- 1Johns Hopkins Hospital, Osler Medical Residency, Johns Hopkins University, Baltimore, MD, United States
- 2Department of Medicine, Weill Cornell Medicine-Qatar, Doha, Qatar
- 3Biostatistics Core, Weill Cornell Medicine-Qatar, Doha, Qatar
- 4Heart Hospital, Hamad Medical Corporation, Doha, Qatar
- 5Department of Internal Medicine, University of Texas Medical Branch (UTMB), Galveston, TX, United States
- 6Joan and Sanford I. Weill Department of Medicine, Weill Cornell Medicine, New York, NY, United States
Aims: We aimed to assess the impact of diabetes on sudden cardiac arrest (SCA) in US patients hospitalized for ST-elevation myocardial infarction (STEMI).
Methods: We used the National Inpatient Sample (2005–2017) data to identify adult patients with STEMI. The primary outcome was in-hospital SCA. Secondary outcomes included in-hospital mortality, ventricular tachycardia (VT), ventricular fibrillation (VF), cardiogenic shock (CS), acute renal failure (ARF), and the revascularization strategy in SCA patients.
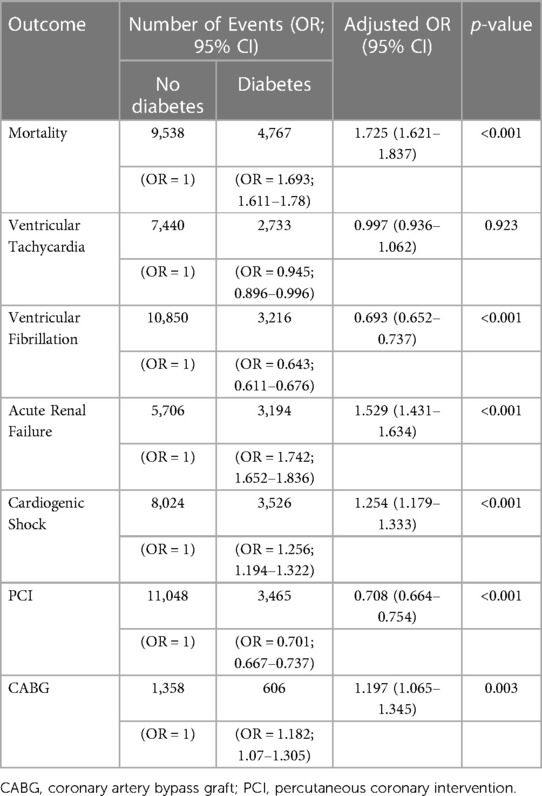
Results: SCA significantly increased from 4% in 2005 to 7.6% in 2018 in diabetes patients and from 3% in 2005 to 4.6% in 2018 in non-diabetes ones (p < 0.001 for both). Further, diabetes was associated with an increased risk of SCA [aOR = 1.432 (1.336–1.707)]. In SCA patients with diabetes, the mean age (SD) decreased from 68 (13) to 66 (11) years old, and mortality decreased from 65.7% to 49.3% during the observation period (p < 0.001). Compared to non-diabetes patients, those with T2DM had a higher adjusted risk of mortality, ARF, and CS [aOR = 1.72 (1.62–1.83), 1.52 (1.43–1.63), 1.25 (1.17–1.33); respectively] but not VF or VT. Those patients were more likely to undergo revascularization with CABG [aOR = 1.197 (1.065–1.345)] but less likely to undergo PCI [aOR = 0.708 (0.664–0.754)].
Conclusion: Diabetes is associated with an increased risk of sudden cardiac arrest in ST-elevation myocardial infarction. It is also associated with a higher mortality risk in SCA patients. However, the recent temporal mortality trend in SCA patients shows a steady decline, irrespective of diabetes.
1. Introduction
Sudden cardiac arrest (SCA) is the most common cause of death in the United States and worldwide. In the general population, SCA accounts for approximately 10% of total mortality and 40% of mortality from coronary artery disease (CAD) (1, 2). Mortality rates from SCA increase with age and are reported to be higher in males compared to females and higher in African-Americans compared to Caucasians (3, 4).
SCA is often the consequence of lethal arrhythmias, most commonly ventricular fibrillation (5). Inciting events could be due to an acute atherosclerotic plaque rupture, electrolyte imbalance, and increased sympathetic activity in patients with underlying structural and coronary heart disease (6).
During the past two decades, type 2 diabetes mellitus (T2DM) reached epidemic proportions worldwide. Diabetes is an established risk factor for cardiovascular disease; it is also associated with higher mortality and morbidity in patients with established atherosclerotic cardiovascular disease (ASCVD) (7). However, the landscape of diabetes has changed over the past decade. Despite the rise in the incidence of diabetes and its related complication, mortality in the general population is overall decreasing (8); which is also the case in clinical conditions such as heart failure, stroke, valvular heart disease, and hypertrophic cardiomyopathy (9–12).
In this study, we report a temporal increase in the number of in-hospital SCA in STEMI patients. However, in-hospital mortality in those patients is on a descending slope. Nonetheless, diabetes is still associated with a higher risk of SCA and mortality.
2. Methods
2.1. Data source
The National Inpatient Sample (NIS) database was used to obtain patient data from 2005 to 2017. The NIS is an administrative and de-identified database designed by the Healthcare Cost and Utilization Project (HCUP) to produce US regional and national estimates of inpatient utilization, access, charges, quality, and outcomes. It accounts for 20% of all US community hospitals. Each entry in the database entails demographic details such as age, sex, race, etiology of admissions, outcomes, and procedures while using safeguards to protect the privacy of patients, physicians, and hospitals (13). Each admission consists of unique identifiers in the database, preventing repeat admissions for the same patient from being distinguished. The study did not require institutional review board approval but an exempt determination (number 18-00017).
2.2. Study population
The ICD-9-CM and ICD-10-CM (International Classification of Diseases, Ninth and Tenth Revisions, Clinical Modification) codes were used to identify admissions of patients from the years 2005–2017 with a primary diagnosis of STEMI who developed in-hospital SCA. Further, patients were categorized according to the presence of diabetes. Patients less than 18 years of age and those with incomplete or missing data were excluded.
2.3. Outcomes
The primary outcome of this study was in-hospital SCA. Secondary outcomes include in-hospital mortality, ventricular fibrillation (VF), ventricular tachycardia (VT), acute renal failure (ARF), and cardiogenic shock in SCA patients. We further assessed interventional procedures: percutaneous coronary intervention (PCI) and coronary artery bypass graft (CABG). Diagnoses were based on ICD-9 and ICD-10 codes.
2.4. Analysis plan and statistics
Data were weighted as mandated by the HCUP to generate national estimates of admissions each year (14). We first analyzed the characteristics and outcomes of all STEMI patients who develop SCA. We then stratified them into two groups according to the presence of T2DM. Further, we merged both groups for intercomparison during the observation period. Finally, we assessed the predictors of mortality in patients with diabetes. Data are presented as mean (SD), median (IQR), number (%), or OR (95% CI). A simple linear regression was used to identify trends. A Chi-squared test, student T-test, or ANOVA was used to compare groups, as appropriate. Binary logistic regression was used to calculate the unadjusted odds ratios of outcomes, which were further adjusted for all baseline characteristics that were different among both groups. Multivariate logistic regression was performed to determine predictors of mortality. It included age, gender, race, hypertension, obesity, smoking, dyslipidemia, chronic kidney disease (CKD), and CAD. The significance level was set at 5%; all analyses were done using SPSS version 26 (IBM SPSS Statistics, IBM, Armonk, New York).
3. Results
3.1. Study group
A total of 543,094 patients were hospitalized with the primary diagnosis of STEMI between 2005 and 2017. After excluding patients below 18 years old and those with missing or incomplete records, the dataset consisted of 534,567 patients (Figure 1). Weighted, 3,412,202 patients with STEMI were included in the initial analysis. Among those, 20.8% had diabetes.
3.2. Temporal trend in characteristics and outcomes of patients with STEMI who develop SCA
During the observation period, SCA was recorded in 6% of diabetes patients and 4.1% of non-diabetes patients (Figure 1); hence, diabetes significantly increased the risk of SCA [OR = 1.451, 95% CI (1.439–1.477)] even after correcting for confounding factors [adjusted OR = 1.432 (1.336–1.707)]. SCA significantly increased from 4% in 2005 to 7.6% in 2018 in diabetes patients and from 3% in 2005 to 4.6% in 2018 in non-diabetes ones (p < 0.001 for both) (Figure 2). Over the observation period, the mean age (SD) of patients with STEMI, SCA, and T2DM decreased from 68 (13) to 66 (11) (Table 1, p = 0.003). The proportion of patients in the age categories of <55, 55–64, and 65–74 increased over time, while that of patients in the age categories of 75–84 and >85 decreased (p < 0.001 for all). The patients were predominantly males throughout the years, and the proportion of male patients increased from 59.0% to 69.1% (p trend < 0.001). The proportion of Caucasian patients decreased, whereas that of African-Americans rose from 8.0% to 12.1% (p trend <0.001). The prevalence of comorbidities such as hypertension, obesity, smoking, dyslipidemia, CAD, and CKD also increased over time (p < 0.001). Mortality decreased from 65.7% to 49.3%, which was also observed in-non diabetes patients (Figure 3, p < 0.001 for both). There was a temporal increase in the proportions of patients who developed VT, VF, acute renal failure, and cardiogenic shock (p < 0.001 for all). As expected, a temporal increase in the prevalence of PCI was observed, parallel to a decrease in the rate of CABG (p < 0.001). The same baseline characteristics and outcomes trend was also observed in non-diabetes patients (Table 2).
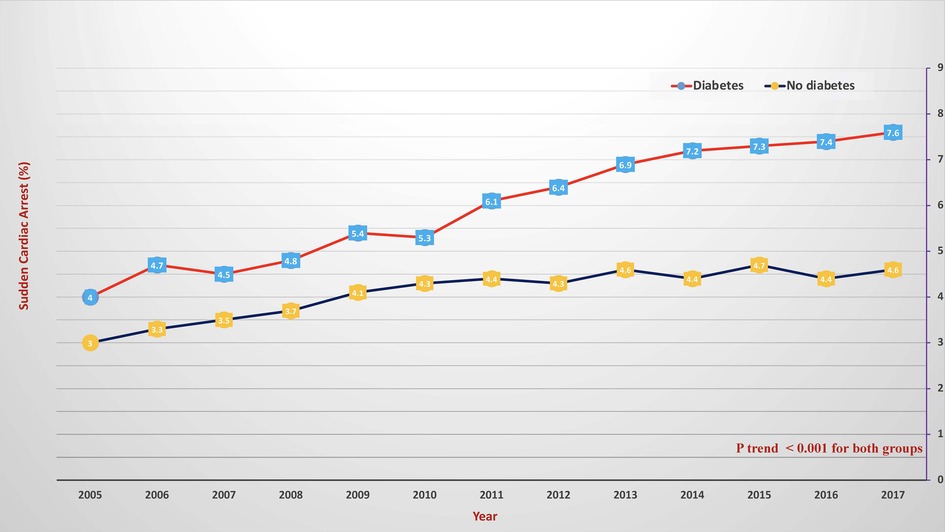
Figure 2. Temporal changes of in-hospital sudden cardiac arrest in patients with diabetes (red color) and without diabetes (blue color). The X-axis represents the percentage of sudden cardiac arrest. The Y-axis represents the year.
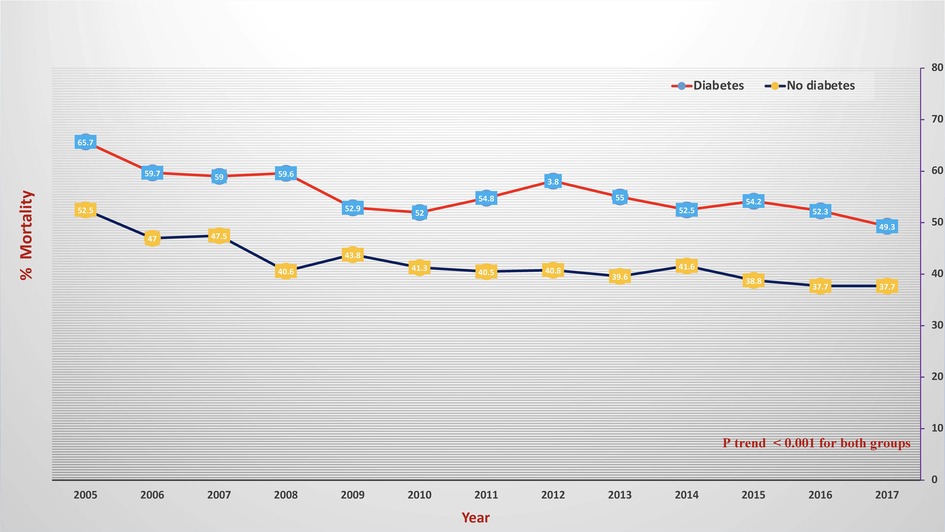
Figure 3. Temporal change of in-hospital mortality in patients with sudden cardiac arrest and diabetes (red color) and without diabetes (blue). The X-axis represents the percentage of sudden mortality. The Y-axis represents the year.
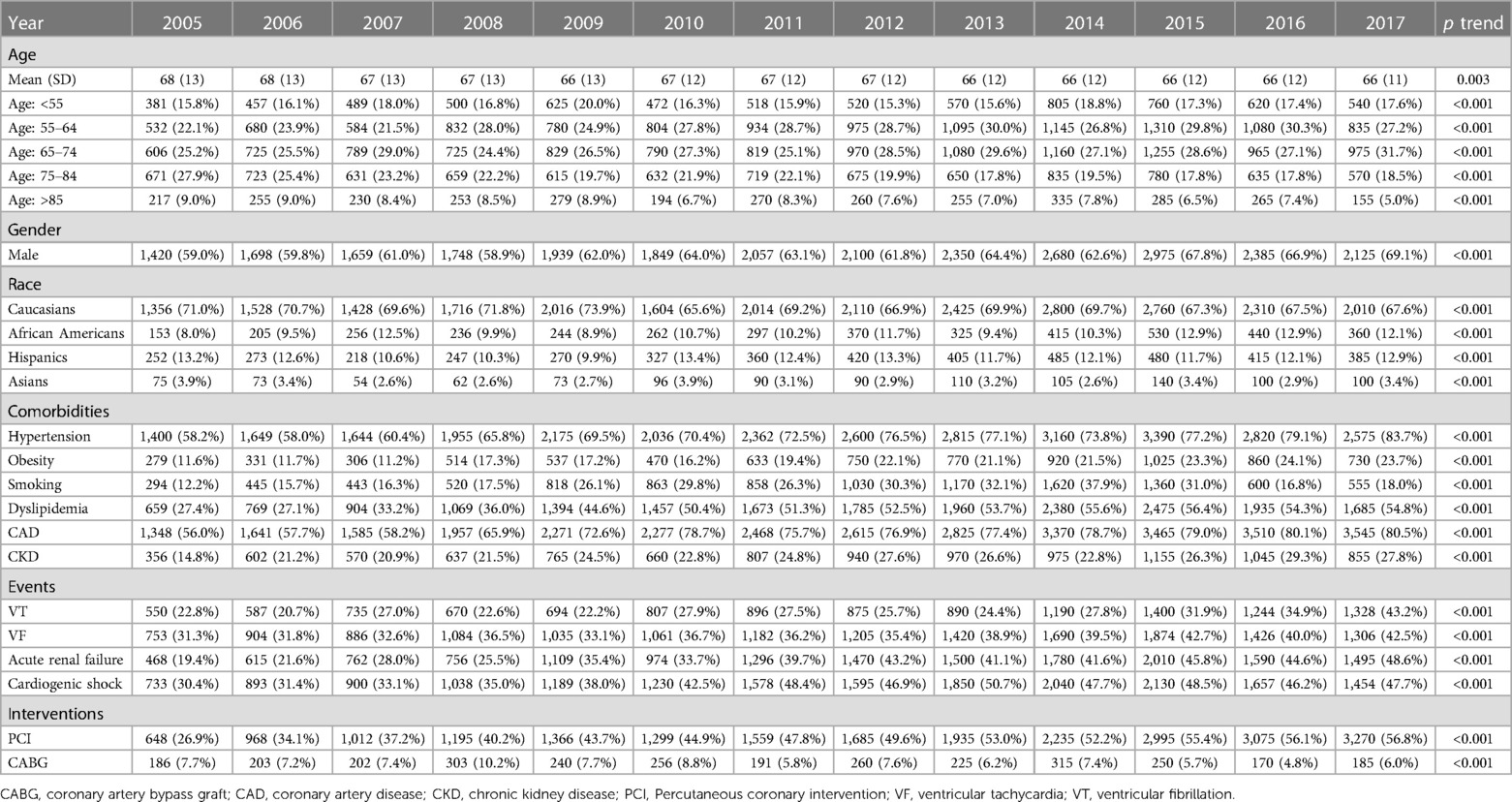
Table 1. Trends in demographics, comorbidities, outcomes, and interventions in patients with STEMI, SCA, and diabetes.
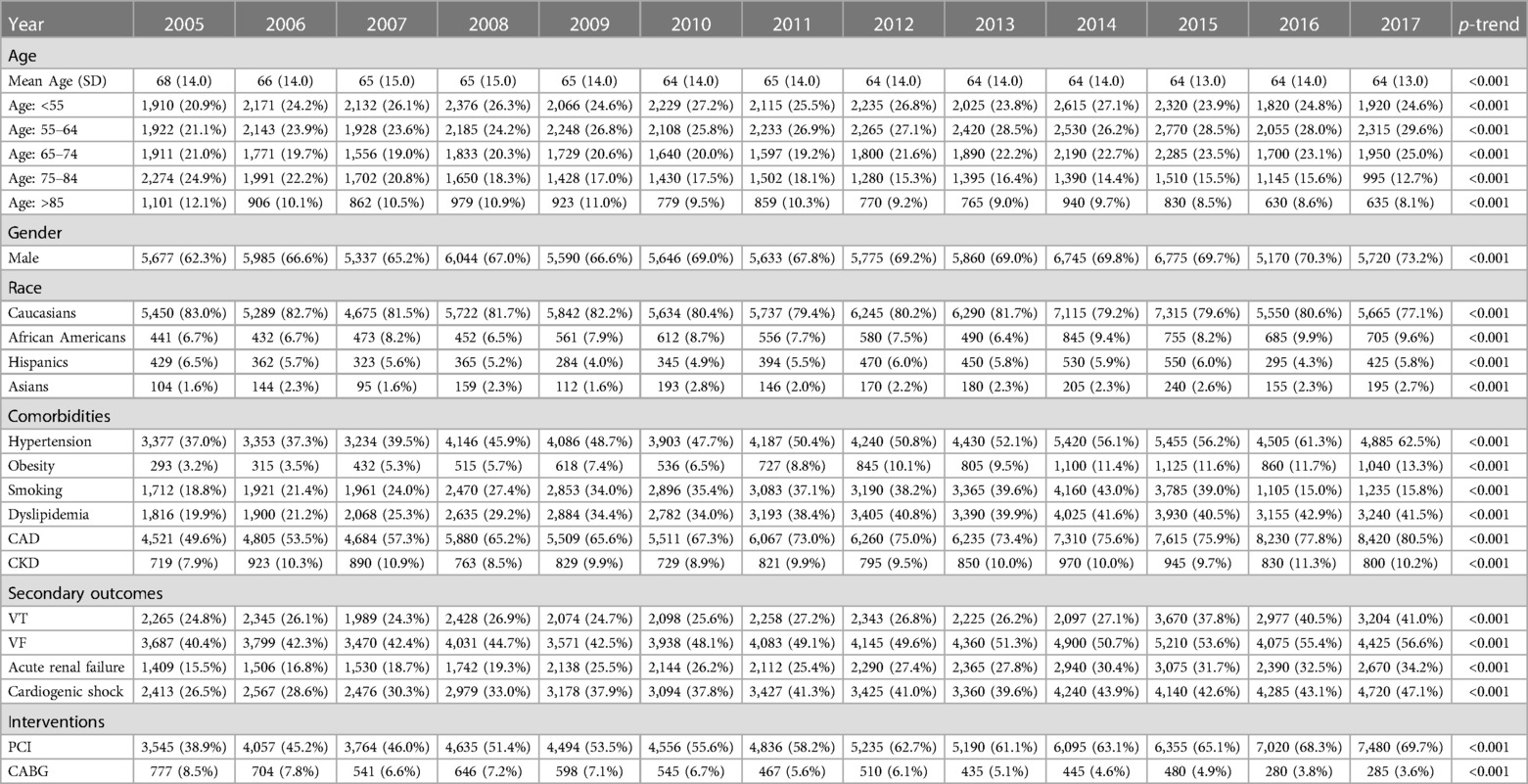
Table 2. Trends in demographics, comorbidities, outcomes, and interventions in patients with STEMI and SCA, without diabetes.
3.3. Comparison of both groups
Diabetes patients with SCA were older, more likely to be females, and belonged to ethnic minorities (Table 3, p < 0.001). They were also more likely to have comorbidities but smoked less. Diabetes was associated with a higher adjusted risk of mortality [aOR = 1.725 (1.621–1.837)], acute renal failure [aR = 1.529 (1.431–1.634)], and cardiogenic shock [aOR = 1.254 (1.179–1.333)] (Table 4). Unexpectedly, ventricular fibrillation was significantly lower in the diabetes group [aOR = 0.693 (0.652–0.737)]. Patients were also more likely to undergo revascularization with CABG [aOR = 1.197 (1.065–1.345)] but less likely to undergo PCI [aOR = 0.708 (0.664–0.754)].
3.4. Predictors of mortality in patients with diabetes
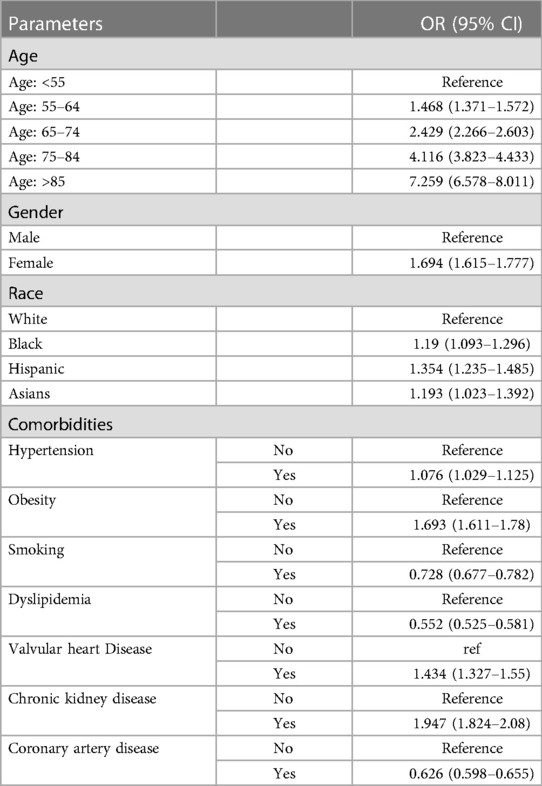
The risk of mortality increases with age (Table 5). For instance, patients >85 years old have a 7-fold increased mortality risk [OR = 7.529 (6.578–8.011)], and so do females [OR = 1.694 (1.615–1.777)]. Compared to Caucasians, African-Americans, Hispanics, and Asians had higher mortality risk [ORs = 1.19 (1.093–1.296), 1.354 (1.235–1.485), 1.193 (1.023–1.392); respectively]. Comorbidities associated with increased mortality risk included hypertension, obesity, valvular heart disease, and renal failure. Paradoxically, smoking, dyslipidemia, and coronary artery disease had a protective effect.
4. Discussion
The pathogenesis of SCA in patients with diabetes is believed to be due to acute atherosclerotic CAD. However, other mechanisms, including diabetic autonomic neuropathy, QT interval prolongation, diabetic cardiomyopathy, diabetic nephropathy, poor diabetes control, and hypoglycemia, may independently contribute to SCA. Diabetic autonomic neuropathy, a common complication of diabetes, is associated with a decrease in parasympathetic and an increase in sympathetic tone, increasing the susceptibility to arrhythmias. Patients with diabetic autonomic neuropathy may also develop electrocardiographic abnormalities such as QT prolongation, predisposing them to lethal ventricular arrhythmias (15, 16). Whitsel et al. showed that diabetes patients without diagnosed pre-existing heart disease in the upper quartile of the QT index (QTI > 107%) had a 3-fold increased risk of SCA (17). Diabetic autonomic neuropathy is also associated with resting tachycardia, exercise intolerance, intraoperative and perioperative cardiovascular instability, silent myocardial ischemia, and increased mortality (18). Diabetic cardiomyopathy is a condition in which pathologic changes in the myocardium develop without cardiovascular disease. It could contribute to ECG abnormalities, such as non-specific ST-T wave changes and QT prolongation. Autopsy of patients with diabetic cardiomyopathy revealed areas of myocardial fibrosis resulting from microvascular disease (19). Diabetic Nephropathy (DN) is the leading cause of end-stage renal disease and is associated with an increased risk of all-cause and cardiovascular mortality (20) The development of DN is characterized by activation of metabolic, inflammatory, and hemodynamic pathways by chronic hyperglycemia (21). The Rochester diabetic neuropathy study reported a 2-fold increase of sudden cardiac death (SCD) in patients with DN, independently of other comorbidities (22).
High HbA1c levels, associated with poor glycemic control, increase the risk of SCD (23, 24). Lastly, stringent glycemic control in diabetes patients often results in hypoglycemic episodes, which may also contribute to the pathogenesis of SCA. We have recently shown that hypoglycemia increases the risk of mortality and arrhythmias in equally both diabetes and non-diabetes STEMI patients (25). A meta-analysis of prospective studies by Goto et al. suggested increased cardiovascular risk with severe hypoglycemia (26). Hypoglycemia activates the sympathetic nervous response, producing catecholamine-mediated adverse effects on the myocardium. Catecholamine excess driven by hypoglycemia is also associated with platelet activation, leukocyte mobilization, and coagulation that may result in ischemic damage to the myocardium and endothelial dysfunction (27, 28).
Our study demonstrated that diabetes patients with STEMI were 1.7 times more likely to die from SCA than non-diabetes patients. This is consistent with previous studies which assessed diabetes and SCA in different populations other than STEMI. In the Paris Prospective Study I of middle-aged working-class men, the risk of SCD was increased in patients with diabetes (29). The prospective, population-based ARIC study in the US also found an association between diabetes and increased risk of SCD (30). Meta-analyses by Aune et al. and Zaccardi et al. suggested a 2-fold increase in the risk of SCD in those patients (31, 32). In a nationwide study in Denmark, Lynge et al. reported a significantly higher incidence rate of SCD in diabetic patients within 1–49 years of age than in non-diabetic patients of the same age (33).
Risk factors associated with increased mortality also align with previous studies that assessed the link between diabetes and SCA. The mortality risk increased with age, consistent with previous studies (3, 34, 35). Although male patients predominantly experienced SCA in the setting of diabetes, the mortality risk was higher among female patients. In the Framingham study, the association between SCA and diabetes was more robust in females (36). In a case-control study by Escobedo et al., diabetes was associated with SCD in individuals with pre-existing coronary heart disease, and women with a history of diabetes were more strongly associated with SCD than men (37).
It has already been shown that mortality rates are different among ethnicities. Benjamins et al. reported that all-cause mortality is 20% higher in African-Americans compared to Caucasians (38). The increased mortality due to SCA among African-American patients has also been described previously (3, 34, 35, 39). Increased odds of cardiovascular mortality have also been reported in South East Asians (40). The higher mortality rates among non-Caucasians may be attributed to socioeconomic and predisposing factors (41). The increased prevalence of comorbidities among non-Caucasians may predispose those patients to poorer outcomes (42–44). Pharmacogenetics and personalized medicine based on genetic clusters might help reduce CVD risk disparities among different ethnicities (45). Race and genetic information are progressively shown to be important determinants of risk prediction (46). Further, current CVD guidelines emphasize the importance of ethnic differences in treating cardiovascular risk factors. The HELIUS study recently reported a beneficial effect of earlier hypertension treatment in ethnic minorities compared to Caucasians (47).
Interestingly, cardiovascular risk factors, including smoking, dyslipidemia, and CAD, had a protective effect, a finding that we and others already reported in the NIS database (9, 10, 12, 25, 48, 49). The paradoxical protective effect may be due to confounding factors not accounted for in our study, such as medications. Patients with known CAD may be more likely to undergo regular screening and follow-up for diabetes control and cardiovascular disease. However, it has been shown that hibernating myocardium in chronic CAD induces sympathetic remodeling and nerve sprouting, which were associated with VF and VT (50, 51). Further, myocyte adaptation in the hibernating myocardium, secondary to chronic ischemia, results in a substrate with enhanced vulnerability to lethal arrhythmias and SCD (52).
The decline in mortality related to SCA could be due to advances in cardiopulmonary resuscitation and post-resuscitation care (53), and potentially due to novel hypoglycemic agents such as SGLT-2 inhibitors and GLP-1 agonists. Those medications have been implicated in reducing the risk of cardiac arrhythmias. SGLT-2 inhibitors have a pleiotropic effect in patients with diabetes, irrespective of their impact on glycemic control (54). In a recent meta-analysis of over 30 studies, SGLT-2 inhibitors reduced the risk of arrhythmias by almost 20% (55). Although the exact mechanisms are not clear, it is believed that SGLT-2 inhibitors improve the electrical stability of the heart by decreasing sympathetic activity and attenuating the pro-arrhythmogenic sodium current (late INa) (56). However, there is conflicting data on the effectiveness of these agents in the risk reduction of SCA and SCD in particular. A meta-analysis of 34 randomized controlled trials by Fernandes et al. suggested that treatment with an SGLT2 inhibitor was associated with a decreased risk in patients with diabetes (55). Whereas other meta-analyses by Li et al. and Sfairopoulos et al. showed a reduction in the risk of SCD in diabetic patients with SGLT-2 inhibitors, this reduction was not statistically significant (57, 58).
Similarly, a meta-analysis by Liu et al. of 5 randomized controlled trials suggested that there was no statistically significant difference in the risk of SCD between diabetes patients treated with GLP-1 receptor agonists and the placebo group (59). Lipid-lowering agents and statins are commonly used in patients with dyslipidemia, peripheral vascular disease, and CAD. Statins have been implicated in having antiarrhythmic properties in several studies (60). Statin use could underlie the protective effect of these comorbidities.
The results of our study must be interpreted within its limitations. The data analyzed in our study was extracted using ICD-9-CM and ICD-10-CM codes; hence, the transition could have led to extraction errors and variations in the number of cases and events. Additionally, the results are subject to administrative errors, such as issues with reporting and coding inaccuracies. As the NIS data relies on discharge diagnoses, the data lacks in-hospital temporality. Furthermore, due to the unavailability of patient-level data, factors such as medications, glycemic control, and duration and severity of comorbidities could not be included.
Further, the NIS is a retrospective observational database, and due to the absence of randomization, only correlations can be made, and no causality can be established. Our analysis only included hospitalized patients, which may represent a sicker cohort with an increased prevalence of adverse outcomes compared to the general population. Lastly, due to the unavailability of data following patient discharge or readmission data, follow-up of patients was not possible. Despite these limitations, our results are derived from a large sample representative of the US population, which is ideal for assessing temporal trends and potential associations.
5. Conclusion
Although diabetes is associated with an increased risk of sudden cardiac arrest in ST-elevation myocardial infarction, in-hospital mortality in those patients is declining despite an increase in cardiometabolic comorbidities. While decreasing mortality is reassuring, more has to be done since diabetes is still associated with worse cardiovascular outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved for exempt determination from the institutional review board at Weill Cornell Medicine-Qatar (number 18-00017). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
CAK conceived the study concept and design. OM and KP acquired data and performed statistical analyses with SD. OM, KP, JS, HJ, and CAK analyzed and interpreted data. OM and KP wrote the first draft and conducted the literature search. All authors contributed to the critical revision of the manuscript. CAK is the guarantor of this work and, as such, has full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zimmerman DS, Tan HL. Epidemiology and risk factors of sudden cardiac arrest. Curr Opin Crit Care. (2021) 27:613–6. doi: 10.1097/MCC.0000000000000896
2. Koivunen M, Tynkkynen J, Oksala N, Eskola M, Hernesniemi J. Incidence of sudden cardiac arrest and sudden cardiac death after unstable angina pectoris and myocardial infarction. Am Heart J. (2022) 257:9–19. doi: 10.1016/j.ahj.2022.11.009
3. Okin PM, Kjeldsen SE, Julius S, Dahlof B, Devereux RB. Racial differences in sudden cardiac death among hypertensive patients during antihypertensive therapy: the LIFE study. Heart Rhythm. (2012) 9:531–7. doi: 10.1016/j.hrthm.2011.11.008
4. Jimenez-Pavon D, Artero EG, Lee DC, Espana-Romero V, Sui X, Pate RR, et al. Cardiorespiratory fitness and risk of sudden cardiac death in men and women in the United States: a prospective evaluation from the aerobics center longitudinal study. Mayo Clin Proc. (2016) 91:849–57. doi: 10.1016/j.mayocp.2016.04.025
5. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart rhythm society. J Am Coll Cardiol. (2018) 72:e91–e220. doi: 10.1016/j.jacc.2017.10.054
6. Ha ACT, Doumouras BS, Wang CN, Tranmer J, Lee DS. Prediction of sudden cardiac arrest in the general population: review of traditional and emerging risk factors. Can J Cardiol. (2022) 38:465–78. doi: 10.1016/j.cjca.2022.01.007
7. Huang D, Refaat M, Mohammedi K, Jayyousi A, Al Suwaidi J, Abi Khalil C. Macrovascular complications in patients with diabetes and prediabetes. Biomed Res Int. (2017) 2017:7839101. doi: 10.1155/2017/7839101
8. Abi Khalil C, Roussel R, Mohammedi K, Danchin N, Marre M. Cause-specific mortality in diabetes: recent changes in trend mortality. Eur J Prev Cardiol. (2012) 19:374–81. doi: 10.1177/1741826711409324
9. Mekhaimar M, Dargham S, El-Shazly M, Al Suwaidi J, Jneid H, Abi Khalil C. Diabetes-related cardiovascular and economic burden in patients hospitalized for heart failure in the US: a recent temporal trend analysis from the national inpatient sample. Heart Fail Rev. (2021) 26:289–300. doi: 10.1007/s10741-020-10012-6
10. Tabbalat A, Dargham S, Al Suwaidi J, Aboulsoud S, Al Jerdi S, Abi Khalil C. Mortality and socio-economic outcomes among patients hospitalized for stroke and diabetes in the US: a recent analysis from the national inpatient sample. Sci Rep. (2021) 11:8204. doi: 10.1038/s41598-021-87320-w
11. Khan S, Dargham S, Al Suwaidi J, Jneid H, Abi Khalil C. Trends and outcomes of aortic valve replacement in patients with diabetes in the US. Front Cardiovasc Med. (2022) 9:844068. doi: 10.3389/fcvm.2022.844068
12. Mekhaimar M, Al Mohannadi M, Dargham S, Al Suwaidi J, Jneid H, Abi Khalil C. Diabetes outcomes in heart failure patients with hypertrophic cardiomyopathy. Front Physiol. (2022) 13:976315. doi: 10.3389/fphys.2022.976315
13. Steiner C, Elixhauser A, Schnaier J. The healthcare cost and utilization project: an overview. Eff Clin Pract. (2002) 5:143–51.12088294
14. (Nis), N.I.S. Healthcare cost and utilization project (HCUP). Rockville, MD: Agency for Healthcare Research and Quality (2023).
15. Jungen C, Scherschel K, Flenner F, Jee H, Rajendran P, De Jong KA, et al. Increased arrhythmia susceptibility in type 2 diabetic mice related to dysregulation of ventricular sympathetic innervation. Am J Physiol Heart Circ Physiol. (2019) 317:H1328–41. doi: 10.1152/ajpheart.00249.2019
16. Jiang AJ, Gu H, Feng ZR, Ding Y, Xu XH, Yin GP, et al. Heart rate-corrected QT interval: a novel diagnostic biomarker for diabetic peripheral neuropathy. J Diabetes Investig. (2022) 13:850–7. doi: 10.1111/jdi.13738
17. Whitsel EA, Boyko EJ, Rautaharju PM, Raghunathan TE, Lin D, Pearce RM, et al. Electrocardiographic QT interval prolongation and risk of primary cardiac arrest in diabetic patients. Diabetes Care. (2005) 28:2045–7. doi: 10.2337/diacare.28.8.2045
18. Sudo SZ, Montagnoli TL, Rocha BS, Santos AD, De Sa MPL, Zapata-Sudo G. Diabetes-induced cardiac autonomic neuropathy: impact on heart function and prognosis. Biomedicines. (2022) 10(12):3258. doi: 10.3390/biomedicines10123258
19. Parim B, Sathibabu Uddandrao VV, Saravanan G. Diabetic cardiomyopathy: molecular mechanisms, detrimental effects of conventional treatment, and beneficial effects of natural therapy. Heart Fail Rev. (2019) 24:279–99. doi: 10.1007/s10741-018-9749-1
20. Go AS, Chertow GM, Fan D, Mcculloch CE, Hsu C-Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. (2004) 351:1296–305. doi: 10.1056/NEJMoa041031
21. Tervaert TW, Mooyaart AL, Amann K, Cohen AH, Cook HT, Drachenberg CB, et al. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. (2010) 21:556–63. doi: 10.1681/ASN.2010010010
22. Suarez GA, Clark VM, Norell JE, Kottke TE, Callahan MJ, O'brien PC, et al. Sudden cardiac death in diabetes mellitus: risk factors in the Rochester diabetic neuropathy study. J Neurol Neurosurg Psychiatry. (2005) 76:240–5. doi: 10.1136/jnnp.2004.039339
23. Martinez M, Santamarina J, Pavesi A, Musso C, Umpierrez GE. Glycemic variability and cardiovascular disease in patients with type 2 diabetes. BMJ Open Diabetes Res Care. (2021) 9(1):e002032. doi: 10.1136/bmjdrc-2020-002032
24. Aroda VR, Eckel RH. Reconsidering the role of glycaemic control in cardiovascular disease risk in type 2 diabetes: a 21st century assessment. Diabetes Obes Metab. (2022) 24:2297–308. doi: 10.1111/dom.14830
25. Humos B, Mahfoud Z, Dargham S, Al Suwaidi J, Jneid H, Abi Khalil C. Hypoglycemia is associated with a higher risk of mortality and arrhythmias in ST-elevation myocardial infarction, irrespective of diabetes. Front Cardiovasc Med. (2022) 9:940035. doi: 10.3389/fcvm.2022.940035
26. Goto A, Arah OA, Goto M, Terauchi Y, Noda M. Severe hypoglycaemia and cardiovascular disease: systematic review and meta-analysis with bias analysis. Br Med J. (2013) 347:f4533. doi: 10.1136/bmj.f4533
27. Katsiki N, Kotsa K, Stoian AP, Mikhailidis DP. Hypoglycaemia and cardiovascular disease risk in patients with diabetes. Curr Pharm Des. (2020) 26:5637–49. doi: 10.2174/1381612826666200909142658
28. Papachristoforou E, Lambadiari V, Maratou E, Makrilakis K. Association of glycemic indices (hyperglycemia, glucose variability, and hypoglycemia) with oxidative stress and diabetic complications. J Diabetes Res. (2020) 2020:7489795. doi: 10.1155/2020/7489795
29. Balkau B, Jouven X, Ducimetiere P, Eschwege E. Diabetes as a risk factor for sudden death. Lancet. (1999) 354:1968–9. doi: 10.1016/S0140-6736(99)04383-4
30. Kucharska-Newton AM, Couper DJ, Pankow JS, Prineas RJ, Rea TD, Sotoodehnia N, et al. Diabetes and the risk of sudden cardiac death, the atherosclerosis risk in communities study. Acta Diabetol. (2010) 47(Suppl 1):161–8. doi: 10.1007/s00592-009-0157-9
31. Zaccardi F, Khan H, Laukkanen JA. Diabetes mellitus and risk of sudden cardiac death: a systematic review and meta-analysis. Int J Cardiol. (2014) 177:535–7. doi: 10.1016/j.ijcard.2014.08.105
32. Aune D, Schlesinger S, Norat T, Riboli E. Diabetes mellitus and the risk of sudden cardiac death: a systematic review and meta-analysis of prospective studies. Nutr Metab Cardiovasc Dis. (2018) 28:543–56. doi: 10.1016/j.numecd.2018.02.011
33. Lynge TH, Svane J, Pedersen-Bjergaard U, Gislason G, Torp-Pedersen C, Banner J, et al. Sudden cardiac death among persons with diabetes aged 1-49 years: a 10-year nationwide study of 14 294 deaths in Denmark. Eur Heart J. (2020) 41:2699–706. doi: 10.1093/eurheartj/ehz891
34. Albert CM, Chae CU, Grodstein F, Rose LM, Rexrode KM, Ruskin JN, et al. Prospective study of sudden cardiac death among women in the United States. Circulation. (2003) 107:2096–101. doi: 10.1161/01.CIR.0000065223.21530.11
35. Bertoia ML, Allison MA, Manson JE, Freiberg MS, Kuller LH, Solomon AJ, et al. Risk factors for sudden cardiac death in post-menopausal women. J Am Coll Cardiol. (2012) 60:2674–82. doi: 10.1016/j.jacc.2012.09.031
36. Kannel WB, Wilson PW, D'agostino RB, Cobb J. Sudden coronary death in women. Am Heart J. (1998) 136:205–12. doi: 10.1053/hj.1998.v136.90226
37. Escobedo LG, Caspersen CJ. Risk factors for sudden coronary death in the United States. Epidemiology. (1997) 8:175–80. doi: 10.1097/00001648-199703000-00009
38. Benjamins MR, Silva A, Saiyed NS, De Maio FG. Comparison of all-cause mortality rates and inequities between black and white populations across the 30 most populous US cities. JAMA Netw Open. (2021) 4:e2032086. doi: 10.1001/jamanetworkopen.2020.32086
39. Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. (2001) 104:2158–63. doi: 10.1161/hc4301.098254
40. Patel M, Abatcha S, Uthman OA. Ethnic differences between south asians and white caucasians in cardiovascular disease-related mortality in developed countries: a systematic literature review protocol. BMJ Open. (2022) 12:e052487. doi: 10.1136/bmjopen-2021-052487
41. Deo R, Albert CM. Epidemiology and genetics of sudden cardiac death. Circulation. (2012) 125:620–37. doi: 10.1161/CIRCULATIONAHA.111.023838
42. Becker LB, Han BH, Meyer PM, Wright FA, Rhodes KV, Smith DW, et al. Racial differences in the incidence of cardiac arrest and subsequent survival. The CPR Chicago project. N Engl J Med. (1993) 329:600–6. doi: 10.1056/NEJM199308263290902
43. Cowie MR, Fahrenbruch CE, Cobb LA, Hallstrom AP. Out-of-hospital cardiac arrest: racial differences in outcome in Seattle. Am J Public Health. (1993) 83:955–9. doi: 10.2105/AJPH.83.7.955
44. Chan PS, Nichol G, Krumholz HM, Spertus JA, Jones PG, Peterson ED, et al. Racial differences in survival after in-hospital cardiac arrest. J Am Med Assoc. (2009) 302:1195–201. doi: 10.1001/jama.2009.1340
45. Ortega VE, Meyers DA. Pharmacogenetics: implications of race and ethnicity on defining genetic profiles for personalized medicine. J Allergy Clin Immunol. (2014) 133:16–26. doi: 10.1016/j.jaci.2013.10.040
46. Tada H, Fujino N, Nomura A, Nakanishi C, Hayashi K, Takamura M, et al. Personalized medicine for cardiovascular diseases. J Hum Genet. (2021) 66:67–74. doi: 10.1038/s10038-020-0818-7
47. Perini W, Snijder MB, Agyemang C, Peters RJ, Kunst AE, Van Valkengoed IG. Eligibility for cardiovascular risk screening among different ethnic groups: the HELIUS study. Eur J Prev Cardiol. (2020) 27:1204–11. doi: 10.1177/2047487319866284
48. Ahmed B, Davis HT, Laskey WK. In-hospital mortality among patients with type 2 diabetes mellitus and acute myocardial infarction: results from the national inpatient sample, 2000-2010. J Am Heart Assoc. (2014) 3(4):e001090. doi: 10.1161/JAHA.114.001090
49. Ali B, Dargham S, Al Suwaidi J, Jneid H, Abi Khalil C. Temporal trends in outcomes of ST-elevation myocardial infarction patients with heart failure and diabetes. Front Physiol. (2022) 13:803092. doi: 10.3389/fphys.2022.803092
50. Chen PS, Chen LS, Cao JM, Sharifi B, Karagueuzian HS, Fishbein MC. Sympathetic nerve sprouting, electrical remodeling and the mechanisms of sudden cardiac death. Cardiovasc Res. (2001) 50:409–16. doi: 10.1016/S0008-6363(00)00308-4
51. Fernandez SF, Ovchinnikov V, Canty JM, Fallavollita JA. Hibernating myocardium results in partial sympathetic denervation and nerve sprouting. Am J Physiol Heart Circ Physiol. (2013) 304:H318–327. doi: 10.1152/ajpheart.00810.2011
52. Canty JM, Suzuki G, Banas MD, Verheyen F, Borgers M, Fallavollita JA. Hibernating myocardium: chronically adapted to ischemia but vulnerable to sudden death. Circ Res. (2004) 94:1142–9. doi: 10.1161/01.RES.0000125628.57672.CF
53. Field JM, Hazinski MF, Sayre MR, Chameides L, Schexnayder SM, Hemphill R, et al. Part 1: executive summary: 2010 American heart association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. (2010) 122:S640–656. doi: 10.1161/CIRCULATIONAHA.110.970889
54. Satoh H. Pleiotropic effects of SGLT2 inhibitors beyond the effect on glycemic control. Diabetol Int. (2018) 9:212–4. doi: 10.1007/s13340-018-0367-x
55. Fernandes GC, Fernandes A, Cardoso R, Penalver J, Knijnik L, Mitrani RD, et al. Association of SGLT2 inhibitors with arrhythmias and sudden cardiac death in patients with type 2 diabetes or heart failure: a meta-analysis of 34 randomized controlled trials. Heart Rhythm. (2021) 18:1098–105. doi: 10.1016/j.hrthm.2021.03.028
56. Kowalska K, Wilczopolski P, Bulawska D, Mlynarska E, Rysz J, Franczyk B. The importance of SGLT-2 inhibitors as both the prevention and the treatment of diabetic cardiomyopathy. Antioxidants (Basel). (2022) 11(12):2500. doi: 10.3390/antiox11122500
57. Li HL, Lip GYH, Feng Q, Fei Y, Tse YK, Wu MZ, et al. Sodium-glucose cotransporter 2 inhibitors (SGLT2i) and cardiac arrhythmias: a systematic review and meta-analysis. Cardiovasc Diabetol. (2021) 20:100. doi: 10.1186/s12933-021-01293-8
58. Sfairopoulos D, Zhang N, Wang Y, Chen Z, Letsas KP, Tse G, et al. Association between sodium-glucose cotransporter-2 inhibitors and risk of sudden cardiac death or ventricular arrhythmias: a meta-analysis of randomized controlled trials. Europace. (2022) 24:20–30. doi: 10.1093/europace/euab177
59. Liu Z, Bian N, Wu S, Fan Y, Li H, Yu J, et al. A meta-analysis evaluating indirectly GLP-1 receptor agonists and arrhythmias in patients with type 2 diabetes and myocardial infarction. Front Cardiovasc Med. (2022) 9:1019120. doi: 10.3389/fcvm.2022.1019120
Keywords: ST elevation myocardial infarction, sudden cardiac arrest, diabetes, mortality, cardiovascular disease
Citation: Mhaimeed O, Pillai K, Dargham S, Al Suwaidi J, Jneid H and Abi Khalil C (2023) Type 2 diabetes and in-hospital sudden cardiac arrest in ST-elevation myocardial infarction in the US. Front. Cardiovasc. Med. 10:1175731. doi: 10.3389/fcvm.2023.1175731
Received: 28 February 2023; Accepted: 31 May 2023;
Published: 3 July 2023.
Edited by:
Gennaro Galasso, University of Salerno, ItalyReviewed by:
Monica Verdoia, University of Eastern Piedmont, ItalyMichele Bellino, University of Salerno, Italy
© 2023 Mhaimeed, Pillai, Dargham, Al Suwaidi, Jneid and Abi Khalil. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Charbel Abi Khalil Y2hhMjAyMkBtZWQuY29ybmVsbC5lZHU=
†These authors have contributed equally to this work
 Omar Mhaimeed1,2,†
Omar Mhaimeed1,2,† Soha Dargham
Soha Dargham Charbel Abi Khalil
Charbel Abi Khalil