader P, Pieper K, Thomas L, Camm AJ, et al. International trends in clinical characteristics and oral anticoagulation treatment for patients with atrial fibrillation: results from the GARFIELD-AF, ORBIT-AF I, and ORBIT-AF II registries. Am Heart J. (2017) 194:132–40. doi: 10.1016/j.ahj.2017.08.011
25. Camm AJ, Accetta G, Ambrosio G, Atar D, Bassand JP, Berge E, et al. Evolving antithrombotic treatment patterns for patients with newly diagnosed atrial fibrillation. Heart. (2017) 103:307–14. doi: 10.1136/heartjnl-2016-309832
26. Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KG, et al. 2021 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace. (2021) 23(10):1612–76. doi: 10.1093/europace/euab065
27. Voskoboinik A, Kalman JM, De Silva A, Nicholls T, Costello B, Nanayakkara S, et al. Alcohol abstinence in drinkers with atrial fibrillation. N Engl J Med. (2020) 382:20–8. doi: 10.1056/NEJMoa1817591
28. Voskoboinik A, Prabhu S, Ling LH, Kalman JM, Kistler PM. Alcohol and atrial fibrillation: a sobering review. J Am Coll Cardiol. (2016) 68:2567–76. doi: 10.1016/j.jacc.2016.08.074
29. Delesie M, Knaepen L, Hendrickx B, Huygen L, Verbraecken J, Weytjens K, et al. The value of screening questionnaires/scoring scales for obstructive sleep apnoea in patients with atrial fibrillation. Arch Cardiovasc Dis. (2021) 114(11):737–47. doi: 10.1016/j.acvd.2021.08.002
30. Pluymaekers N, Hermans ANL, van der Velden RMJ, Gawalko M, den Uijl DW, Buskes S, et al. Implementation of an on-demand app-based heart rate and rhythm monitoring infrastructure for the management of atrial fibrillation through teleconsultation: TeleCheck-AF. Europace. (2021) 23:345–52. doi: 10.1093/europace/euaa201
31. Varma N, Cygankiewicz I, Turakhia MP, Heidbuchel H, Hu YF, Chen LY, et al. . 2021 ISHNE/HRS/EHRA/APHRS expert collaborative statement on mHealth in arrhythmia management: digital medical tools for heart rhythm professionals: from the international society for holter and noninvasive electrocardiology/heart rhythm society/European heart rhythm association/Asia-pacific heart rhythm society. Circ Arrhythm Electrophysiol. (2021) 14:e009204. doi: 10.1161/CIRCEP.120.009204.33573393
Keywords: atrial fibrillation, integrated care, demographics, education, cardiovascular comorbidities
Citation: Delesie M, Knaepen L, Dendale P, Vijgen J, Ector J, Desteghe L and Heidbuchel H (2023) Baseline demographics of a contemporary Belgian atrial fibrillation cohort included in a large randomised clinical trial on targeted education and integrated care (AF-EduCare/AF-EduApp study). Front. Cardiovasc. Med. 10:1186453. doi: 10.3389/fcvm.2023.1186453
Received: 14 March 2023; Accepted: 9 May 2023;
Published: 2 June 2023.
Edited by:
Federico Migliore, University of Padua, ItalyReviewed by:
Bernadette Corica, Sapienza University of Rome, ItalyStefan Simović, University of Kragujevac, Serbia
© 2023 Delesie, Knaepen, Dendale, Vijgen, Ector, Desteghe and Heidbuchel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michiel Delesie bS5kZWxlc2llQGdtYWlsLmNvbQ==
 Michiel Delesie
Michiel Delesie Lieselotte Knaepen
Lieselotte Knaepen Paul Dendale2,3
Paul Dendale2,3 Johan Vijgen
Johan Vijgen Joris Ector
Joris Ector