
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 09 June 2023
Sec. Coronary Artery Disease
Volume 10 - 2023 | https://doi.org/10.3389/fcvm.2023.1175287
Background and aims: The risk factors of perioperative and long-term cardiovascular events in patients undergoing coronary artery bypass grafting (CABG) with adjunctive coronary endarterectomy (CE) are not well determined. This study evaluated the clinical value of coronary plaque burden, coronary anatomic stenosis, and serum biomarkers for predicting perioperative cardiovascular events after off-pump CABG + CE.
Methods: This retrospective cohort single-center study enrolled 125 patients undergoing off-pump CABG + CE between February 2018 and September 2021 in China. Coronary plaque burden was reflected by the length of plaque removed by CE. Plaque length-max, which represents the plaque length in patients undergoing single-vessel CE and the maximum plaque length in patients undergoing multivessel CE, was calculated. The primary endpoint was perioperative myocardial infraction (PMI).
Results: Plaque length-max was significantly higher in patients with PMI than in those without PMI (2.4 ± 1.5 vs. 1.6 ± 0.9, p = .001). A threshold plaque length-max of 1.15 cm was an independent predictor of PMI (area under the curve: 0.67; sensitivity 87.9%; specificity 59.8%; p = .005). Patients with plaque length-max ≥1.15 had a > 5-fold increase in PMI after adjusting for confounding factors (odds ratio = 5.89; p = .002). Furthermore, interleukin-6 (Beta = .32: p = .028), CD68 (Beta = .34; p = .045), and osteopontin (Beta = .43; p = .008) were significantly correlated with plaque length-max.
Conclusions: Plaque length-max was superior to clinical cardiovascular risk factors in predicting PMI occurrence after off-pump CABG + CE, which might be associated with systemic and plaque inflammation state.
Coronary artery bypass grafting (CABG) is the main surgical intervention for coronary revascularization in patients with multivessel coronary artery diseases; however, it fails to achieve satisfactory outcomes in patients with diffuse coronary artery disease (DCAD) due to incomplete revascularization (1, 2). Coronary endarterectomy (CE), as an adjunct to CABG, has been used to treat patients with DCAD in whom CABG alone could not achieve satisfactory outcomes (3, 4). While the occurrence of perioperative cardiovascular events after CE, including perioperative myocardial infarction (PMI) and mortality, remains up to 19%, which is 2–3 folds higher than that after CABG alone (5–8). These risks of perioperative adverse events are still a major challenge for CABG with adjunctive CE (CABG + CE).
Previous studies have attempted to identify specific biomarkers for predicting PMI (9–11), including serum biomarkers, SYNTAX score, and EUROScore. The prognostic value of serum biomarkers, especially inflammatory biomarkers, such as interleukin-6 (IL-6), tumor necrosis factor, and C-reactive protein, are associated with the occurrence of cardiac events in acute coronary syndrome patients or patients undergoing CABG alone, while these value in DCAD patients after CE is influenced by multi-factors. The endothelial dysfunction or residual lesions induced by coronary plaque removal during CE and vulnerable plaque rupture may all account for cardiac events in patients with DCAD (12–14).
The associations between inflammation, plaque burden, and cardiac events after CE are still unclear. Theoretically, coronary endothelium damage and distal residual lesions may correlate with the length of plaque removed by CE, and plaque length can also reflect the plaque burden of the coronary artery. In addition, pathological analysis of plaques can reveal the inflammatory microenvironment. Therefore, the purpose of this study was to evaluate the clinical value of plaque length, coronary anatomic stenosis (SYNTAX score), and serum biomarker for predicting perioperative cardiovascular events after off-pump CABG + CE.
This was a retrospective cohort study of 512 patients with coronary artery disease (CAD) who visited the outpatient clinic of Beijing Anzhen Hospital between February 2018 and September 2021. Of these, 143 patients with DCAD were scheduled for off-pump CABG with adjunctive CE (2). Patients with recent myocardial infarction (MI) (within 4 weeks of surgery), history of malignancy, inflammatory or autoimmune disease, or history of cardiac surgery were excluded. Finally, 125 patients were enrolled. The primary endpoint was PMI, which was defined as an elevation of troponin I (TNI) values >10× 99th percentile upper reference limit within 7 days of surgery, in patients together with either: (a) new pathologic Q waves on the electrocardiogram, or (b) new abnormal segment wall motion of the ventricular wall on echocardiography, according to the fourth universal definition of myocardial infarction (2018) by expert consensus.
This study was conducted in accordance with the Declaration of Helsinki. The trial was approved by the Beijing Anzhen Hospital Medical Ethics Committee (No. 2015012X) and was a retrospective sub-analysis of the prospective trial registered with the Chinese Clinical Trial Registry (ChiCTR1900022527). Written informed consent was obtained from all patients without stipend.
Off-pump CABG with adjunctive CE was performed in at least one artery by two experienced cardiologists (CG, YY) if patients with DCAD were unsuitable for off-pump CABG alone (15). Closed-CE involved a smaller arteriotomy than open-CE to remove the atherosclerotic plaque by applying steady, gentle traction on the plaque proximally and distally. If the distal target vessel or its side branches were insufficiently revascularized, a second, concurrent arteriotomy was performed to remove the total distal end of the plaque after angioplasty. Graft flow was measured using the Veri Q flow meter (Medi-Stim Inc., Oslo, Norway) after conventional off-pump CABG to guarantee patency.
The geometry of 144 plaques obtained from all 125 patients undergoing CE, including the length and diameter of plaques, was routinely measured by pathologists. We defined plaque length as the length of the coronary plaque obtained through CE. Plaque length-max and plaque diameter-max, which represent the plaque length and plaque diameter, respectively, in patients undergoing single-vessel CE and the maximum plaque length and maximum plaque diameter, respectively, in patients undergoing multivessel CE, were calculated. Systemic heparinization was initiated post-surgery to avoid thromboembolic complications or early occlusion of the coronary arteries. When the postoperative bleeding rate was <50 mL/h, heparin infusion was initiated 4 h post-surgery. Aspirin (100 mg) and clopidogrel (75 mg) were administered daily starting on postoperative day 1 (16).
The SYNTAX score was calculated for individual arteries and lesions using an online calculator (http://www.syntaxscore.com/calculator/start.htm). The EUROScore II was calculated for each patient using an online calculator (http://www.euroscore.org/calc.html). Both SYNTAX score and EUROScore II were conducted by two cardiologists (MG, HL) who were blinded to patient data.
Two cardiologists (MG, HL) determined PMI occurrence according to the plasma TNI levels, electrocardiograms, and echocardiography results (17). PMI was defined as an elevation of TNI values >10× the 99th percentile upper reference limit within 7 days of surgery, in patients together with either (a) new pathologic Q waves on the electrocardiogram or (b) new abnormal segment wall motion of the ventricular wall on echocardiography (17). Arrhythmia was defined as the occurrence of ventricular tachycardia, fibrillation, or atrial fibrillation within 7 days post-surgery.
With the patients' consent, we performed additional analysis of pathological features in 37 coronary plaques obtained from 27 patients. Immunohistochemical staining was performed for CD68, osteopontin, and osteocalcin, and the average optical values were assessed. Plaque pathological features were determined from the hematoxylin and eosin slides according to the American Heart Association classification (18). The selected specimens were decalcified in ethylenediaminetetraacetic acid (after being stored in 4% paraformaldehyde for 24 h), embedded in paraffin, and sawed into 5-mm-thick sections for the analysis of their morphological characteristics. Hematoxylin and eosin (H&E) staining was performed. Immunohistochemical staining for CD68 (mouse anti-human ab955, Abcam, Cambridge, UK) and osteopontin (rabbit anti-human ab214050) was performed, and the average optical values were assessed, as described below. Slides were scanned using Leica Biosystems software (Germany) and assessed using CaseViewer software (Pannoramic MIDI, 3D HISTECH, Hungary). Masson's trichrome histochemical staining (Sigma Aldrich, St. Louis, MO) was performed for collagen and fibrosis analysis.
The H&E slides were analyzed by two experienced pathologists. Morphological features, including a large necrotic core, thin fibrous cap, loose fibroelastic tissue, intra-plaque hemorrhage, calcification, and microcalcifications, were identified according to the American Heart Association classifications.
The average optical value analysis of CD68 and osteopontin was performed using Image-pro plus 6.0 software (Media Cybernetics, Inc., Rockville, MD). We randomly screenshotted three 400-fold fields of each slice, carefully ensuring consistent background lighting in all photographs. A brownish yellow color was selected as the unified standard for evaluating the positive expression of all photographs; the integral optical density and pixel area of each photograph were determined. The average optical value is expressed as integral optical density/pixel area; a large average optical value indicates a great positive expression level.
Statistical analyses were performed using SPSS software (version 25, SPSS, Inc., Chicago, IL). Data were tested for normality using the Shapiro-Wilk test. Continuous variables with a normal distribution are presented as means ± standard deviations; non-normal variables are reported as medians [interquartile ranges (IQRs)]. Data were compared using a two-sample t-test or Mann–Whitney U tests. Categorical variables are presented as absolute numbers (percentages) and were compared using a χ2 test (with a Yates correction or Fisher exact test for smaller sample sizes).
Univariate and multivariable logistic regression analyses (forced entry method) were performed to assess the association between plaque length and PMI occurrence. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated using logistic regression analyses. In addition, univariate linear regression analyses (forced entry method) were performed to assess the association between preoperative plasma biomarker levels, plaque length, and all pathological features. Beta and 95% CIs were estimated using linear regression analyses. Receiving operator curves (ROC) were calculated for the prediction of PMI based on plaque length and the optimal plaque length cut-off value for predicting PMI was determined. A two-sided p value<.05 was considered statistically significant.
This study recruited 143 patients with DCAD. Eleven patients with a recent MI, one with malignancy, and six with chronic hepatitis were excluded (Figure 1). Accordingly, the final study population consisted of 125 patients who had undergone CE (102 male; mean age: 61.9 ± 8.3 years), of whom 107 (85.6%) underwent single-vessel CE and 18 (14.4%) underwent multivessel CE (Table 1). Thirty-three patients (26.4%) experienced PMI, including three (9.1%) with PMIanterior, four (12.1%) with PMIlateral, 23 (69.7%) with PMIinferior, one (3.0%) with both PMIanterior and PMIlateral, and two (6.1%) with both PMIanterior and PMIinferior.
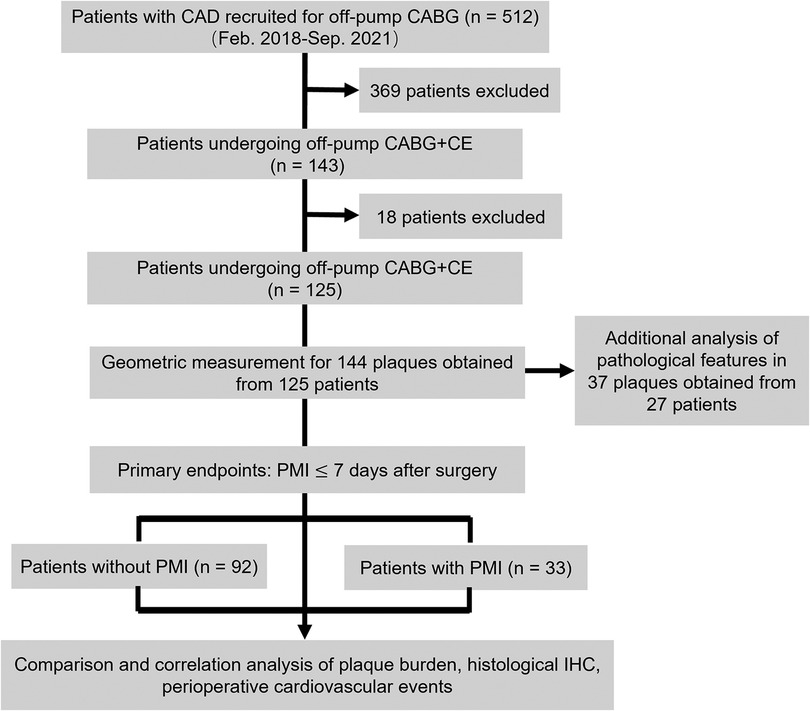
Figure 1. Study design flowchart. CAD, coronary artery disease; CABG, coronary artery bypass grafting; CE, coronary endarterectomy; IHC, immunohistochemistry; PMI, postoperative myocardial infarction.
Plaque length-max was significantly higher in patients who experienced PMI than in those who did not (2.4 ± 1.5 vs. 1.6 ± 0.9, p = .001) (Table 2). ROC curves analyses were constructed to evaluate the predictive values of plaque length-max, SYNTAX score, EUROScore II, and operative time for the identification of PMI (Figure 2). A threshold plaque length-max of 1.15 cm was a significant predictor of total PMI (area under the curve: 0.67; sensitivity 87.9%; specificity 59.8%; p = .005). The curve area of SYNTAX score, EUROScore II, and operative time were 0.57 (p = 0.353), 0.61 (p = 0.166), and 0.64 (p = 0.089), respectively. After adjusting for confounding factors of age, sex, body mass index (BMI), patients with plaque length-max ≥1.15 had a >5-fold increase in PMI [odds ratio (OR) = 5.89; p = .002]. Moreover, multivariate logistic regression analyses revealed that CE of the posterior descending artery had no significant correlation with PMIinferior occurrence (OR = 3.05; p = .06). Patients with plaque length-max ≥ 1.15 had a > 5-fold increase in PMIinferior after adjusting for confounding factors (OR = 5.84; p = .026) (Table 3).
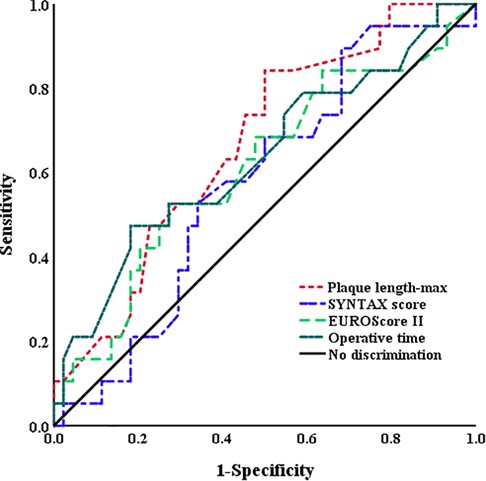
Figure 2. Receiving operator curves using plaque length-max, SYNTAX score, EUROScore II, and operative time for the identification of PMI. The AUC for the plaque length-max (0.67) was higher than SYNTAX score (0.57), EUROScore II (0.61), and operative time (0.64). AUC, area under curve; PMI, postoperative myocardial infarction.
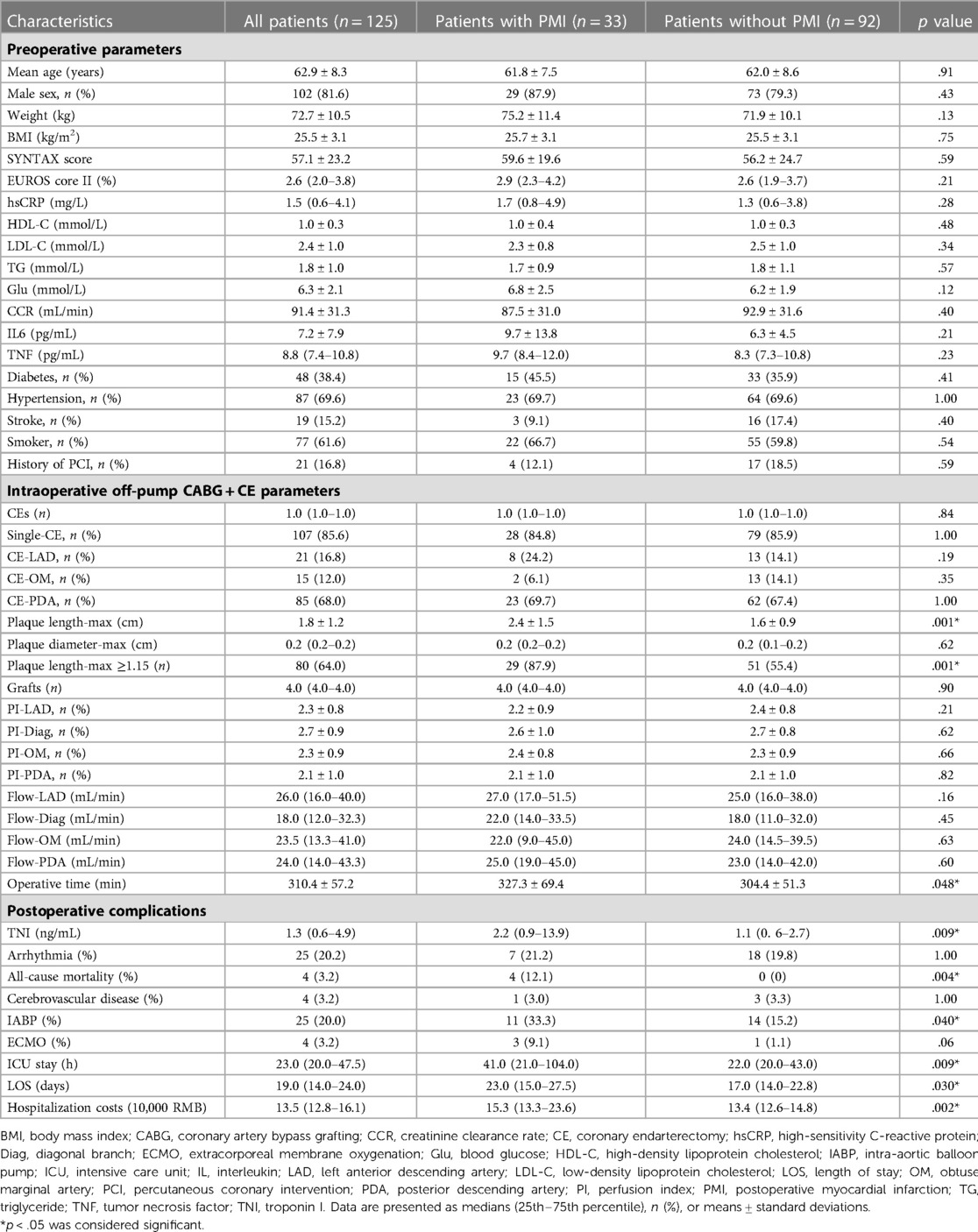
Table 2. Distribution of all clinical parameters in patients with and without postoperative myocardial infarction.
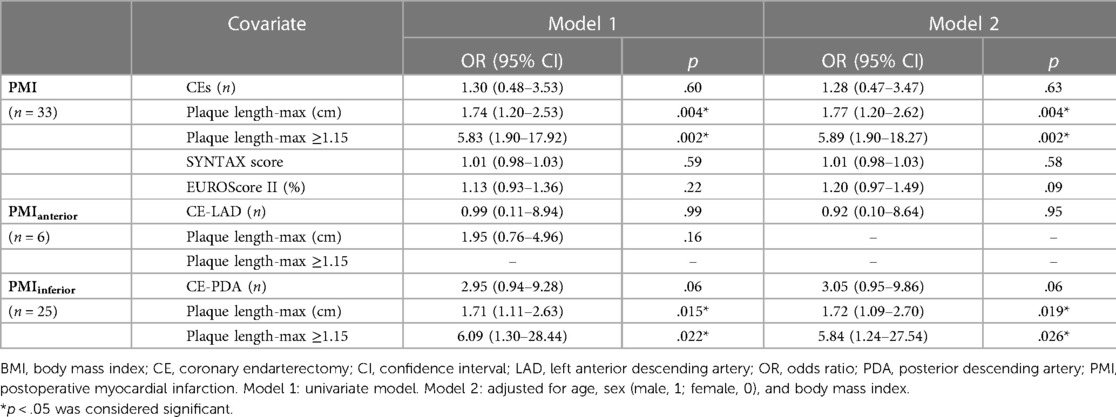
Table 3. Association between PMI, PMIanterior, PMIinferior, and clinical parameters in univariate and multivariable logistic regression models.
PMI significantly increased TNI (2.2 [0.9–13.9] ng/mL vs. 1.1 [0.6–2.7] ng/mL; p = .009), all-cause mortality (4 [12.1] vs. 0 [0]; p = .004), use of an intra-aortic balloon pump (IABP) (11 [33.3] vs. 14 [15.2]; p = .040), rate of intensive care unit (ICU) stay (41.0 [21.0–104.0] h vs. 22.0 [20.0–43.0] h; p = .009), length of stay (LOS) (23.0 [15.0–27.5] h vs. 17.0 [14.0–22.8] h; p = .030), and hospitalization cost (15.3 [13.3–23.6] 10,000 RMB vs. 13.4 [12.6–14.8] 10,000 RMB; p = .002) (Table 2). There was no significant difference in serum biomarkers, SYNTAX score, EUROScore II, or intraoperative parameters between patients with and without PMI (all p > .05) (Table 2).
As shown in Table 4, linear regression analysis revealed that IL-6 was significantly correlated with plaque length-max (Beta = 0.32: p = .028), whereas the other clinical variables were not (all p > .05).
The average length of all 37 plaque specimens obtained through CE was 1.25 (IQR: 1.00–2.00) cm. Linear regression analysis revealed that CD68 (Beta = .34; p = .045), osteopontin (Beta = 0.43; p = .008), and calcium score (r = .52; p = .001) were significantly correlated with plaque length-max (Table 5). Patients with plaque length-max ≥1.15 cm had increased average optical values of osteopontin co-localized in areas with higher CD68 expression. In plaques of length <1.15 cm, a portion of osteopontin was localized in microcalcifications adjacent to the initial calcification and margins of large macrocalcifications (Figure 3).
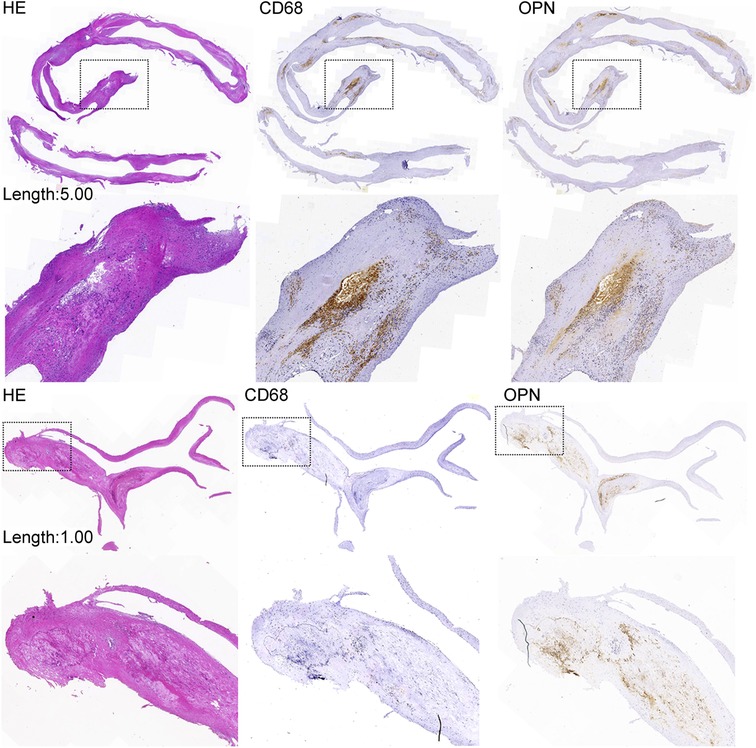
Figure 3. Coronary plaque length and immunochemical examination. The expression of OPN in plaques of length ≥1.15 cm is increased and correlated with increased CD68 expression. The expression of OPN in plaques of length <1.15 cm is decreased and correlated with decreased CD68 expression. The expression of OPN was also increased in microcalcifications adjacent to the initial calcification and at the margins of large macrocalcifications. HE, hematoxylin and eosin; OPN, osteopontin.
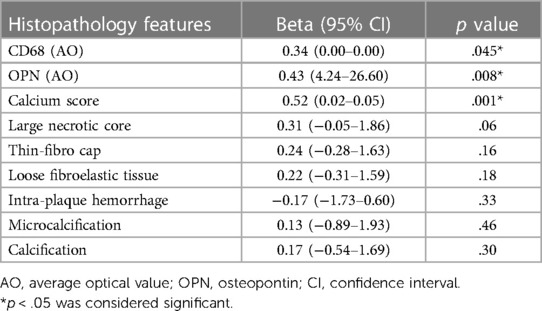
Table 5. Association between histopathology features and plaque length in 37 coronary specimens in linear regression model.
We analyzed the predictive value of plaque length for occurrence of PMI after off-pump CABG with adjunctive CE. We found that plaque length-max correlated with PMI occurrence and a plaque length-max threshold of 1.15 cm was an independent predictor for PMI. Furthermore, we also found a correlation between focal plaque length and IL-6, a systemic inflammatory biomarker, and validated the correlation between plaque length in vivo and plaque histopathologic features in vitro. To the best of our knowledge, this is the first study to predict perioperative cardiovascular events by coronary plaque burden in patients who underwent off-pump CABG with adjunctive CE (Figure 4).
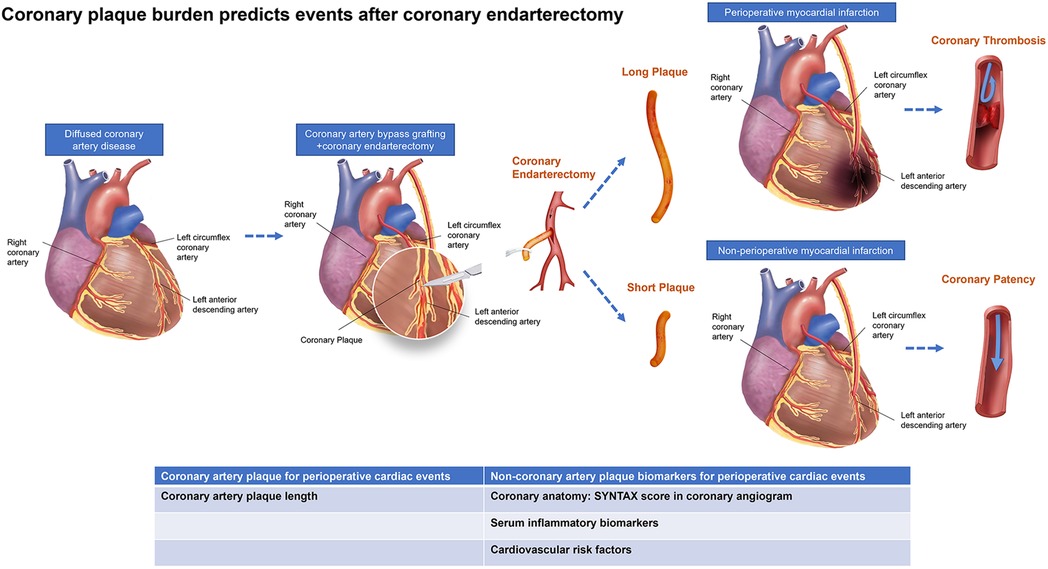
Figure 4. Central illustration. Schematic illustration of the occurrence of adverse events shows vascular occlusions after off-pump coronary artery bypass grafting compatible with coronary endarterectomy in patients with diffused coronary artery disease. Multifactorial validation on both coronary arteries’ anatomy, plaque morphology, and serum biomarkers may yield further insights into the interplay of alternative morphological and molecular processes, including coronary stenosis, inflammation, osteogenesis, and metabolic processes.
CABG is the standard treatment strategy for complete revascularization in patients with complex CAD and incomplete revascularization is a critical negative factor influencing long-term mortality and morbidity (2, 19). DCAD accounts for 25% of patients with complex CAD, and complete revascularization is rarely achieved by CABG alone in these patients (20, 21). Therefore, CE is recommended as an adjunct to CABG for DCAD patients to achieve complete revascularization via the removal of coronary plaques in the target vessels if complete revascularization could not be achieved (5, 15, 22). However, a consequent rise in PMI occurrence and 30-day outcomes is worse in patients undergoing CABG adjunct with CE than in those undergoing CABG alone (23, 24). PMI rate was 24.7% in our study, which was higher than the rate of 2.8% in patients undergoing CABG alone reported in previous literature (6).
Compared with closed-CE, open-CE typically achieves complete plaque removal, prevents postoperative thrombotic occlusions, and reduces perioperative cardiac events, particularly in patients with DCAD in the left coronary descending artery (LAD) (7, 8, 25–27). Nevertheless, based on empirical data for patients with DCAD in other target arteries that are harder to access than the LAD, closed-CE is a good choice to achieve complete revascularization. In this study, all 125 patients underwent closed-CE, and a second CE was performed to remove the total distal end of the plaque if the graft patency of the distal target vessel or its side branches was insufficiently revascularized, as judged by the Veri Q flow meter. The prevalence of target vessels for CABG adjunct with CE was 16.8% in LAD, 18.4% in diagonal branches of the LAD, 12.0% in left circumflex, and 68.0% in posterior descending artery. This may explain why the incidence of total PMI, particularly PMIinferior, was higher in this study than in a previous study (28). Moreover, patients with PMI had higher rates of perioperative all-cause mortality and IABP procedure, longer ICU stay and LOS, and greater hospitalization costs than those without PMI. It indicated that the identification of high-risk patients who are more likely to develop PMI is very important.
Intact coronary endothelium can produce vasoactive biomarkers to neutralize leukocyte adhesion and to inhibit platelet aggregation, consequently reducing inflammation and thrombosis in a target artery (29, 30). The poor short-term postoperative outcomes in our current study patients with DCAD undergoing CABG + CE may be due to endothelial cell injury, loss of cytoprotective factors, unsteady blood flow or shear stress, and distal residual lesions caused by closed-CE (12–14). Acute PMI, a key complication associated with CE failure, is also associated with thrombosis, which can be induced by endothelial lining removal (13, 14, 31). Theoretically, endothelium damage and distal residual lesions are correlated with the plaque length removed by CE. Surgeons always try to reduce endothelium damage by shortening plaque length during CE. However, there are no criteria for surgeons to determine the location and length of the CE. The key issue is that the association of plaque length with acute perioperative clinical outcomes remained unclear. We found that increased plaque length was significantly associated with PMI occurrence. Patients with plaque length-max ≥1.15 had a >5-fold increase in PMI and a >5-fold increase in PMIinferior. Therefore, surgeons should adequately assess the benefits and adverse effects of CE and preoperatively determine the location of the CE to achieve satisfactory clinical outcomes. Although the AUROC of plaque length-max in predicting PMI was 0.67, it was still more powerful in predicting events than other risk factors in this relatively rare sample.
The elevation of serum systemic inflammatory biomarkers post-surgery, including IL-6, tumor necrosis factor, and C-reactive protein, is correlated with PMI incidence. While the prognostic significance of serum biomarkers remains controversial (32–35). We found that preoperative serum biomarkers were not correlated with PMI occurrence. This suggests that serum biomarkers have limited early prognostic value due to a lack of organ specificity and less sensitivity for predicting postoperative cardiac events. Recent studies have found that plasma levels of sLOX-1 can predict fatal events at 1 year in ACS patients and JCAD can promote the formation of arterial thrombosis in STEMI patients by selectively regulating coagulation and fibrinolysis. Whereas, the power of sLOX-1 predicting cardiovascular events after CABG or JCAD preventing the formation of platelet thrombosis after CABG remains to be investigated (36, 37). IL-6 plays a central role as a mediator of the inflammatory response and is essential for the initiation and progression of the atherosclerotic process (38, 39). We found a linear correlation between IL-6 and plaque length-max. It suggested that IL-6 could reflect the severity of inflammation in total atherosclerotic plaques and can be considered as an indirect parameter for the prediction of PMI. In a previous intracoronary imaging study with optical coherence tomography (OCT) that investigated outcomes of patients with non-culprit OCT-defined vulnerable plaques, serum levels of C-reactive protein were not associated with intraplaque accumulation of inflammatory cells (OCT-defined macrophages). This is probably due to the more specific role of IL-6 compared with that of C-reactive protein. Furthermore, a positive association between intraplaque inflammatory cells and adverse clinical events was found in the paper by Gatto et al., further corroborating results obtained in the present study (40).
Osteopontin is expressed in human atherosclerotic plaques and is strongly correlated with vulnerable plaques. Carotid plaque osteopontin and serum osteopontin levels have been reported to predict cardiovascular events (41, 42). Systemic inflammation biomarker (IL-6) may trigger IL-6 trans-signaling, contributing to the upregulation of osteopontin in macrophages (43, 44). Moreover, osteopontin expression is associated with macrophages and foam cells within atherosclerotic lesions (45). In our study, osteopontin expression in coronary plaques >1.15 cm in length was significantly increased. Osteopontin and CD68 also were correlated with plaque length. Thus, it could be explained that both systemic inflammation (IL-6) and regional inflammation (CD68) possibly contribute to plaque vulnerability and lead to coronary plaque progression. We postulated that, in patients with increased plaque length who underwent off-pump CABG + CE, the inflammatory microenvironment within the damaged coronary artery activates processes, such as thrombosis processes, subsequently leading to PMI, and it may affect the long-term cardiac events.
First, the sample size was relatively small; and it was not sufficient to evaluate the association between the CE location and local PMI. Second, recruiting a homogeneous group of patients with severe CAD may have biased the CABG indications. Third, contrast-enhanced coronary CT or coronary angiography should have been performed in patients with PMI to confirm the graft patency of affected vessels despite the fact that most patients with PMI had stable hemodynamics. Finally, a long-term follow-up study is warranted to validate the prognostic value of plaque length for long-term outcomes in these patients.
Plaque length-max was an independent predictor for PMI occurrence in patients undergoing off-pump CABG + CE, which might be associated with systemic and regional plaque inflammation.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
The trial was approved by the Beijing Anzhen Hospital Medical Ethics Committee (No. 2015012X) and registered at the Chinese Clinical Trial Committee (ChiCTR1900022527). Written informed consent was obtained from all patients without stipend. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
MG and WW have contributed equally to this work. MG, XZ, YY and HL: study concept and design. MG, WW and CG: acquisition, analysis, or interpretation of data. MG, WW and XZ: drafting of the manuscript. MG, YY and HL: obtained funding. All authors: critical revision of the manuscript for important intellectual content, read and approved the final manuscript.
This study is supported by grants from Beijing Municipal Science & Technology Commission (NO. Z221100007422015), Beijing Municipal Hospital Administration Youth Plan (NO. QML20230603), the Science and Technology Development Fund of Beijing Anzhen Hospital (NO. AZ2022), Scientific Research Common Program of Beijing Municipal Commission of Education (NO. KM202110025014), Beijing Hospitals Authority Clinical medicine Development of special funding support (NO. XMLX202107), the Beijing Hospitals Authority's Ascent Plan (NO. DFL20220605) and National Natural Science Foundation of China (NO. 82070364). These funders had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
We wish to thank Ziwei Zhu, Department of Nuclear Medicine, Zhongshan Hospital affiliated to Fudan University, for her contribution to the histopathological analyses and advice that helped improve the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer LW declared a shared affiliation with the authors to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Fukui T, Takanashi S, Hosoda Y. Long segmental reconstruction of diffusely diseased left anterior descending coronary artery with left internal thoracic artery with or without endarterectomy. Ann Thorac Surg. (2005) 80:2098–105. doi: 10.1016/j.athoracsur.2005.06.047
2. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. (2019) 40:87–165. doi: 10.1093/eurheartj/ehy394
3. Laaksonen R, Ekroos K, Sysi-Aho M, Hilvo M, Vihervaara T, Kauhanen D, et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur Heart J. (2016) 37:1967–76. doi: 10.1093/eurheartj/ehw148
4. Wallace EL, Morgan TM, Walsh TF, Dall'Armellina E, Ntim W, Hamilton CA, et al. Dobutamine cardiac magnetic resonance results predict cardiac prognosis in women with known or suspected ischemic heart disease. JACC Cardiovasc Imaging. (2009) 2:299–307. doi: 10.1016/j.jcmg.2008.10.015
5. Wang J, Gu C, Yu W, Gao M, Yu Y. Short- and long-term patient outcomes from combined coronary endarterectomy and coronary artery bypass grafting: a meta-analysis of 63,730 patients (PRISMA). Medicine (Baltim). (2015) 94:e1781. doi: 10.1097/MD.0000000000001781
6. Soylu E, Harling L, Ashrafian H, Casula R, Kokotsakis J, Athanasiou T. Adjunct coronary endarterectomy increases myocardial infarction and early mortality after coronary artery bypass grafting: a meta-analysis. Interact Cardiovasc Thorac Surg. (2014) 19:462–73. doi: 10.1093/icvts/ivu157
7. Soylu E, Harling L, Ashrafian H, Athanasiou T. Does coronary endarterectomy technique affect surgical outcome when combined with coronary artery bypass grafting? Interact Cardiovasc Thorac Surg. (2014) 19:848–55. doi: 10.1093/icvts/ivu261
8. Taşdemir O, Kiziltepe U, Karagöz HY, Yamak B, Korkmaz S, Bayazit K. Long-term results of reconstructions of the left anterior descending coronary artery in diffuse atherosclerotic lesions. J Thorac Cardiovasc Surg. (1996) 112:745–54. doi: 10.1016/S0022-5223(96)70061-2
9. Budoff MJ, Mayrhofer T, Ferencik M, Bittner D, Lee KL, Lu MT, et al. Prognostic value of coronary artery calcium in the PROMISE study (prospective multicenter imaging study for evaluation of chest pain). Circulation. (2017) 136:1993–2005. doi: 10.1161/CIRCULATIONAHA.117.030578
10. Serruys PW, Onuma Y, Garg S, Sarno G, van den Brand M, Kappetein AP, et al. Assessment of the SYNTAX score in the syntax study. EuroIntervention. (2009) 5:50–6. doi: 10.4244/EIJV5I1A9
11. Cho Y, Shimura S, Aki A, Furuya H, Okada K, Ueda T. The SYNTAX score is correlated with long-term outcomes of coronary artery bypass grafting for complex coronary artery lesions. Interact Cardiovasc Thorac Surg. (2016) 23:125–32. doi: 10.1093/icvts/ivw057
12. LaPar DJ, Anvari F, Irvine JN Jr, Kern JA, Swenson BR, Kron IL, et al. The impact of coronary artery endarterectomy on outcomes during coronary artery bypass grafting. J Card Surg. (2011) 26:247–53. doi: 10.1111/j.1540-8191.2011.01247.x
13. Lawrie GM, Morris GC Jr, Silvers A, Wagner WF, Baron AE, Beltangady SS, et al. The influence of residual disease after coronary bypass on the 5-year survival rate of 1274 men with coronary artery disease. Circulation. (1982) 66:717–23. doi: 10.1161/01.CIR.66.4.717
14. Marinelli G, Chiappini B, Di Eusanio M, Di Bartolomeo R, Caldarera I, Marrozzini C, et al. Bypass grafting with coronary endarterectomy: immediate and long-term results. J Thorac Cardiovasc Surg. (2002) 124:553–60. doi: 10.1067/mtc.2002.124670
15. Stavrou A, Gkiousias V, Kyprianou K, Dimitrakaki IA, Challoumas D, Dimitrakakis G. Coronary endarterectomy: the current state of knowledge. Atherosclerosis. (2016) 249:88–98. doi: 10.1016/j.atherosclerosis.2016.03.036
16. Schmitto JD, Kolat P, Ortmann P, Popov AF, Coskun KO, Friedrich M, et al. Early results of coronary artery bypass grafting with coronary endarterectomy for severe coronary artery disease. J Cardiothorac Surg. (2009) 4:52. doi: 10.1186/1749-8090-4-52
17. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Circulation. (2018) 138:e618–651. doi: 10.1161/CIR.0000000000000617
18. Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Jr IW, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the committee on vascular lesions of the council on arteriosclerosis, American heart association. Circulation. (1995) 92:1355–74. doi: 10.1161/01.CIR.92.5.1355
19. Bangalore S, Guo Y, Samadashvili Z, Blecker S, Hannan EL. Revascularization in patients with multivessel coronary artery disease and severe left ventricular systolic dysfunction: everolimus-eluting stents versus coronary artery bypass graft surgery. Circulation. (2016) 133:2132–40. doi: 10.1161/CIRCULATIONAHA.115.021168
20. Santini F, Casali G, Lusini M, D'Onofrio A, Barbieri E, Rigatelli G, et al. Mid-term results after extensive vein patch reconstruction and internal mammary grafting of the diffusely diseased left anterior descending coronary artery. Eur J Cardiothorac Surg. (2002) 21:1020–5. doi: 10.1016/S1010-7940(02)00074-X
21. Kim YH, Park DW, Lee JY, Kim WJ, Yun SC, Ahn JM, et al. Impact of angiographic complete revascularization after drug-eluting stent implantation or coronary artery bypass graft surgery for multivessel coronary artery disease. Circulation. (2011) 123:2373–81. doi: 10.1161/CIRCULATIONAHA.110.005041
22. Sirivella S, Gielchinsky I, Parsonnet V. Results of coronary artery endarterectomy and coronary artery bypass grafting for diffuse coronary artery disease. Ann Thorac Surg. (2005) 80:1738–44. doi: 10.1016/j.athoracsur.2005.05.034
23. Silberman S, Dzigivker I, Merin O, Shapira N, Deeb M, Bitran D. Does coronary endarterectomy increase the risk of coronary bypass? J Card Surg. (2002) 17:267–71. doi: 10.1111/j.1540-8191.2001.tb01138.x
24. Tiruvoipati R, Loubani M, Lencioni M, Ghosh S, Jones PW, Patel RL. Coronary endarterectomy: impact on morbidity and mortality when combined with coronary artery bypass surgery. Ann Thorac Surg. (2005) 79:1999–2003. doi: 10.1016/j.athoracsur.2004.12.041
25. Soylu E, Harling L, Ashrafian H, Athanasiou T. Should we consider off-pump coronary artery bypass grafting in patients undergoing coronary endarterectomy? Interact Cardiovasc Thorac Surg. (2014) 19:295–301. doi: 10.1093/icvts/ivu116
26. Papakonstantinou NA, Baikoussis NG, Apostolakis E. Coronary endarterectomy: new flavors from old recipes. J Cardiol. (2014) 63:397–401. doi: 10.1016/j.jjcc.2014.02.005
27. Qiu Z, Auchoybur Merveesh L, Xu Y, Jiang Y, Wang L, Xu M, et al. The midterm results of coronary endarterectomy in patients with diffuse coronary artery disease. J Cardiothorac Surg. (2018) 13:90. doi: 10.1186/s13019-018-0776-8
28. Vohra HA, Kanwar R, Khan T, Dimitri WR. Early and late outcome after off-pump coronary artery bypass graft surgery with coronary endarterectomy: a single-center 10-year experience. Ann Thorac Surg. (2006) 81:1691–6. doi: 10.1016/j.athoracsur.2005.12.028
29. Selemidis S, Cocks TM. Endothelium-dependent hyperpolarization as a remote anti-atherogenic mechanism. Trends Pharmacol Sci. (2002) 23:213–20. doi: 10.1016/S0165-6147(02)01998-3
30. Mugge A, Forstermann U, Lichtlen PR. Platelets, endothelium-dependent responses and atherosclerosis. Ann Med. (1991) 23:545–50. doi: 10.3109/07853899109150516
31. Choi BJ, Matsuo Y, Aoki T, Kwon TG, Prasad A, Gulati R, et al. Coronary endothelial dysfunction is associated with inflammation and vasa vasorum proliferation in patients with early atherosclerosis. Arterioscler Thromb Vasc Biol. (2014) 34:2473–7. doi: 10.1161/ATVBAHA.114.304445
32. Podgoreanu MV, White WD, Morris RW, Mathew JP, Stafford-Smith M, Welsby IJ, et al. Inflammatory gene polymorphisms and risk of postoperative myocardial infarction after cardiac surgery. Circulation. (2006) 114:1275–81. doi: 10.1161/CIRCULATIONAHA.105.001032
33. Padayachee L, Rodseth RN, Biccard BM. A meta-analysis of the utility of C-reactive protein in predicting early, intermediate-term and long term mortality and major adverse cardiac events in vascular surgical patients. Anaesthesia. (2009) 64:416–24. doi: 10.1111/j.1365-2044.2008.05786.x
34. Wypasek E, Undas A, Sniezek-Maciejewska M, Kapelak B, Plicner D, Stepien E, et al. The increased plasma C-reactive protein and interleukin-6 levels in patients undergoing coronary artery bypass grafting surgery are associated with the interleukin-6-174G>C gene polymorphism. Ann Clin Biochem. (2010) 47:343–9. doi: 10.1258/acb.2010.090305
35. Jouan J, Golmard L, Benhamouda N, Durrleman N, Golmard JL, Ceccaldi R, et al. Gene polymorphisms and cytokine plasma levels as predictive factors of complications after cardiopulmonary bypass. J Thorac Cardiovasc Surg. (2012) 144:467–73. 473.e1–2. doi: 10.1016/j.jtcvs.2011.12.022
36. Kraler S, Wenzl FA, Georgiopoulos G, Obeid S, Liberale L, von Eckardstein A, et al. Soluble lectin-like oxidized low-density lipoprotein receptor-1 predicts premature death in acute coronary syndromes. Eur Heart J. (2022) 43:1849–60. doi: 10.1093/eurheartj/ehac143
37. Liberale L, Puspitasari YM, Ministrini S, Akhmedov A, Kraler S, Bonetti NR, et al. JCAD promotes arterial thrombosis through PI3K/Akt modulation: a translational study. Eur Heart J. (2023) 44:1818–33. doi: 10.1093/eurheartj/ehac641
38. Held C, White HD, Stewart RAH, Budaj A, Cannon CP, Hochman JS, et al. Inflammatory biomarkers interleukin-6 and C-reactive protein and outcomes in stable coronary heart disease: experiences from the STABILITY (stabilization of atherosclerotic plaque by initiation of darapladib therapy) trial. J Am Heart Assoc. (2017) 6:e005077. doi: 10.1161/JAHA.116.005077
39. Tyrrell DJ, Goldstein DR. Ageing and atherosclerosis: vascular intrinsic and extrinsic factors and potential role of IL-6. Nat Rev Cardiol. (2021) 18:58–68. doi: 10.1038/s41569-020-0431-7
40. Gatto L, Alfonso F, Paoletti G, Burzotta F, La Manna A, Budassi S, et al. Relationship betweeen the amount and location of macrophages and clinical outcome: subanalysis of the CLIMA-study. Int J Cardiol. (2022) 346:8–12. doi: 10.1016/j.ijcard.2021.11.042
41. Carbone F, Rigamonti F, Burger F, Roth A, Bertolotto M, Spinella G, et al. Serum levels of osteopontin predict major adverse cardiovascular events in patients with severe carotid artery stenosis. Int J Cardiol. (2018) 255:195–9. doi: 10.1016/j.ijcard.2018.01.008
42. Icer MA, Gezmen-Karadag M. The multiple functions and mechanisms of osteopontin. Clin Biochem. (2018) 59:17–24. doi: 10.1016/j.clinbiochem.2018.07.003
43. Wang CQ, Sun HT, Gao XM, Ren N, Sheng YY, Wang Z, et al. Interleukin-6 enhances cancer stemness and promotes metastasis of hepatocellular carcinoma via up-regulating osteopontin expression. Am J Cancer Res. (2016) 6:1873–89. PMID: 27725896; PMCID: PMC504310027725896
44. Uchibori T, Matsuda K, Shimodaira T, Sugano M, Uehara T, Honda T. IL-6 trans-signaling is another pathway to upregulate osteopontin. Cytokine. (2017) 90:88–95. doi: 10.1016/j.cyto.2016.11.006
Keywords: coronary artery bypass, coronary artery bypass grafting, endarterectomy, myocardial infarction, prognosis
Citation: Gao M, Wen W, Gu C, Zhang X, Yu Y and Li H (2023) Coronary plaque burden predicts perioperative cardiovascular events after coronary endarterectomy. Front. Cardiovasc. Med. 10:1175287. doi: 10.3389/fcvm.2023.1175287
Received: 1 March 2023; Accepted: 23 May 2023;
Published: 9 June 2023.
Edited by:
Michio Shimabukuro, Fukushima Medical University, JapanReviewed by:
Flavio Giuseppe Biccirè, Sapienza University of Rome, Italy© 2023 Gao, Wen, Gu, Zhang, Yu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiyang Li b2NlYW4wMjAzQDE2My5jb20= Yang Yu MTM5MTE1MjQxMDFAMTYzLmNvbQ== XiaoLi Zhang eGx6aGFuZzY4QDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.